The Effect of Festulolium Silage-Based Diets on the Content of Tocopherols, β-Carotene and Retinol in Meat from Young Rams
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Silages
- Third-harvest herbage of hybrid ryegrass cv. Bakus (group RB);
- Third-harvest herbage of three Festulolium cultivars:
- Becva (Festuca arudinacea × Lolium multiflorum) (group FB);
- Felopa (Festuca pratensis × Lolium multiflorum) (group FF);
- Paulita (Festuca pratensis × Lolium multiflorum) (group FP).
- Seeds of hybrid ryegrass and three Festulolium cultivars were sown in experimental plots with an area of 544 m2 (16 m × 34 m; seeding rate-approx. 4.65 g seeds/m2) on 11 April 2015. Before sowing, all plots were fertilized with N, P and K (at 2.0, 4.0 and 3.6 g/m2, respectively). In the first growing season, grass was cut at the end of August 2016, and left in the field. After 24 h of wilting, herbage was harvested with a round baler (Kverneland Group, Klepp, Norway). Six layers of plastic film were used to wrap the bales (30 μm × 750 mm) with a stationary bale wrapper (Sipma S.A., Lublin, Poland). The time between baling and wrapping did not exceed 60 min.
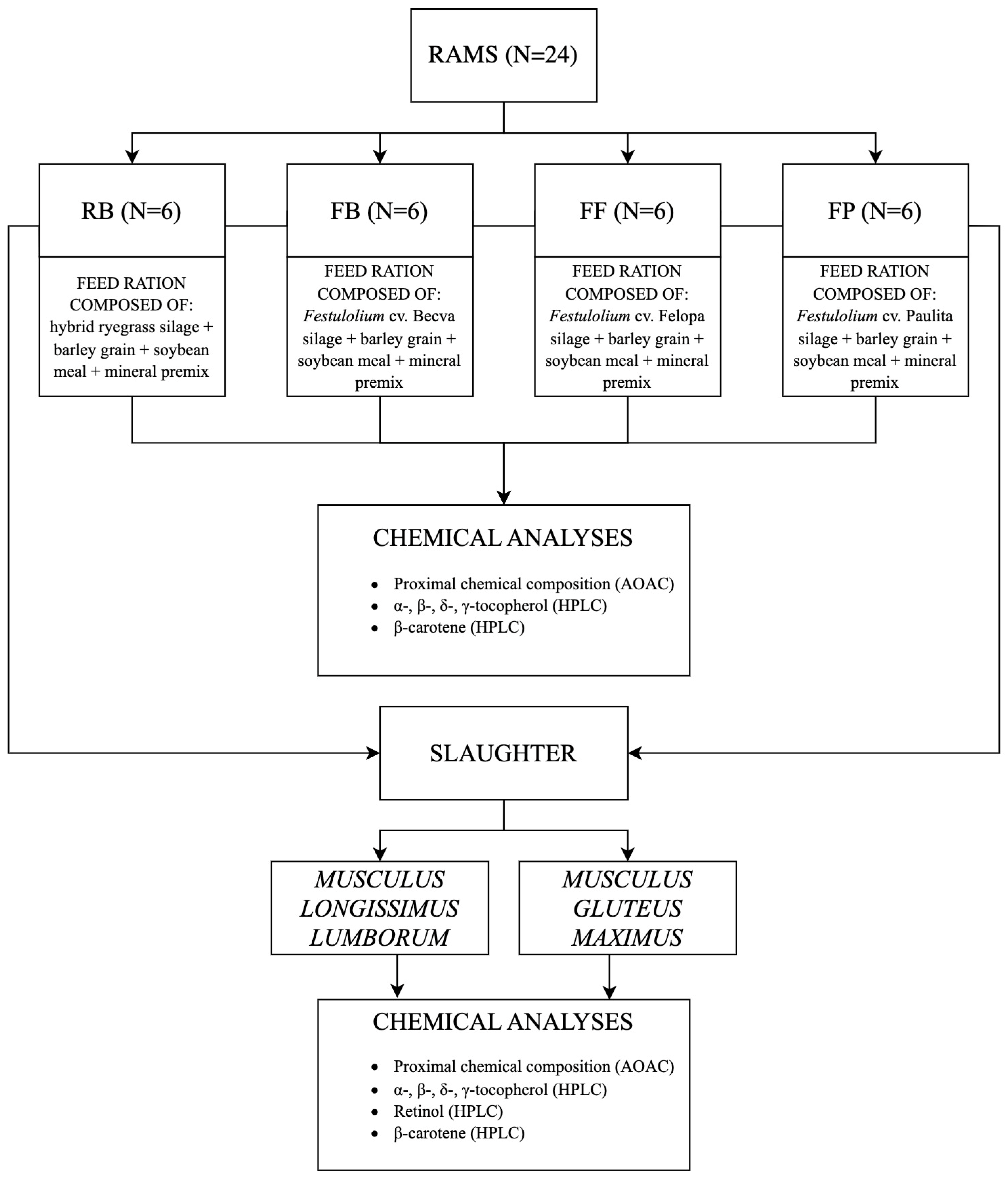
2.2. Animals, Experimental Design and Diets
2.3. Feed Sampling and Analyses
2.4. Meat Sampling and Analyses
2.5. Statistical Analysis
3. Results
4. Discussion
4.1. Tocopherol Concentrations in Meat from Rams (Musculus Gluteus Maximus and Musculus Longissimus Lumborum) Fed Festulolium Silage-Based Diets
4.2. Retinol Concentration in Meat from Rams (Musculus Gluteus Maximus and Musculus Longissimus Lumborum) Fed Festulolium Silage-Based Diets
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Waweru Mwendia, S.; Maass, B.; Njenga, D.; Notenbaert, A. Perrenial ryegrass and novel festulolium forage grasses in the tropical highlands of Central Kenya: Preliminary assessment. Trop. Grassl. 2019, 7, 234–243. [Google Scholar] [CrossRef]
- Grønbæk, O. Festulolium in Scandinavia. Combining yield, forage quality and persistence. In Kvalita Píce z Travních Porostů a Chov Skotu v Měnících se Ekonomických Podmínkách, Sborník z Celostátní Vědecké Konference s Mezinárodní účastí Konané 14. října 2010 v Sále Zámku; Kunín, A., Ed.; Výzkumný ústav Rostlinné Výroby: Prague, Czech Republic, 2010; pp. 5–7. [Google Scholar]
- Rzeźnik, A.; Goliński, P. Osiągnięcia w hodowli mieszańców× Festulolium. Łąkarstwo. Pol. 2013, 16, 79–98. [Google Scholar]
