Evaluation of Immune Nanoparticles for Rapid and Non-Specific Activation of Antiviral and Antibacterial Immune Responses in Cattle, Swine, and Poultry
Abstract
Simple Summary
Abstract
1. Introduction
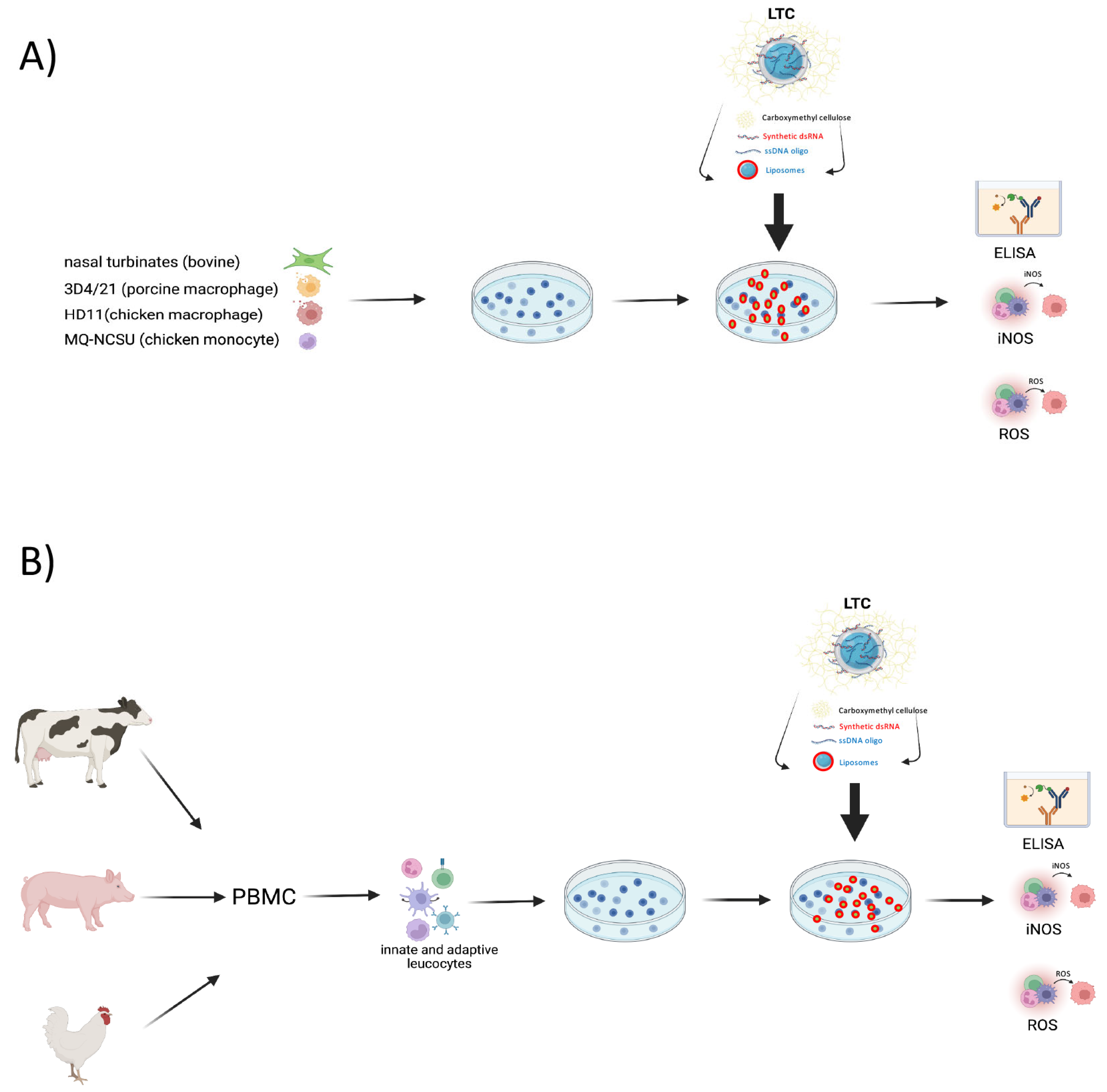
2. Materials and Methods
2.1. Preparation of Liposome-TLR Complexes
2.2. Cell Lines and Antibodies
2.3. Separation of Peripheral Blood Mononuclear Cells (PBMC)
2.4. Generation of Primary Cultures of Bovine Monocyte-Derived Macrophages (MDM)
2.5. Assessment of NO Production
2.6. Assessment of ROS Generation
2.7. ELISA Assay for Measurement of Cytokine Production
2.8. RT-PCR Assays for Measurement of IFNβ Production by Cells from Poultry
2.9. Statistical Analysis
3. Results
3.1. Immune Response of Bovine Nasal Turbinate Epithelial Cells to Treatment with LTC
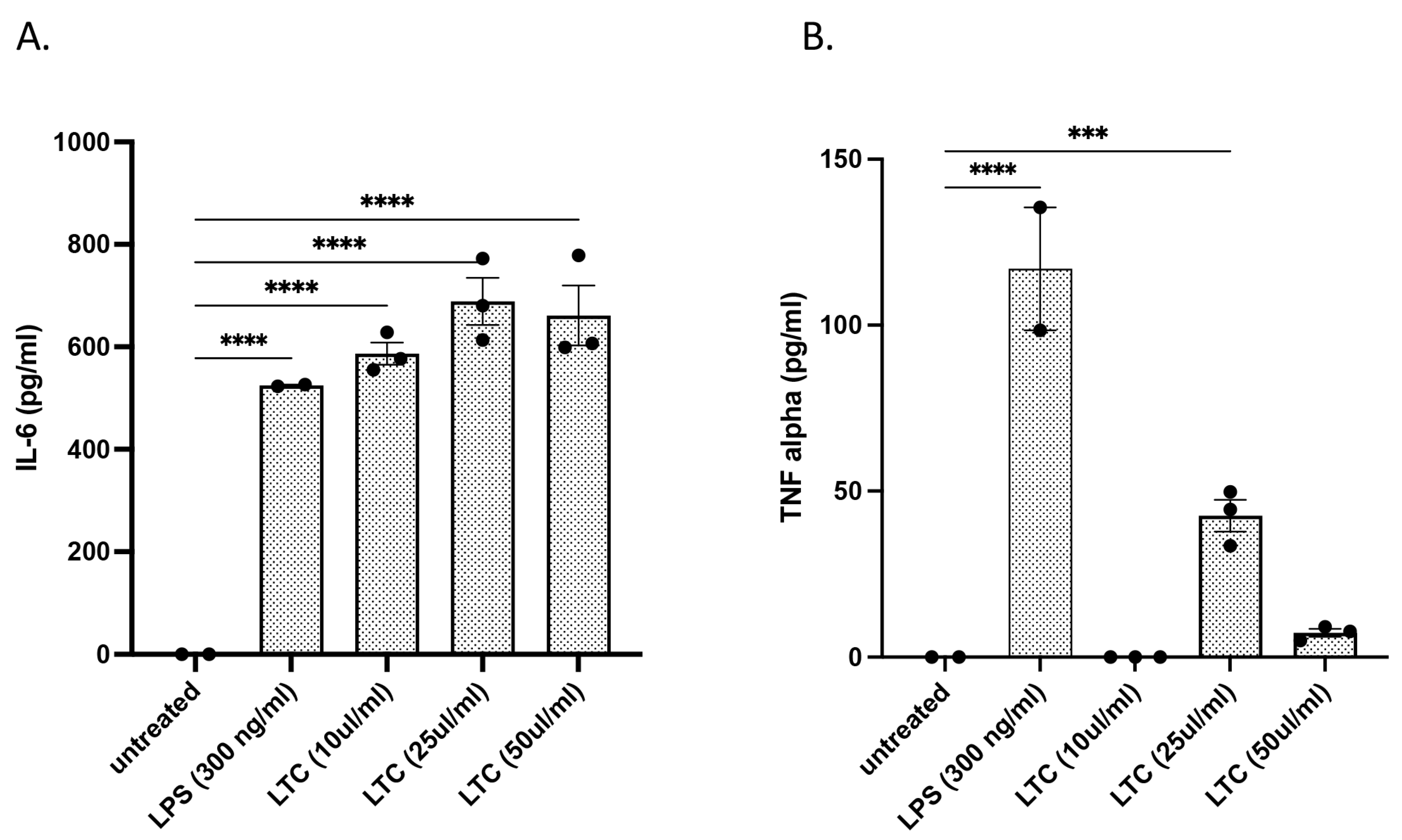
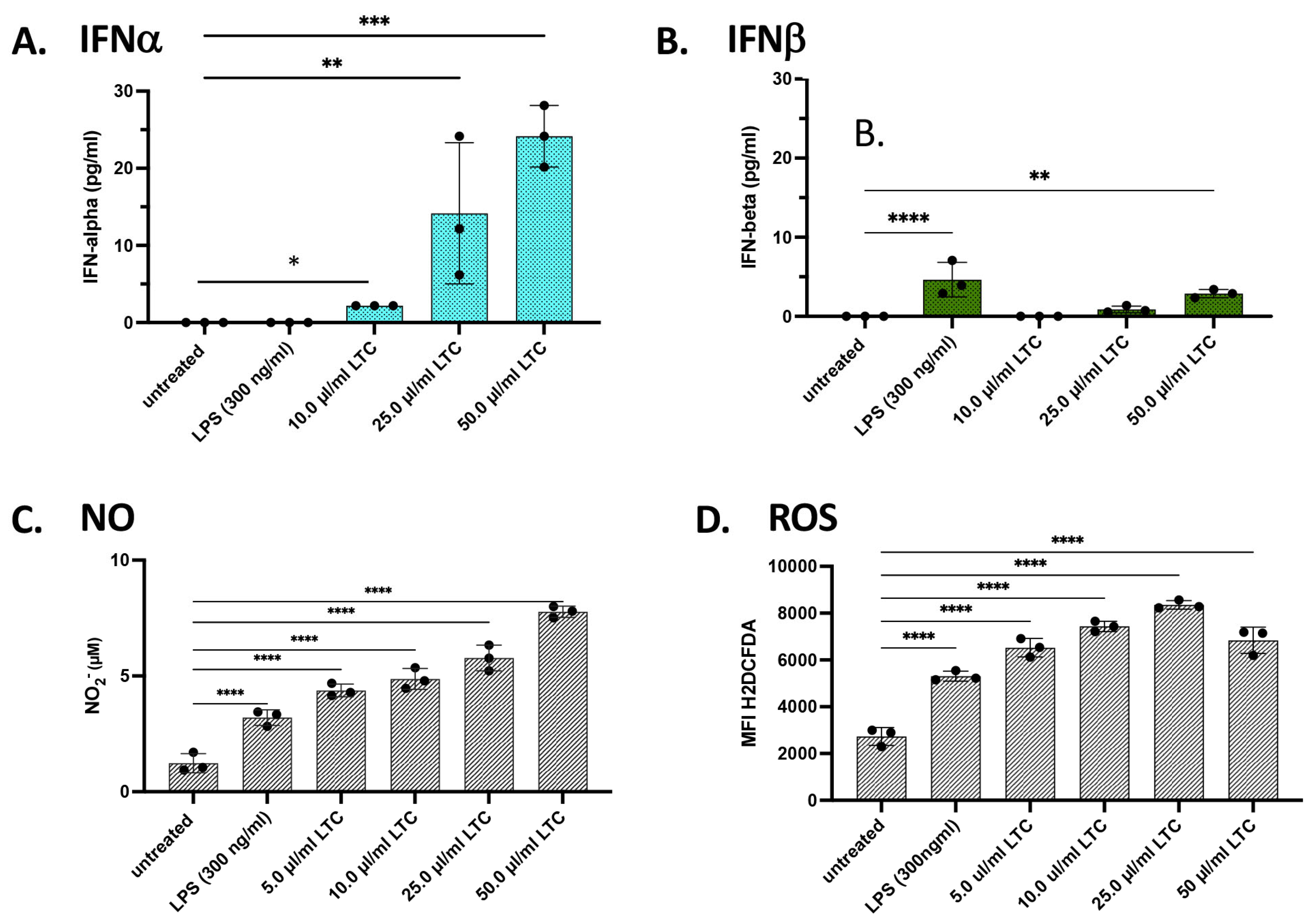
3.2. In Vitro Treatment of Bovine PBMC with LTC Stimulated Significant Increases in Protective Innate Immunity Mediators
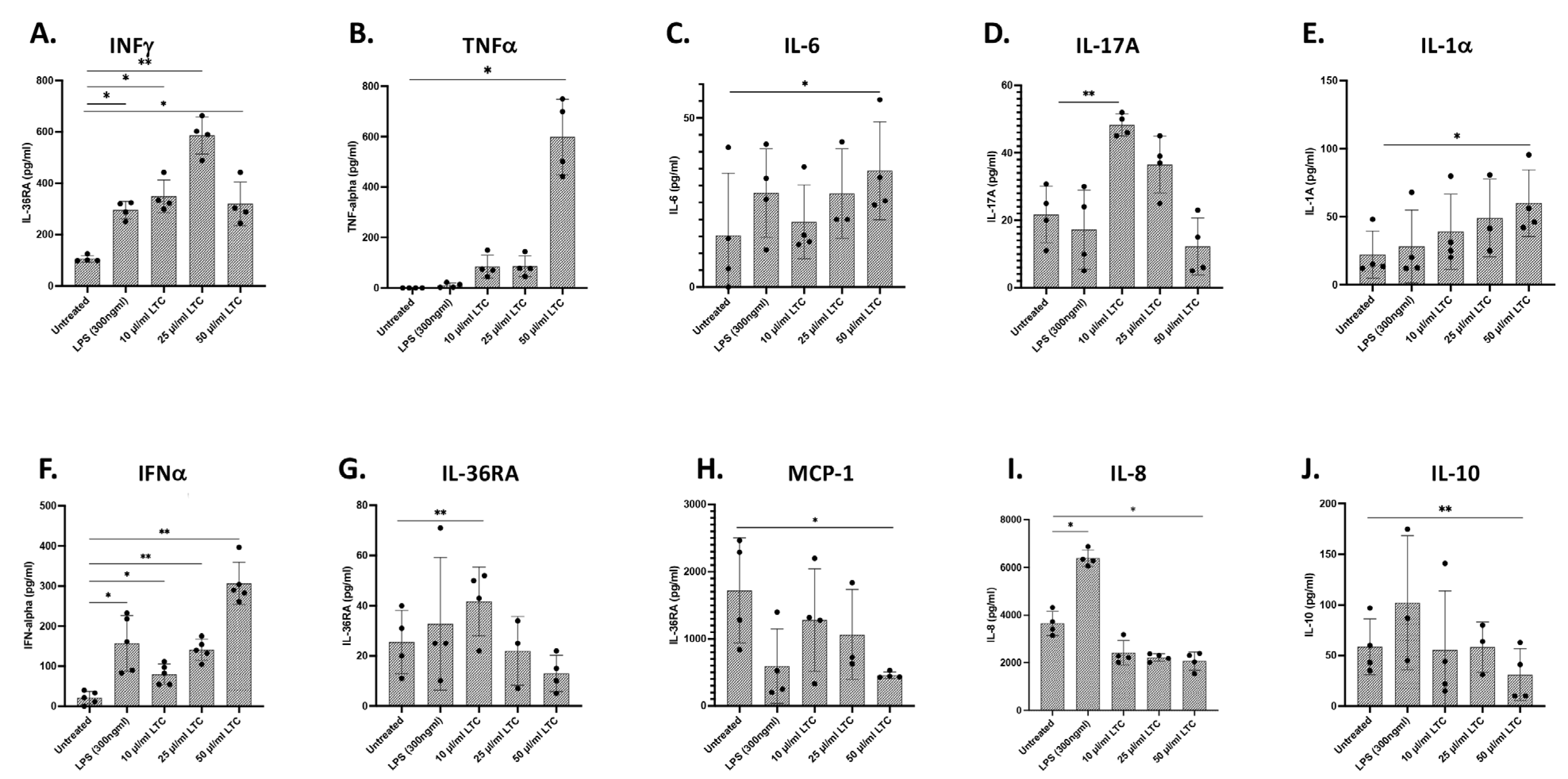
3.3. Treatment of Bovine Monocyte-Derived Macrophages (MDM) with LTC Stimulated Multiple Innate Immune Pathways
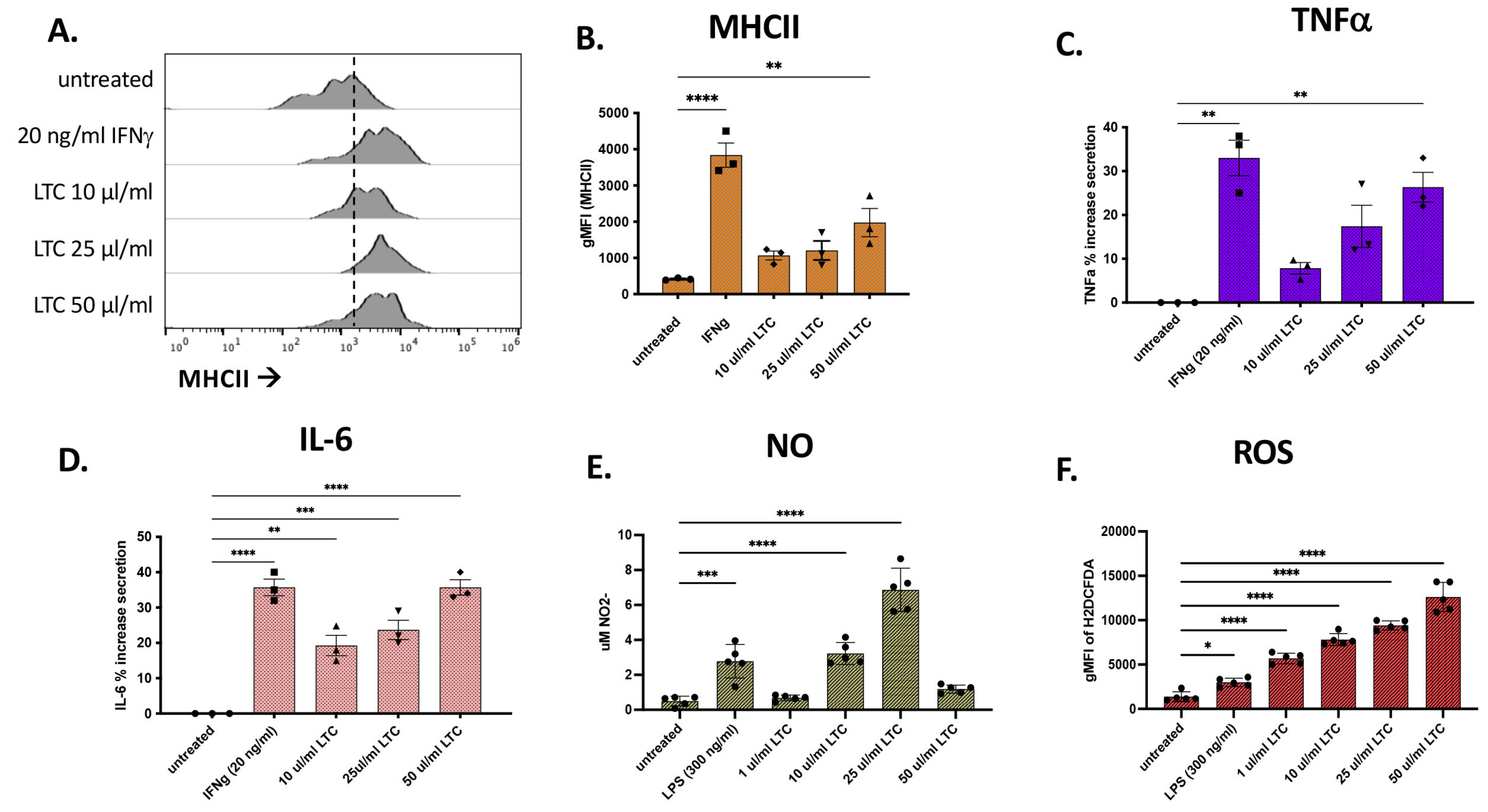
3.4. Porcine Macrophage Responses to LTC Stimulation
3.5. Responses of Porcine Leukocytes to LTC Activation
3.6. LTC Responses by Macrophages from Poultry
