Comparative Transcriptome Analysis Reveals the Innate Immune Response to Mycoplasma gallisepticum Infection in Chicken Embryos and Newly Hatched Chicks
Abstract
Simple Summary
Abstract
1. Introduction
| Animal | Sample | Groups | Pathways | Reference |
|---|---|---|---|---|
| 5-week SPF female chicken | Tracheal epithelial cells | CG vs. MG Rlow, Rhigh, Rlow LAMP, and Rhigh LAMP | Up: TLRs, TNF-α/NF-kB, adipogenesis, senescence and autophagy, MAPK, apoptosis, EGFR1, Type II interferon | [11] |
| 5-week SPF chicken | 1, 3, 5, and 7 days after challenge Tracheal | CG vs. MG Rlow | Up: apoptosis, TLRs, MAPK, JAK-SAT, NKC mediated cytotoxicity, CCRI, intestinal immune network for IgA production | [12] |
| 5-week SPF chicken | 1 and 2 days after infection Tracheal lumen cell | CG vs. MG Rlow, GT5, and Mg7 | Up: CCRI, MAPK, NOD receptor, JAK-SAT, TLRs | [13] |
| 5-week SPF chicken | 6 days after infection Tracheal rings | CG/H3N8 vs. MGRlow/H3N8 | Up: phagosome, cell adhesion molecules, intestinal immune network for IgA production, JAK/STAT, TLRs Down: cardiac muscle contraction, purine metabolism, oxidative phosphorylation | [14] |
| 10d chick | 3 days after infection Lung tissue | CG vs. MG Rlow/E. coli O78 | Up: CCRI, phagosome, IL-17, NF-κB, cell adhesion molecules | [15] |
| 70-week or 4-week SPF chicken | Two weeks after challenge Tracheal tissues | CG vs. MG Asp3A | Up: DNA replication, cell cycle, CCRI, TLRs, phagosome, Down: formation and motor movement of the cilia, adherens junctions, tight junctions | [5] |
| 3-week SPF chicken | 15 days after infection Tracheal | CG vs. MG-HY | Up: cell adhesion molecules, phagosome, MAPK, calcium, and PPAR Down: Tight, ECM-receptor interaction, focal, AMPK, p53, Rap1, regulation of actin | [16] |