- Macleod, C.K.J.; Humphreys, M.W.; Whalley, W.R.; Turner, L.; Binley, A.; Watts, C.W.; Skøt, L.; Joynes, A.; Hawkins, S.; King, I.P.; et al. A novel grass hybrid to reduce flood generation in temperate regions. Sci. Rep. 2013, 3, 1683. [Google Scholar] [CrossRef] [PubMed]
- Østrem, L.; Volden, B.; Larsen, A. Morphology, dry matter yield and phenological characters at different maturity stages of ×Festulolium compared with other grass species. Acta Agric. Scand. Sect. B Soil Plant Sci. 2013, 63, 531–542. [Google Scholar] [CrossRef]
- Hric, P.; Vozár, Ľ.; Kovár, P.; Hric, J. Growth-production parameters of the first Slovak cultivar of Festulolium A. et Gr. Acta Univ. Agric. Silvic. Mendelianae Brun. 2018, 66, 825–828. [Google Scholar] [CrossRef]
- Šimkunas, A.; Valašinaite, S.; Mažeika, V. Peculiarities of various Festulolium braunii cultivars development and overwintering. Vagos 2009, 85, 35–38. [Google Scholar]
- Staniak, M.; Harasim, E. Changes in nutritive value of alfalfa (Medicago × varia T. Martyn) and Festulolium (Festulolium braunii (K. Richt) A. Camus) under drought stress. J. Agron. Crop. Sci. 2018, 204, 456–466. [Google Scholar] [CrossRef]
- Østrem, L.; Novoa-Garrido, M.; Larsen, A. Festulolium—An interesting forage grass for high-latitude regions? Grassl. Sci. Eur. 2013, 18, 270–272. [Google Scholar]
- Fariaszewska, A.; Aper, J.; Van Huylenbroeck, J.; Baert, J.; De Riek, J.; Staniak, M.; Pecio, Ł. Mild drought stress-induced changes in yield, physiological processes and chemical composition in Festuca, Lolium and Festulolium. J. Agro. Crop Sci. 2017, 203, 103–116. [Google Scholar] [CrossRef]
- Nekrošas, S.; Kemešyte, V. Breeding of ryegrass and Festulolium in Lithuania. Zemdirb. Agric. 2007, 94, 29–39. [Google Scholar]
- Østrem, L.; Larsen, A. Winter survival, yield performance and forage quality of Festulolium cvs. for Norwegian farming. Grassl. Sci. Eur. 2008, 13, 293–295. [Google Scholar]
- Gutmane, I.; Adamovics, A. Influence of nitrogen fertilization rates on Festulolium and Lolium × boucheanum forage yield and persistency. Grassl. Sci. Eur. 2009, 14, 336–338. [Google Scholar]
- Sosnowski, J. The value of production, energy and food of Festulolium braunii (K. Richt.) A. Camus microbiologically and mineral suppled. Fragm. Agron. 2012, 29, 115–122. [Google Scholar]