3.7. Response of PBMC from Chickens to LTC Activation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sánchez-Cordón, P.J.; Montoya, M.; Reis, A.L.; Dixon, L.K. African swine fever: A re-emerging viral disease threatening the global pig industry. Vet. J. 2018, 233, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Domenech, J.; Lubroth, J.; Sumption, K. Immune Protection in Animals: The Examples of Rinderpest and Foot-and-Mouth Disease. J. Comp. Pathol. 2010, 142 (Suppl. 1), S120–S124. [Google Scholar] [CrossRef] [PubMed]
- Lin, F.; Kitching, R. Swine Vesicular Disease: An Overview. Vet. J. 2000, 160, 192–201. [Google Scholar] [CrossRef] [PubMed]
- Knight-Jones, T.J.; Rushton, J. The economic impacts of foot and mouth disease—What are they, how big are they and where do they occur? Prev. Vet. Med. 2013, 112, 161–173. [Google Scholar] [CrossRef]
- Mao, Q.; Ma, S.; Schrickel, P.L.; Zhao, P.; Wang, J.; Zhang, Y.; Li, S.; Wang, C. Review detection of Newcastle disease virus. Front. Vet. Sci. 2022, 9, 936251. [Google Scholar] [CrossRef]
- Priola, S.A.; Vorberg, I.; Spencer, J.F.T. Molecular Aspects of Disease Pathogenesis in the Transmissible Spongiform Encephalopathies. Methods Mol. Biol. 2004, 268, 517–540. [Google Scholar] [CrossRef]
- Dixon, L.K.; Sun, H.; Roberts, H. African swine fever. Antivir. Res. 2019, 165, 34–41. [Google Scholar] [CrossRef]
- Sauter-Louis, C.; Conraths, F.J.; Probst, C.; Blohm, U.; Schulz, K.; Sehl, J.; Fischer, M.; Forth, J.H.; Zani, L.; Depner, K.; et al. African Swine Fever in Wild Boar in Europe—A Review. Viruses 2021, 13, 1717. [Google Scholar] [CrossRef]