2. Materials and Methods
2.1. Ethics in Animal Experimentation
2.2. Mycoplasma gallisepticum Strain and Culture
2.3. Cell Culture and Treatment
2.4. Experimental Infection of Chicks and Chicken Embryos
2.5. Total RNA Extraction and Real-Time Quantitative PCR (RT-qPCR)
2.6. Illumina Sequencing
2.7. Transcriptome Data Analysis
2.8. Histopathological Evaluation of Trachea, Lungs, and Immune Organs in Birds
2.9. Statistical Analysis
3. Results
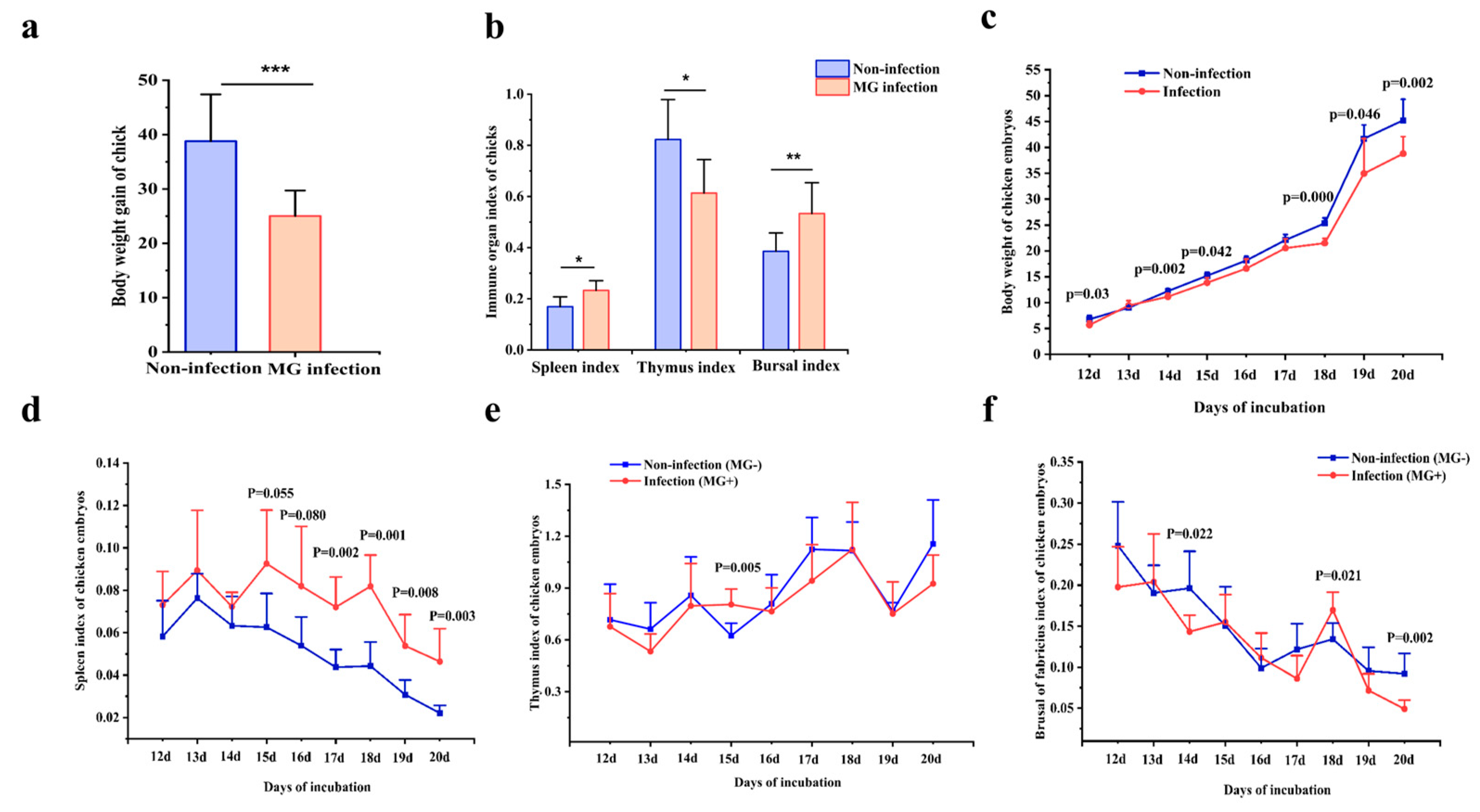
3.1. MG Infection Leads to Reduced Body Weight and Dysregulated Immune Organs Index in Chicken Embryos and Newly Hatched Chicks
3.2. MG-Induced Histopathological Damage in Chicks and Chicken Embryos
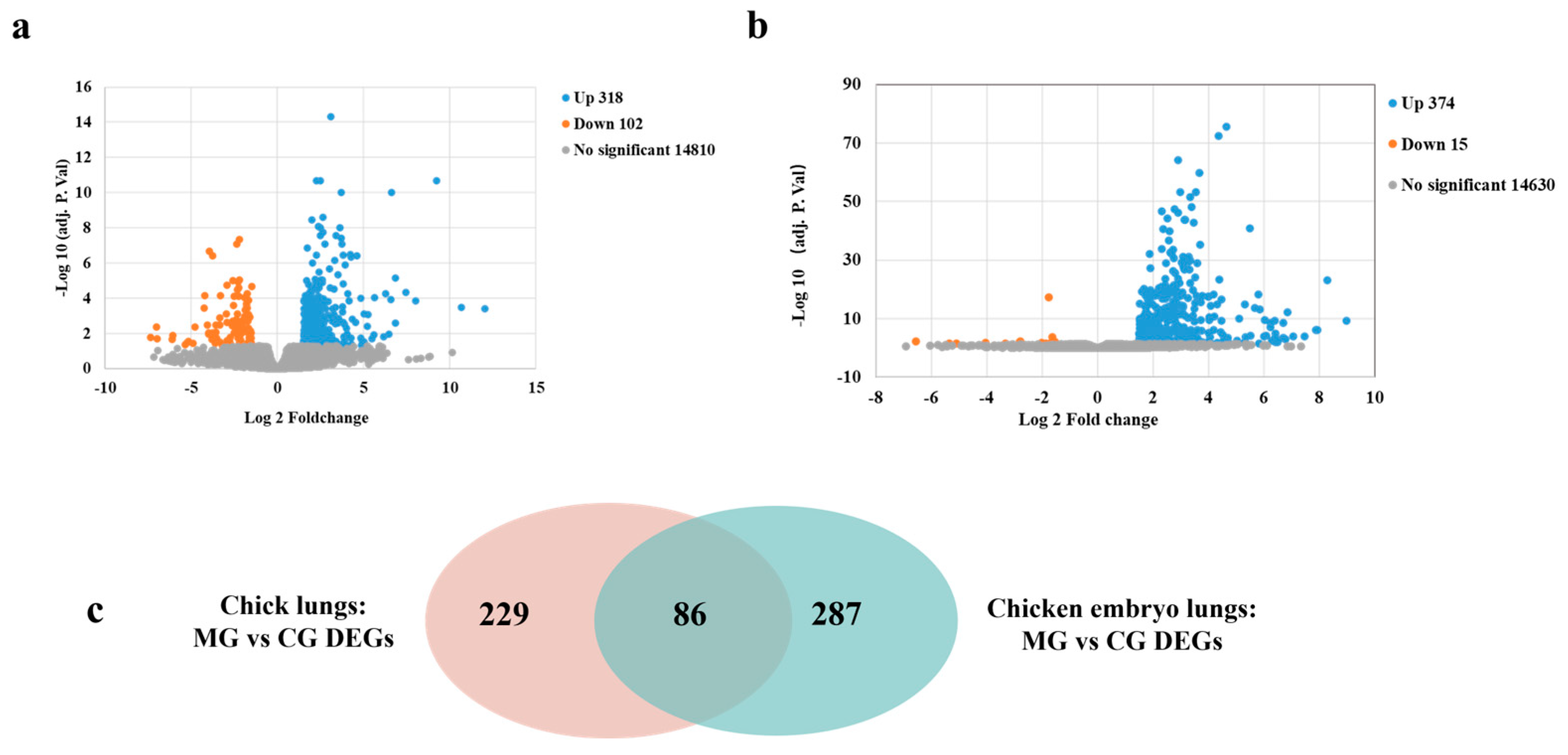
3.3. Transcriptome Sequencing Analysis of Chicks and Chicken Embryos Lung Tissues
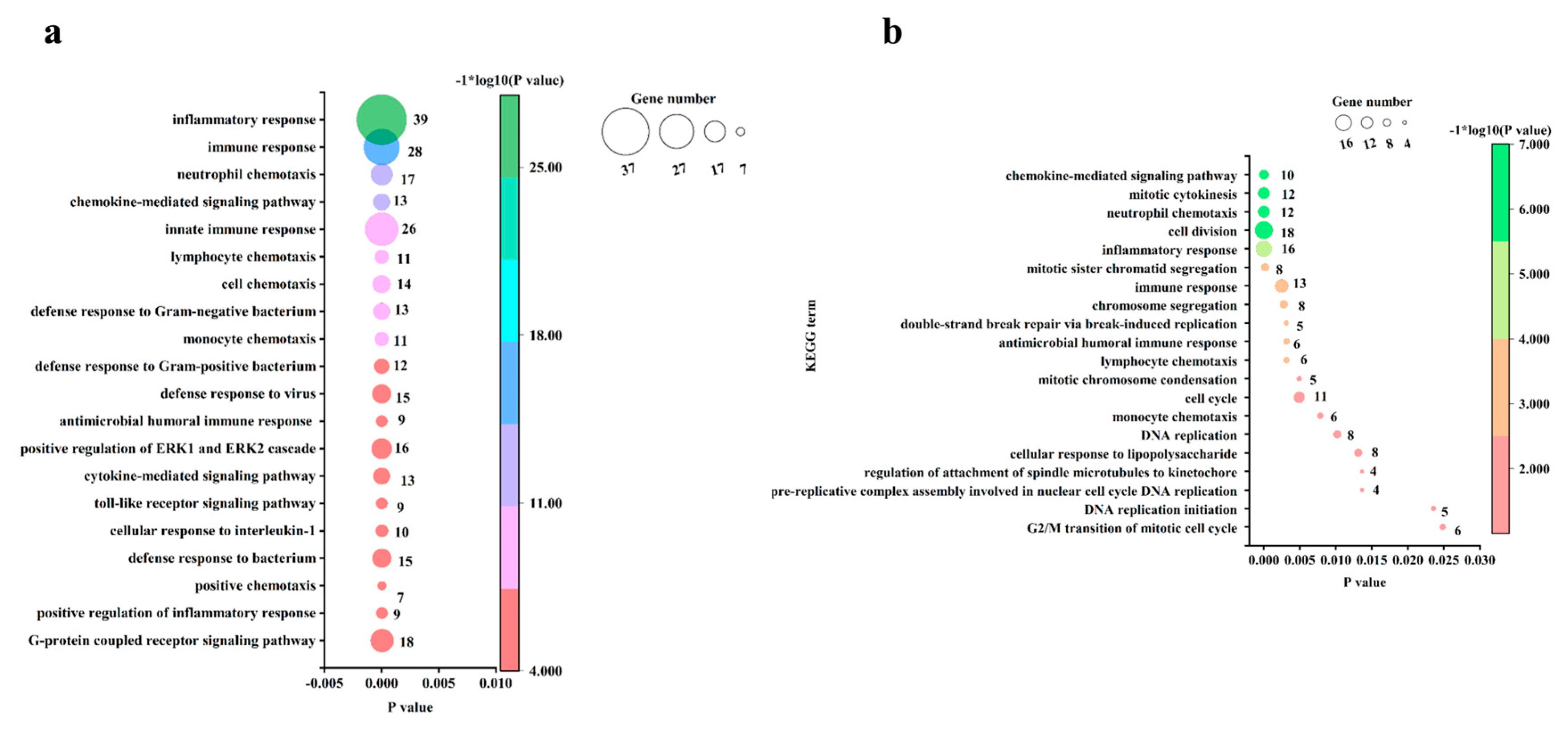
3.4. GO Analysis of the DEGs in Chicken Embryos and Chicks after MG Infection
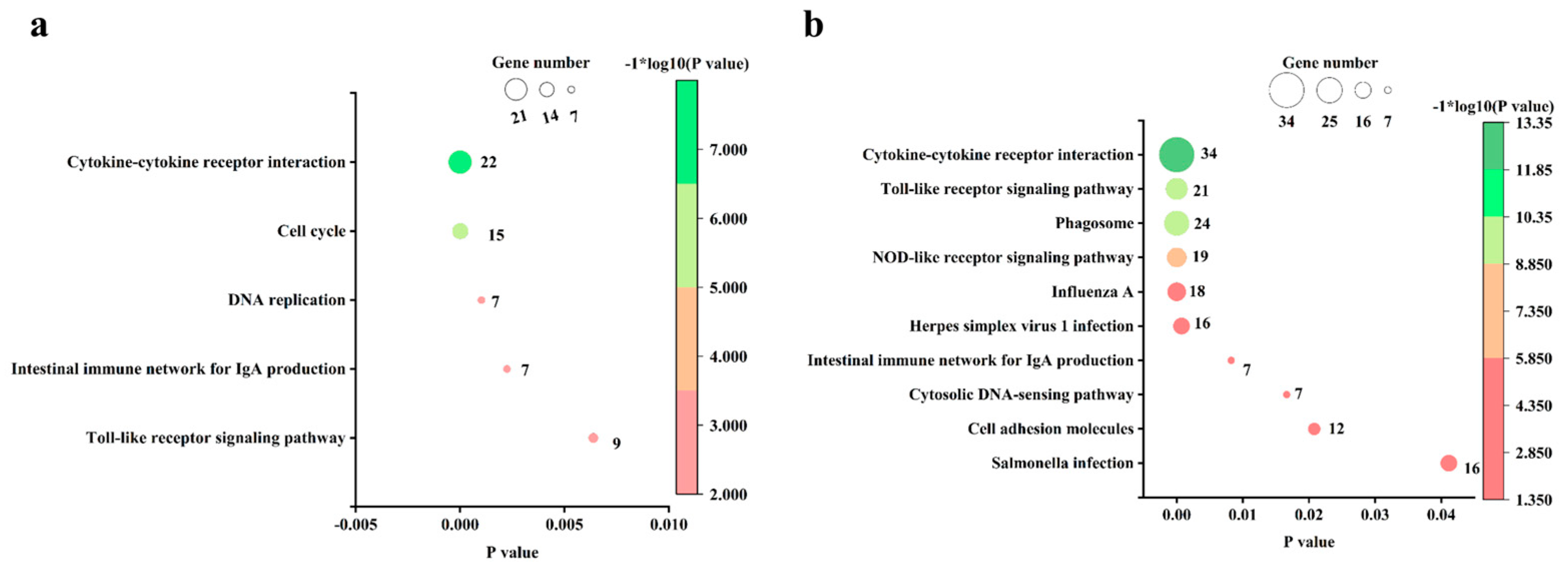
3.5. Pathway Analysis of the DEGs in Chicks and Chicken Embryos after MG Infection
3.6. Validation of CCRs in Both Chicks and Chicken Embryos after MG Infection
3.7. Validation of TLRs in Both Chick and Chicken Embryo after MG Infection
3.8. Analysis of Protein Interaction Network
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Elliott, K.E.C.; Branton, S.L.; Evans, J.D.; Peebles, E.D. Occurrence of horizontal transmission in layer chickens after administration of an in ovo strain F Mycoplasma gallisepticum vaccine 1, 2, 3. Poult. Sci. 2019, 98, 4492–4497. [Google Scholar] [CrossRef] [PubMed]