- Kitczak, T.; Jänicke, H.; Bury, M.; Malinowski, R. The Usefulness of Mixtures with Festulolium braunii for the Regeneration of Grassland under Progressive Climate Change. Agriculture 2021, 11, 537. [Google Scholar] [CrossRef]
- Hopkins, A.; Del Prado, A. Implications of climate change for grassland in Europe: Impacts, adaptations and mitigation options: A review. Grass Forage Sci. 2007, 62, 118–126. [Google Scholar] [CrossRef]
- Staniak, M.; Bojarszczuk, J.; Kraska, P.; Kwiatkowski, C.; Harasim, E. Prolonged drought stress induced changes in yield and physiological processes of Trifolium repens and Festulolium braunii. Biol. Plant. 2020, 64, 701–709. [Google Scholar] [CrossRef]
- Raza, A.; Razzaq, A.; Mehmood, S.; Zou, X.; Zhang, X.; Lv, Y.; Xu, J. Impact of Climate Change on Crops Adaptation and Strategies to Tackle Its Outcome: A Review. Plants. 2019, 8, 34. [Google Scholar] [CrossRef]
- Abdelrahman, M.; Jogaiah, S.; Burritt, D.J.; Tran, L.S.P. Legume genetic resources and transcriptome dynamics under abiotic stress conditions. Plant Cell Environ. 2018, 41, 1972–1983. [Google Scholar] [CrossRef]
- DaMatta, F.M.; Grandis, A.; Arenque, B.C.; Buckeridge, M.S. Impacts of climate changes on crop physiology and food quality. Food Res. Int. 2010, 43, 1814–1823. [Google Scholar] [CrossRef]
- Milewski, S.; Purwin, C.; Pysera, B.; Lipiński, K.; Antoszkiewicz, Z.; Sobiech, P.; Ząbek, K.; Fijałkowska, M.; Tański, Z.; Illek, J. Effect of feeding silages from different plant raw materials on the profile of fatty acids, cholesterol, and vitamins A and E in lamb meat. Acta Vet. Brno 2014, 83, 371–378. [Google Scholar] [CrossRef]
- Dunne, P.G.; Monahan, F.J.; O’mara, F.P.; Moloney, A.P. Colour of bovine subcutaneous adipose tissue: A review of contributory factors, associations with carcass and meat quality and its potential utility in authentication of dietary history. Meat Sci. 2009, 81, 28–45. [Google Scholar] [CrossRef] [PubMed]
- Debier, C.; Larondelle, Y. Vitamins A and E: Metabolism, roles and transfer to offspring. Br. J. Nut. 2005, 93, 153–174. [Google Scholar] [CrossRef] [PubMed]
- Act of 15 January 2015 on the protection of animals used for scientific or educational purposes. J. Laws. 2015, 266.
- Zarudzki, R.; Traczykowski, A.; Mroczko, L. DLG-Tabele Wartości Pokarmowej Pasz i Normy Żywienia Przeżuwaczy; PPH VIT-REA: Kusowo, Poland, 1997. [Google Scholar]
- Council Regulation (EC) No 1099/2009 of 24 September 2009 on the protection of animals at the time of slaughter. J. Laws. 2009, 4, 303.