- Verhagen, J.H.; Fouchier, R.A.M.; Lewis, N. Highly Pathogenic Avian Influenza Viruses at the Wild–Domestic Bird Interface in Europe: Future Directions for Research and Surveillance. Viruses 2021, 13, 212. [Google Scholar] [CrossRef]
- Wille, M.; Barr, I.G. Resurgence of avian influenza virus. Science 2022, 376, 459–460. [Google Scholar] [CrossRef]
- Diamond, M.S.; Kanneganti, T.D. Innate immunity: The first line of defense against SARS-CoV-2. Nat. Immunol. 2022, 23, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Wheat, W.; Chow, L.; Kuzmik, A.; Soontararak, S.; Kurihara, J.; Lappin, M.; Dow, S. Local immune and microbiological responses to mucosal administration of a Liposome-TLR agonist immunotherapeutic in dogs. BMC Vet. Res. 2019, 15, 330. [Google Scholar] [CrossRef] [PubMed]
- Wheat, W.; Chow, L.; Coy, J.; Contreras, E.; Lappin, M.; Dow, S. Activation of upper respiratory tract mucosal innate immune responses in cats by liposomal toll-like receptor ligand complexes delivered topically. J. Vet. Intern. Med. 2019, 33, 838–845. [Google Scholar] [CrossRef] [PubMed]
- Contreras, E.T.; Olea-Popelka, F.; Wheat, W.; Dow, S.; Hawley, J.; Lappin, M.R. Evaluation of liposome toll-like receptor ligand complexes for non-specific mucosal immunoprotection from feline herpesvirus-1 infection. J. Vet. Intern. Med. 2019, 33, 831–837. [Google Scholar] [CrossRef]