- Luo, R.; Wang, Y.; Guo, Q.; Fan, C.; Jiang, G.; Wang, L.; Zou, M.; Wang, T.; Sun, Y.; Peng, X. Andrographolide attenuates Mycoplasma gallisepticum-induced inflammation and apoptosis by the JAK/PI3K/AKT signal pathway in the chicken lungs and primary alveolar type II epithelial cells. Int. Immunopharmacol. 2022, 109, 108819. [Google Scholar] [CrossRef] [PubMed]
- Alqhtani, A.H.; Fatemi, S.A.; Elliott, K.E.C.; Branton, S.L.; Evans, J.D.; Leigh, S.A.; Gerard, P.D.; Peebles, E.D. Effects of the In Ovo Vaccination of the ts-11 Strain of Mycoplasma gallisepticum in Layer Embryos and Posthatch Chicks. Animals 2022, 12, 1120. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Wu, Y.; Zhou, Z.; Xia, X.; Gu, X.; Cai, Q.; Shen, X.; Yang, H.; Ding, H. Pharmacokinetic and Pharmacodynamic Integration and Resistance Analysis of Tilmicosin Against Mycoplasma gallisepticum in an In Vitro Dynamic Model. Front. Pharmacol. 2019, 10, 670. [Google Scholar] [CrossRef]
- Kulappu Arachchige, S.N.; Young, N.D.; Kanci Condello, A.; Omotainse, O.S.; Noormohammadi, A.H.; Wawegama, N.K.; Browning, G.F. Transcriptomic Analysis of Long-Term Protective Immunity Induced by Vaccination With Mycoplasma gallisepticum Strain ts-304. Front. Immunol. 2020, 11, 628804. [Google Scholar] [CrossRef]
- Davison, T.F. The immunologists’ debt to the chicken. Br. Poult. Sci. 2003, 44, 6–21. [Google Scholar] [CrossRef]
- Garcia, P.; Wang, Y.; Viallet, J.; Macek Jilkova, Z. The Chicken Embryo Model: A Novel and Relevant Model for Immune-Based Studies. Front. Immunol. 2021, 12, 791081. [Google Scholar] [CrossRef]
- Radomska, K.A.; Vaezirad, M.M.; Verstappen, K.M.; Wosten, M.M.; Wagenaar, J.A.; van Putten, J.P. Chicken Immune Response after In Ovo Immunization with Chimeric TLR5 Activating Flagellin of Campylobacter jejuni. PLoS ONE 2016, 11, e0164837. [Google Scholar] [CrossRef]