- The European Parliament and the Council of the European Union. Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010 on the Protection of Animals Used for Scientific Purposes. Official Journal of the European Union (OJEU), 20.10.2010, L 276/33. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:32010L0063 (accessed on 30 May 2023).
- Association of Official Analytical Chemists (AOAC). Official Methods of Analysis of AOAC International, 20th ed.; AOAC International: Gaithersburg, MD, USA, 2016. [Google Scholar]
- Van Soest, P.V.; Robertson, J.B.; Lewis, B.A. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef] [PubMed]
- Polish Standard PN-EN ISO 6867; Feedstuffs. Determination of vitamin E by high-performance liquid chromatography. Polish Committee for Standardization: Warsaw, Poland, 2002.
- De Quirós, A.R.B.; Costa, H.S. Analysis of carotenoids in vegetable and plasma samples: A review. J. Food Composit. Anal. 2006, 19, 97–111. [Google Scholar] [CrossRef]
- Högberg, A.; Pickova, J.; Babol, J.; Andersson, K.; Dutta, P.C. Muscle lipids, vitamins E and A, and lipid oxidation as affected by diet and RN genotype in female and castrated male Hampshire crossbreed pigs. Meat Sci. 2002, 60, 411–420. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, S.F.; Pickova, J. Fatty acids and tocopherol levels in M. Longissimus dorsi of beef cattle in Sweden–A comparison between seasonal diets. Meat Sci. 2007, 76, 746–754. [Google Scholar] [CrossRef]
- Xu, Z. Comparison of extraction methods for quantifying vitamin E from animal tissues. Bioresour. Technol. 2008, 99, 8705–8709. [Google Scholar] [CrossRef] [PubMed]
- Polish Standard PN-EN ISO 14565; Feedstuffs. Determination of vitamin A by high-performance liquid chromatography. Polish Committee for Standardization: Warsaw, Poland, 2002.
- Semwogerere, F.; Katiyatiya, C.L.; Chikwanha, O.C.; Marufu, M.C.; Mapiye, C. Bioavailability and bioefficacy of hemp by-products in ruminant meat production and preservation: A review. Front. Vet. Sci. 2020, 7, 572906. [Google Scholar] [CrossRef] [PubMed]
- Luciano, G.; Natalello, A.; Mattioli, S.; Pauselli, M.; Sebastiani, B.; Niderkorn, V.; Copani, G.; Benhissi, H.; Amanpour, A.; Valenti, B. Feeding lambs with silage mixtures of grass, sainfoin and red clover improves meat oxidative stability under high oxidative challenge. Meat Sci. 2019, 156, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Yang, A.; Brewster, M.J.; Lanari, M.C.; Tume, R.K. Effect of vitamin E supplementation on α-tocopherol and β-carotene concentrations in tissues from pasture-and grain-fed cattle. Meat Sci. 2002, 60, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Bellés, M.; Leal, L.N.; Díaz, V.; Alonso, V.; Roncalés, P.; Beltrán, J.A. Effect of dietary vitamin E on physicochemical and fatty acid stability of fresh and thawed lamb. Food Chem. 2018, 239, 1–8. [Google Scholar] [CrossRef]