- Decout, A.; Katz, J.D.; Venkatraman, S.; Ablasser, A. The cGAS-STING pathway as a therapeutic target in inflammatory diseases. Nat. Rev. Immunol. 2021, 21, 548–569. [Google Scholar] [CrossRef]
- Xu, S.; Jin, T.; Weng, J. Endothelial Cells as a Key Cell Type for Innate Immunity: A Focused Review on RIG-I Signaling Pathway. Front. Immunol. 2022, 13, 951614. [Google Scholar] [CrossRef]
- Goodyear, A.; Kellihan, L.; Bielefeldt-Ohmann, H.; Troyer, R.; Propst, K.; Dow, S. Protection from Pneumonic Infection with Burkholderia Species by Inhalational Immunotherapy. Infect. Immun. 2009, 77, 1579–1588. [Google Scholar] [CrossRef]
- Propst, K.L.; Troyer, R.M.; Kellihan, L.M.; Schweizer, H.P.; Dow, S.W. Immunotherapy Markedly Increases the Effectiveness of Antimicrobial Therapy for Treatment of Burkholderia pseudomallei Infection. Antimicrob. Agents Chemother. 2010, 54, 1785–1792. [Google Scholar] [CrossRef]
- Jones, A.; Bosio, C.; Duffy, A.; Goodyear, A.; Schriefer, M.; Dow, S. Protection against pneumonic plague following oral immunization with a non-replicating vaccine. Vaccine 2010, 28, 5924–5929. [Google Scholar] [CrossRef]
- Troyer, R.M.; Propst, K.L.; Fairman, J.; Bosio, C.M.; Dow, S.W. Mucosal immunotherapy for protection from pneumonic infection with Francisella tularensis. Vaccine 2009, 27, 4424–4433. [Google Scholar] [CrossRef]