- Alkie, T.N.; Yitbarek, A.; Hodgins, D.C.; Kulkarni, R.R.; Taha-Abdelaziz, K.; Sharif, S. Development of innate immunity in chicken embryos and newly hatched chicks: A disease control perspective. Avian Pathol. 2019, 48, 288–310. [Google Scholar] [CrossRef]
- Weibel, E.R. On the Tricks Alveolar Epithelial Cells Play to Make a Good Lung. Am. J. Respir. Crit. Care Med. 2015, 191, 504–513. [Google Scholar] [CrossRef]
- Majumder, S.; Zappulla, F.; Silbart, L.K. Mycoplasma gallisepticum lipid associated membrane proteins up-regulate inflammatory genes in chicken tracheal epithelial cells via TLR-2 ligation through an NF-kappaB dependent pathway. PLoS ONE 2014, 9, e112796. [Google Scholar] [CrossRef]
- Beaudet, J.; Tulman, E.R.; Pflaum, K.; Liao, X.; Kutish, G.F.; Szczepanek, S.M.; Silbart, L.K.; Geary, S.J. Transcriptional Profiling of the Chicken Tracheal Response to Virulent Mycoplasma gallisepticum Strain R(low). Infect. Immun. 2017, 85, e00343-17. [Google Scholar] [CrossRef]
- Beaudet, J.; Tulman, E.R.; Pflaum, K.; Canter, J.A.; Silbart, L.K.; Geary, S.J. Immunologic Pathways in Protective versus Maladaptive Host Responses to Attenuated and Pathogenic Strains of Mycoplasma gallisepticum. Infect. Immun. 2019, 87, e00613-18. [Google Scholar] [CrossRef] [PubMed]
- Canter, J.A.; Tulman, E.R.; Beaudet, J.; Lee, D.H.; May, M.; Szczepanek, S.M.; Geary, S.J. Transcriptional and Pathological Host Responses to Coinfection with Virulent or Attenuated Mycoplasma gallisepticum and Low-Pathogenic Avian Influenza A Virus in Chickens. Infect. Immun. 2019, 88, e00607-19. [Google Scholar] [CrossRef]