- Ponnampalam, E.N.; Hopkins, D.L.; Giri, K.; Jacobs, J.L.; Plozza, T.; Lewandowski, P.; Bekhit, A. The use of oxidative stress biomarkers in live animals (in vivo) to predict meat quality deterioration postmortem (in vitro) caused by changes in muscle biochemical components. J. Anim. Sci. 2017, 95, 3012–3024. [Google Scholar] [CrossRef] [PubMed]
- Jose, C.G.; Jacob, R.H.; Pethick, D.W.; Gardner, G.E. Short term supplementation rates to optimise vitamin E concentration for retail colour stability of Australian lamb meat. Meat Sci. 2016, 111, 101–109. [Google Scholar] [CrossRef]
- Al-Mabruk, R.M.; Beck, N.F.G.; Dewhurst, R.J. Effects of silage species and supplemental vitamin E on the oxidative stability of milk. J. Dairy Sci. 2004, 87, 406–412. [Google Scholar] [CrossRef]
- Valenti, B.; Natalello, A.; Vasta, V.; Campidonico, L.; Roscini, V.; Mattioli, S.; Pauselli, M.; Priolo, A.; Lanza, M.; Luciano, G. Effect of different dietary tannin extracts on lamb growth performances and meat oxidative stability: Comparison between mimosa, chestnut and tara. Animal 2019, 13, 435–443. [Google Scholar] [CrossRef]
- Waniek, S.; di Giuseppe, R.; Plachta-Danielzik, S.; Ratjen, I.; Jacobs, G.; Koch, M.; Borggrefe, J.; Both, M.; Müller, H.P.; Kassubek, J.; et al. Association of Vitamin E Levels with Metabolic Syndrome, and MRI-Derived Body Fat Volumes and Liver Fat Content. Nutrients 2017, 9, 1143. [Google Scholar] [CrossRef]
- Green, A.S.; Fascetti, A.J. Meeting the Vitamin A Requirement: The Efficacy and Importance of β-Carotene in Animal Species. Sci. World J. 2016, 2016, 7393620. [Google Scholar] [CrossRef]
- Yang, A.; Larsen, T.W.; Tume, R.K. Carotenoid and retinol concentrations in serum, adipose tissue and liver and carotenoid transport in sheep, goats and cattle. Aust. J. Agric. Res. 1992, 43, 1809–1817. [Google Scholar] [CrossRef]
- Álvarez, R.; Meléndez-Martínez, A.J.; Vicario, I.M.; Alcalde, M.J. Carotenoids and fat-soluble vitamins in horse tissues: A comparison with cattle. Animal 2015, 9, 1230–1238. [Google Scholar] [CrossRef] [PubMed]
- Chauveau-Duriot, B.; Doreau, M.; Noziere, P.; Graulet, B. Simultaneous quantification of carotenoids, retinol, and tocopherols in forages, bovine plasma, and milk: Validation of a novel UPLC method. Anal. Bioanal. Chem. 2010, 397, 777–790. [Google Scholar] [CrossRef] [PubMed]
- Röhrle, F.T.; Moloney, A.P.; Osorio, M.T.; Luciano, G.; Priolo, A.; Caplan, P.; Monahan, F.J. Carotenoid, colour and reflectance measurements in bovine adipose tissue to discriminate between beef from different feeding systems. Meat Sci. 2011, 88, 347–353. [Google Scholar] [CrossRef] [PubMed]
- Cardinault, N.; Doreau, M.; Poncet, C.; Noziere, P. Digestion and absorption of carotenoids in sheep given fresh red clover. Anim. Sci. 2006, 82, 49–55. [Google Scholar] [CrossRef]
- Kasapidou, E.; Enser, M.; Wood, J.D.; Richardson, R.I.; Wilkinson, R.G.; Sinclair, L.A. Influence of vitamin E supplementation and basal diet on the vitamin E status, performance and tissue fatty acid concentration in lambs. Animal 2009, 3, 516–526. [Google Scholar] [CrossRef]