- Logue, C.H.; Phillips, A.T.; Mossel, E.C.; Ledermann, J.P.; Welte, T.; Dow, S.W.; Olson, K.E.; Powers, A.M. Treatment with cationic liposome–DNA complexes (CLDCs) protects mice from lethal Western equine encephalitis virus (WEEV) challenge. Antivir. Res. 2010, 87, 195–203. [Google Scholar] [CrossRef]
- Wheat, W.; Chow, L.; Rozo, V.; Herman, J.; Brooks, K.S.; Colbath, A.; Hunter, R.; Dow, S. Non-specific protection from respiratory tract infections in cattle generated by intranasal administration of an innate immune stimulant. PLoS ONE 2020, 15, e0235422. [Google Scholar] [CrossRef] [PubMed]
- Hause, B.M.; Huntimer, L.; Falkenberg, S.; Henningson, J.; Lechtenberg, K.; Halbur, T. An inactivated influenza D virus vaccine partially protects cattle from respiratory disease caused by homologous challenge. Vet. Microbiol. 2017, 199, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Uprety, T.; Sreenivasan, C.C.; Bhattarai, S.; Wang, D.; Kaushik, R.S.; Li, F. Isolation and development of bovine primary respiratory cells as model to study influenza D virus infection. Virology 2021, 559, 89–99. [Google Scholar] [CrossRef]
- Alfi, O.; From, I.; Yakirevitch, A.; Drendel, M.; Wolf, M.; Meir, K.; Zakay-Rones, Z.; Nevo, Y.; Elgavish, S.; Ilan, O.; et al. Human Nasal Turbinate Tissues in Organ Culture as a Model for Human Cytomegalovirus Infection at the Mucosal Entry Site. J. Virol. 2020, 94, e01258-20. [Google Scholar] [CrossRef] [PubMed]