- Wu, Z.; Ding, L.; Bao, J.; Liu, Y.; Zhang, Q.; Wang, J.; Li, R.; Ishfaq, M.; Li, J. Co-infection of Mycoplasma gallisepticum and Escherichia coli Triggers Inflammatory Injury Involving the IL-17 Signaling Pathway. Front. Microbiol. 2019, 10, 2615. [Google Scholar] [CrossRef]
- Wu, C.; Zhong, L.; Zhang, L.; Li, W.; Liu, B.; Huang, B.; Luo, Z.; Wu, Y. Study on the mechanism of Mycoplasma gallisepticum infection on chicken tracheal mucosa injury. Avian Pathol. 2022, 51, 361–373. [Google Scholar] [CrossRef] [PubMed]
- Zou, M.; Yang, W.; Niu, L.; Sun, Y.; Luo, R.; Wang, Y.; Peng, X. Polydatin attenuates Mycoplasma gallisepticum (HS strain)-induced inflammation injury via inhibiting the TLR6/ MyD88/NF-kappaB pathway. Microb. Pathog. 2020, 149, 104552. [Google Scholar] [CrossRef]
- Zhao, Y.; Fu, Y.; Zou, M.; Sun, Y.; Yin, X.; Niu, L.; Gong, Y.; Peng, X. Analysis of deep sequencing exosome-microRNA expression profile derived from CP-II reveals potential role of gga-miRNA-451 in inflammation. J. Cell. Mol. Med. 2020, 24, 6178–6190. [Google Scholar] [CrossRef] [PubMed]
- Zou, M.; Yang, L.; Niu, L.; Zhao, Y.; Sun, Y.; Fu, Y.; Peng, X. Baicalin ameliorates Mycoplasma gallisepticum-induced lung inflammation in chicken by inhibiting TLR6-mediated NF-kappaB signalling. Br. Poult. Sci. 2021, 62, 199–210. [Google Scholar] [CrossRef]
- Kim, D.; Langmead, B.; Salzberg, S.L. HISAT: A fast spliced aligner with low memory requirements. Nat. Methods 2015, 12, 357–360. [Google Scholar] [CrossRef]
- Hu, F.; Luo, R.; Duan, S.; Guo, Q.; Wang, L.; Jiang, G.; Fan, C.; Zou, M.; Wang, T.; Wang, Y.; et al. Evaluation of Glycyrrhizic Acid Therapeutic Effect and Safety in Mycoplasma gallisepticum (HS Strain)-Infected Arbor Acres Broilers. Animals 2022, 12, 1285. [Google Scholar] [CrossRef] [PubMed]