- Kilinç, Ü.; Çerçi, İ.H.; Seven, P.T.; Gürdoğan, F.; Bahsi, M.; Yilmaz, Ö.; Özçelik, M.; Benzer, F.; Erişir, Z.; Seven, İ. The Effect of Alfalfa on Retinol, α-Tocopherol and Cholesterol Levels in Muscle and Tail Fat Tissues in Yearling Sheep. F. Ü. Sağ. Bil. Vet. Derg. 2011, 25, 119–123. [Google Scholar]
- Jordan, H.A.; Smith, G.S.; Neumann, A.L.; Zimmerman, J.E.; Breniman, G.W. Vitamin A nutrition of beef cattle fed corn silages. J. Anim. Sci. 1963, 22, 738–745. [Google Scholar] [CrossRef]
| Specification | Groups | |||
|---|---|---|---|---|
| RB | FB | FF | FP | |
| Diet composition, % DM | ||||
| Silage made from hybrid ryegrass cv. Bakus | 60 | - | - | - |
| Silage made from Festulolium cv. Becva | - | 60 | - | - |
| Silage made from Festulolium cv. Felopa | - | - | 60 | - |
| Silage made from Festulolium cv. Paulita | - | - | - | 60 |
| Barley grain | 35 | 35 | 35 | 35 |
| Soybean meal | 5 | 5 | 5 | 5 |
| Minerals and vitamins (g/d/animal) | 20 | 20 | 20 | 20 |
| Specification | Groups | SEM | |||
|---|---|---|---|---|---|
| RB | FB | FF | FP | ||
| DM, g kg−1 | 429 | 435 | 430 | 431 | 0.342 |
| OM | 905 | 932 | 938 | 936 | 0.054 |
| CP | 149 | 148 | 130 | 134 | 0.202 |
| EE | 24 | 27 | 29 | 26 | 0.260 |
| NDF | 395 | 394 | 445 | 409 | 0.352 |
| α-Tocopherol | 5.89 | 4.44 | 5.10 | 5.52 | 0.220 |
| β-Tocopherol | 0.75 | 0.51 | 0.54 | 0.56 | 0.035 |
| γ-Tocopherol | 1.16 | 1.12 | 0.79 | 1.37 | 0.085 |
| δ-Tocopherol | 0.89 | 0.55 | 0.52 | 1.12 | 0.091 |
| Total tocopherols | 8.69 | 6.62 | 6.95 | 8.57 | 0.346 |
| β-Carotene | 278.39 | 241.84 | 253.68 | 294.34 | 0.497 |
| Specification | Groups | SEM | p-Value | |||
|---|---|---|---|---|---|---|
| RB | FB | FF | FP | |||
| DM | 1155 | 1075 | 1080 | 1120 | 3.617 | 0.104 |
| CP | 172 | 159 | 140 | 150 | 4.767 | 0.119 |
| EE | 28 | 29 | 31 | 29 | 1.372 | 0.920 |
| α-Tocopherol | 6.8 A | 4.8 B | 5.5 B | 6.2 AB | 0.269 | <0.001 |
| β-Tocopherol | 0.87 A | 0.55 B | 0.58 B | 0.63 B | 0.040 | <0.001 |
| γ-Tocopherol | 1.34 ABC | 1.2 B | 0.85 D | 1.54 A | 0.087 | <0.001 |
| δ-Tocopherol | 1.03 A | 0.59 B | 0.56 B | 1.25 A | 0.094 | <0.001 |
| Total tocopherols | 10.04 A | 7.12 B | 7.51 B | 9.6 A | 0.431 | 0.002 |
| β-Carotene | 322 | 260 | 274 | 330 | 4.409 | 0.239 |
| Specification | Groups | SEM | p-Value | |||
|---|---|---|---|---|---|---|
| RB | FB | FF | FP | |||
| DM | 26.42 | 26.82 | 26.41 | 24.53 | 0.373 | 0.054 |
| CA | 1.11 | 1.14 | 1.08 | 1.03 | 0.014 | 0.060 |
| CP | 20.08 | 20.71 | 19.87 | 20.03 | 0.179 | 0.388 |
| EE | 5.23 | 4.97 | 5.46 | 3.47 | 0.412 | 0.330 |
| α-Tocopherol | 1.38 B | 1.56 B | 2.30 A | 2.03 A | 0.090 | <0.001 |
| β-Tocopherol | 0.10 A | 0.10 A | 0.07 B | 0.09 A | 0.002 | <0.001 |
| γ-Tocopherol | 0.27 B | 0.24 B | 0.34 A | 0.32 A | 0.012 | <0.001 |
| δ-Tocopherol | 0.17 B | 0.20 | 0.23 A | 0.21 | 0.006 | 0.003 |
| Total tocopherols | 1.92 C | 2.09 C | 2.94 A | 2.65 B | 0.100 | <0.001 |
| β-Carotene | Nd | Nd | Nd | Nd | - | - |