- Al-Rasheed, M.; Ball, C.; Manswr, B.; Leeming, G.; Ganapathy, K. Infectious bronchitis virus infection in chicken: Viral load and immune responses in Harderian gland, choanal cleft and turbinate tissues compared to trachea. Br. Poult. Sci. 2022, 63, 484–492. [Google Scholar] [CrossRef]
- Beug, H.; von Kirchbach, A.; Döderlein, G.; Conscience, J.-F.; Graf, T. Chicken hematopoietic cells transformed by seven strains of defective avian leukemia viruses display three distinct phenotypes of differentiation. Cell 1979, 18, 375–390. [Google Scholar] [CrossRef]
- Qureshi, M.; Miller, L.; Lillehoj, H.; Ficken, M. Establishment and characterization of a chicken mononuclear cell line. Vet. Immunol. Immunopathol. 1990, 26, 237–250. [Google Scholar] [CrossRef]
- Hoagland, D.A.; Møller, R.; Uhl, S.A.; Oishi, K.; Frere, J.; Golynker, I.; Horiuchi, S.; Panis, M.; Blanco-Melo, D.; Sachs, D.; et al. Leveraging the antiviral type I interferon system as a first line of defense against SARS-CoV-2 pathogenicity. Immunity 2021, 54, 557–570.e5. [Google Scholar] [CrossRef]
- Netea, M.G.; Joosten, L.A.B.; Latz, E.; Mills, K.H.G.; Natoli, G.; Stunnenberg, H.G.; O’neill, L.A.J.; Xavier, R.J. Trained immunity: A program of innate immune memory in health and disease. Science 2016, 352, aaf1098. [Google Scholar] [CrossRef]