- Song, B.; Tang, D.; Yan, S.; Fan, H.; Li, G.; Shahid, M.S.; Mahmood, T.; Guo, Y. Effects of age on immune function in broiler chickens. J. Anim. Sci. Biotechnol. 2021, 12, 42. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Li, J.; Zhang, W.; Shah, S.W.A.; Ishfaq, M. Mycoplasma gallisepticum triggers immune damage in the chicken thymus by activating the TLR-2/MyD88/NF-kappaB signaling pathway and NLRP3 inflammasome. Vet. Res. 2020, 51, 52. [Google Scholar] [CrossRef]
- Ishfaq, M.; Wu, Z.; Wang, J.; Li, R.; Chen, C.; Li, J. Baicalin alleviates Mycoplasma gallisepticum-induced oxidative stress and inflammation via modulating NLRP3 inflammasome-autophagy pathway. Int. Immunopharmacol. 2021, 101, 108250. [Google Scholar] [CrossRef]
- Gaunson, J.E.; Philip, C.J.; Whithear, K.G.; Browning, G.F. Age related differences in the immune response to vaccination and infection with Mycoplasma gallisepticum. Vaccine 2006, 24, 1687–1692. [Google Scholar] [CrossRef] [PubMed]
- Majumder, S.; Silbart, L.K. Interaction of Mycoplasma gallisepticum with Chicken Tracheal Epithelial Cells Contributes to Macrophage Chemotaxis and Activation. Infect. Immun. 2016, 84, 266–274. [Google Scholar] [CrossRef]
- Kulappu Arachchige, S.N.; Young, N.D.; Shil, P.K.; Legione, A.R.; Kanci Condello, A.; Browning, G.F.; Wawegama, N.K. Differential Response of the Chicken Trachea to Chronic Infection with Virulent Mycoplasma gallisepticum Strain Ap3AS and Vaxsafe MG (Strain ts-304): A Transcriptional Profile. Infect. Immun. 2020, 88, e00053-20. [Google Scholar] [CrossRef]
- Cao, J.; Xu, R.; Geng, Y.; Xu, S.; Guo, M. Exposure to polystyrene microplastics triggers lung injury via targeting toll-like receptor 2 and activation of the NF-kappaB signal in mice. Environ. Pollut. 2023, 320, 121068. [Google Scholar] [CrossRef]