| Retinol | 0.77 C | 1.11 B | 2.12 A | 2.26 A | 0.150 | <0.001 |
| Specification | Groups | SEM | p-Value | |||
|---|---|---|---|---|---|---|
| RB | FB | FF | FP | |||
| DM | 27.01 | 26.64 | 26.36 | 25.93 | 0.242 | 0.153 |
| CA | 1.08 | 1.10 | 1.03 | 1.06 | 0.018 | 0.601 |
| CP | 21.84 | 21.51 | 20.84 | 21.75 | 0.152 | 0.073 |
| EE | 4.09 | 4.03 | 4.49 | 3.12 | 0.250 | 0.272 |
| α-Tocopherol | 2.09 B | 2.31 B | 3.53 A | 3.05 A | 0.150 | <0.001 |
| β-Tocopherol | 0.09 B | 0.05 B | 0.19 A | 0.21 A | 0.018 | <0.001 |
| γ-Tocopherol | 0.36 C | 0.27 D | 0.50 B | 0.71 A | 0.039 | <0.001 |
| δ-Tocopherol | 0.22 C | 0.27 C | 0.82 A | 0.42 B | 0.056 | <0.001 |
| Total tocopherols | 2.75 B | 2.90 B | 5.03 A | 4.39 A | 0.236 | <0.001 |
| β-Carotene | nd | nd | Nd | nd | - | - |
| Retinol | 0.74 | 1.19 | 0.78 | 1.21 | 0.083 | 0.053 |
| α-Tocopherol | β-Tocopherol | γ-Tocopherol | δ-Tocopherol | Total Tocopherols | β-Carotene | Retinol | |
|---|---|---|---|---|---|---|---|
| α-Tocopherol | 1.00 | ||||||
| β-Tocopherol | −0.94 * | 1.00 | |||||
| γ-Tocopherol | 0.87 * | −0.95 * | 1.00 | ||||
| δ-Tocopherol | 0.90 * | −0.87 * | 0.68 * | 1.00 | |||
| Total tocopherols | 0.99 * | −0.94 * | 0.88 * | 0.89 * | 1.00 | ||
| β-Carotene | - | - | - | - | - | 1.00 | |
| Retinol | 0.93 * | −0.75 * | 0.71 * | 0.75 * | 0.92 * | 0.91 * | 1.00 |
| α-Tocopherol | β-Tocopherol | γ-Tocopherol | δ-Tocopherol | Total Tocopherols | β-Carotene | Retinol | |
|---|---|---|---|---|---|---|---|
| α-Tocopherol | 1.00 | ||||||
| β-Tocopherol | 0.86 * | 1.00 | |||||
| γ-Tocopherol | 0.67 * | 0.94 * | 1.00 | ||||
| δ-Tocopherol | 0.94 * | 0.69 * | 0.41 * | 1.00 | |||
| Total tocopherols | 0.99 * | 0.91 * | 0.73 * | 0.92 * | 1.00 | ||
| β-Carotene | - | - | - | - | - | 1.00 | |
| Retinol | −0.04 | −0.01 | 0.23 | −0.31 | −0.06 | 0.92 * | 1.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Czurgiel, S.; Antoszkiewicz, Z.; Mazur-Kuśnirek, M.; Bogdaszewski, M. The Effect of Festulolium Silage-Based Diets on the Content of Tocopherols, β-Carotene and Retinol in Meat from Young Rams. Animals 2023, 13, 1817. https://doi.org/10.3390/ani13111817
Czurgiel S, Antoszkiewicz Z, Mazur-Kuśnirek M, Bogdaszewski M. The Effect of Festulolium Silage-Based Diets on the Content of Tocopherols, β-Carotene and Retinol in Meat from Young Rams. Animals. 2023; 13(11):1817. https://doi.org/10.3390/ani13111817
Chicago/Turabian StyleCzurgiel, Sylwia, Zofia Antoszkiewicz, Magdalena Mazur-Kuśnirek, and Marek Bogdaszewski. 2023. "The Effect of Festulolium Silage-Based Diets on the Content of Tocopherols, β-Carotene and Retinol in Meat from Young Rams" Animals 13, no. 11: 1817. https://doi.org/10.3390/ani13111817
APA StyleCzurgiel, S., Antoszkiewicz, Z., Mazur-Kuśnirek, M., & Bogdaszewski, M. (2023). The Effect of Festulolium Silage-Based Diets on the Content of Tocopherols, β-Carotene and Retinol in Meat from Young Rams. Animals, 13(11), 1817. https://doi.org/10.3390/ani13111817