- McNab, F.; Mayer-Barber, K.; Sher, A.; Wack, A.; O’Garra, A. Type I interferons in infectious disease. Nat. Rev. Immunol. 2015, 15, 87–103. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Chen, V.; Shannon, C.P.; Wei, X.-S.; Xiang, X.; Wang, X.; Wang, Z.-H.; Tebbutt, S.J.; Kollmann, T.R.; Fish, E.N. Interferon-α2b Treatment for COVID-19. Front. Immunol. 2020, 11, 1061. [Google Scholar] [CrossRef]
- Weigert, A.; von Knethen, A.; Fuhrmann, D.; Dehne, N.; Brüne, B. Redox-signals and macrophage biology. Mol. Asp. Med. 2018, 63, 70–87. [Google Scholar] [CrossRef] [PubMed]
- Cinelli, M.A.; Do, H.T.; Miley, G.P.; Silverman, R.B. Inducible nitric oxide synthase: Regulation, structure, and inhibition. Med. Res. Rev. 2019, 40, 158–189. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, P. Nitric oxide and immune response. Indian J. Biochem. Biophys. 2007, 44, 310–319. [Google Scholar]
- Bogdan, C. Nitric oxide and the immune response. Nat. Immunol. 2001, 2, 907–916. [Google Scholar] [CrossRef]
- Thwe, P.M.; Amiel, E. The role of nitric oxide in metabolic regulation of Dendritic cell immune function. Cancer Lett. 2017, 412, 236–242. [Google Scholar] [CrossRef]
- Nathan, C.; Cunningham-Bussel, A. Beyond oxidative stress: An immunologist’s guide to reactive oxygen species. Nat. Rev. Immunol. 2013, 13, 349–361. [Google Scholar] [CrossRef]
- Dickson, K.B.; Zhou, J. Role of reactive oxygen species and iron in host defense against infection. Front. Biosci. 2020, 25, 1600–1616. [Google Scholar]