- Cui, J.; Zhang, Y.; Liu, L.; Zhang, Q.; Xu, S.; Guo, M.Y. Polystyrene microplastics induced inflammation with activating the TLR2 signal by excessive accumulation of ROS in hepatopancreas of carp (Cyprinus carpio). Ecotoxicol. Environ. Saf. 2023, 251, 114539. [Google Scholar] [CrossRef] [PubMed]
- Ahn, M.Y.; Yoon, H.E.; Park, J.H.; Lee, J.; Min, S.K.; Ahn, S.G.; Yoon, J.H. Characterization of NODs and TLRs in innate immune response of human cementoblast cells. Oral Dis. 2013, 19, 374–380. [Google Scholar] [CrossRef]
- Kim, S.; Kaiser, P.; Borowska, D.; Vervelde, L. Synergistic effect of co-stimulation of membrane and endosomal TLRs on chicken innate immune responses. Vet. Immunol. Immunopathol. 2018, 199, 15–21. [Google Scholar] [CrossRef]
- Tian, W.; Zhao, C.; Hu, Q.; Sun, J.; Peng, X. Roles of Toll-like receptors 2 and 6 in the inflammatory response to Mycoplasma gallisepticum infection in DF-1 cells and in chicken embryos. Dev. Comp. Immunol. 2016, 59, 39–47. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, L.; Hu, F.; Zou, M.; Luo, R.; Sun, Y.; Wang, T.; Guo, Q.; Peng, X. Extracellular HMGB1 as Inflammatory Mediator in the Progression of Mycoplasma gallisepticum Infection. Cells 2022, 11, 2817. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Genovese, K.J.; Swaggerty, C.L.; MacKinnon, K.M.; Kogut, M.H. Co-stimulation with TLR3 and TLR21 ligands synergistically up-regulates Th1-cytokine IFN-gamma and regulatory cytokine IL-10 expression in chicken monocytes. Dev. Comp. Immunol. 2012, 36, 756–760. [Google Scholar] [CrossRef] [PubMed]
- Boyd, A.C.; Peroval, M.Y.; Hammond, J.A.; Prickett, M.D.; Young, J.R.; Smith, A.L. TLR15 is unique to avian and reptilian lineages and recognizes a yeast-derived agonist. J. Immunol. 2012, 189, 4930–4938. [Google Scholar] [CrossRef]