- Morris, G.; Gevezova, M.; Sarafian, V.; Maes, M. Redox regulation of the immune response. Cell. Mol. Immunol. 2022, 19, 1079–1101. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wheat, W.H.; Chow, L.; Betlach, A.M.; Pieters, M.; Kurihara, J.; Dow, C.; Johnson, V.; Garry, F.B.; Dow, S. Evaluation of Immune Nanoparticles for Rapid and Non-Specific Activation of Antiviral and Antibacterial Immune Responses in Cattle, Swine, and Poultry. Animals 2023, 13, 1686. https://doi.org/10.3390/ani13101686
Wheat WH, Chow L, Betlach AM, Pieters M, Kurihara J, Dow C, Johnson V, Garry FB, Dow S. Evaluation of Immune Nanoparticles for Rapid and Non-Specific Activation of Antiviral and Antibacterial Immune Responses in Cattle, Swine, and Poultry. Animals. 2023; 13(10):1686. https://doi.org/10.3390/ani13101686
Chicago/Turabian StyleWheat, William H., Lyndah Chow, Alyssa M. Betlach, Maria Pieters, Jade Kurihara, Cooper Dow, Valerie Johnson, Franklyn B. Garry, and Steven Dow. 2023. "Evaluation of Immune Nanoparticles for Rapid and Non-Specific Activation of Antiviral and Antibacterial Immune Responses in Cattle, Swine, and Poultry" Animals 13, no. 10: 1686. https://doi.org/10.3390/ani13101686
APA StyleWheat, W. H., Chow, L., Betlach, A. M., Pieters, M., Kurihara, J., Dow, C., Johnson, V., Garry, F. B., & Dow, S. (2023). Evaluation of Immune Nanoparticles for Rapid and Non-Specific Activation of Antiviral and Antibacterial Immune Responses in Cattle, Swine, and Poultry. Animals, 13(10), 1686. https://doi.org/10.3390/ani13101686