- Neves, F.; Munoz-Merida, A.; Machado, A.M.; Almeida, T.; Gaigher, A.; Esteves, P.J.; Castro, L.F.C.; Verissimo, A. Uncovering a 500 million year old history and evidence of pseudogenization for TLR15. Front. Immunol. 2022, 13, 1020601. [Google Scholar] [CrossRef]
- Loes, A.N.; Bridgham, J.T.; Harms, M.J. Coevolution of the Toll-Like Receptor 4 Complex with Calgranulins and Lipopolysaccharide. Front. Immunol. 2018, 9, 304. [Google Scholar] [CrossRef]
- Lu, Y.; Tian, H.; Chen, R.; Liu, Q.; Jia, K.; Hu, D.L.; Chen, H.; Ye, C.; Peng, L.; Fang, R. Synergistic Antimicrobial Effect of Antimicrobial Peptides CATH-1, CATH-3, and PMAP-36 With Erythromycin Against Bacterial Pathogens. Front. Microbiol. 2022, 13, 953720. [Google Scholar] [CrossRef]
- Jang, H.J.; Monson, M.; Kaiser, M.; Lamont, S.J. Induction of Chicken Host Defense Peptides within Disease-Resistant and -Susceptible Lines. Genes 2020, 11, 1195. [Google Scholar] [CrossRef]
- Li, Z.; Ahmed, I.; Xu, Z.; Sun, S.; Li, T.; Gu, D.; Liu, Y.; Zhang, X.; Yan, S.; Hu, W.; et al. Profiles of expression pattern and tissue distribution of host defense peptides genes in different chicken (Gallus gallus) breeds related to body weight. PLoS ONE 2020, 15, e0238675. [Google Scholar] [CrossRef] [PubMed]
- van Harten, R.M.; Tjeerdsma-van Bokhoven, J.L.M.; de Greeff, A.; Balhuizen, M.D.; van Dijk, A.; Veldhuizen, E.J.A.; Haagsman, H.P.; Scheenstra, M.R. d-enantiomers of CATH-2 enhance the response of macrophages against Streptococcus suis serotype 2. J. Adv. Res. 2022, 36, 101–112. [Google Scholar] [CrossRef] [PubMed]

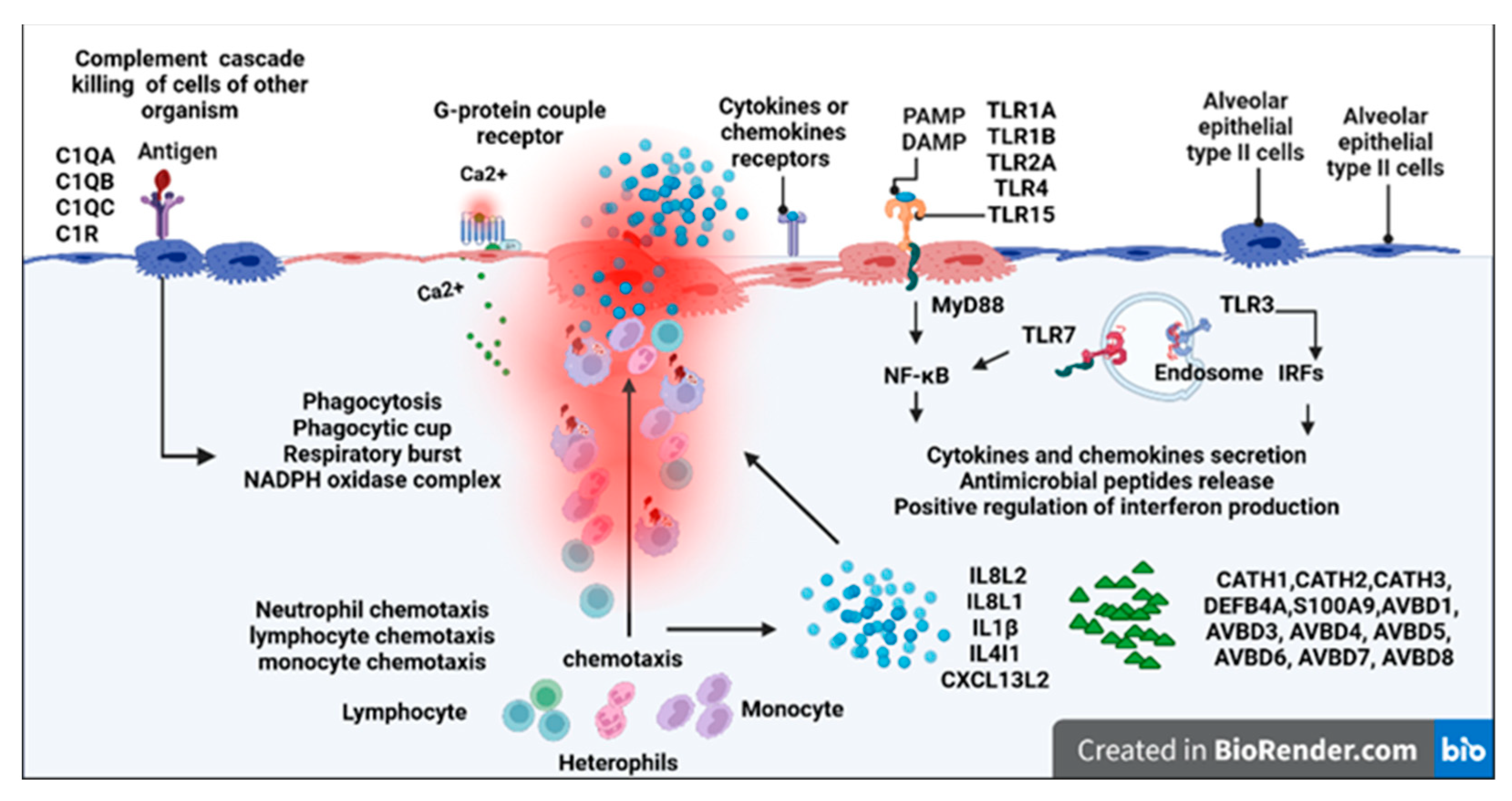
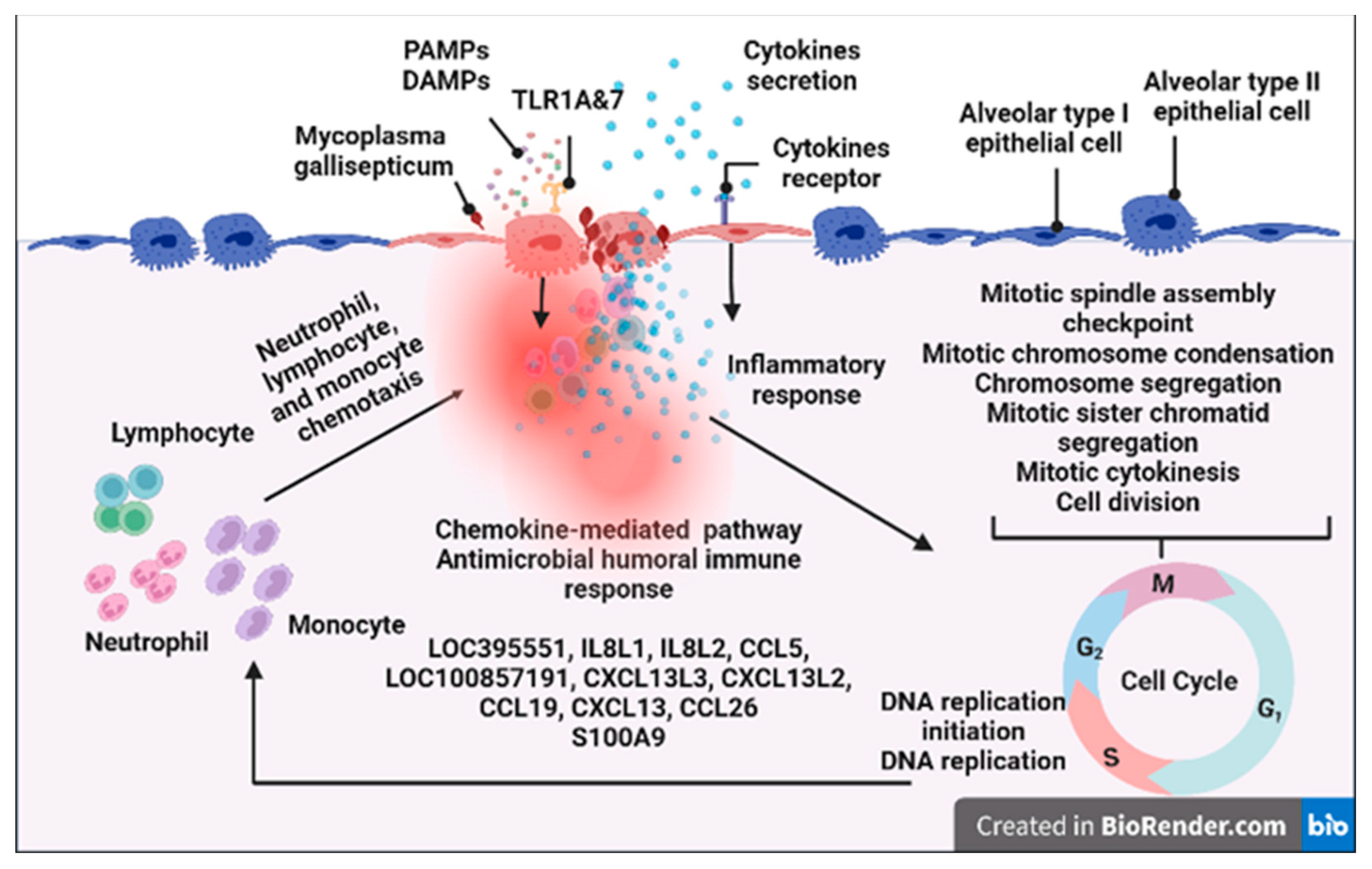
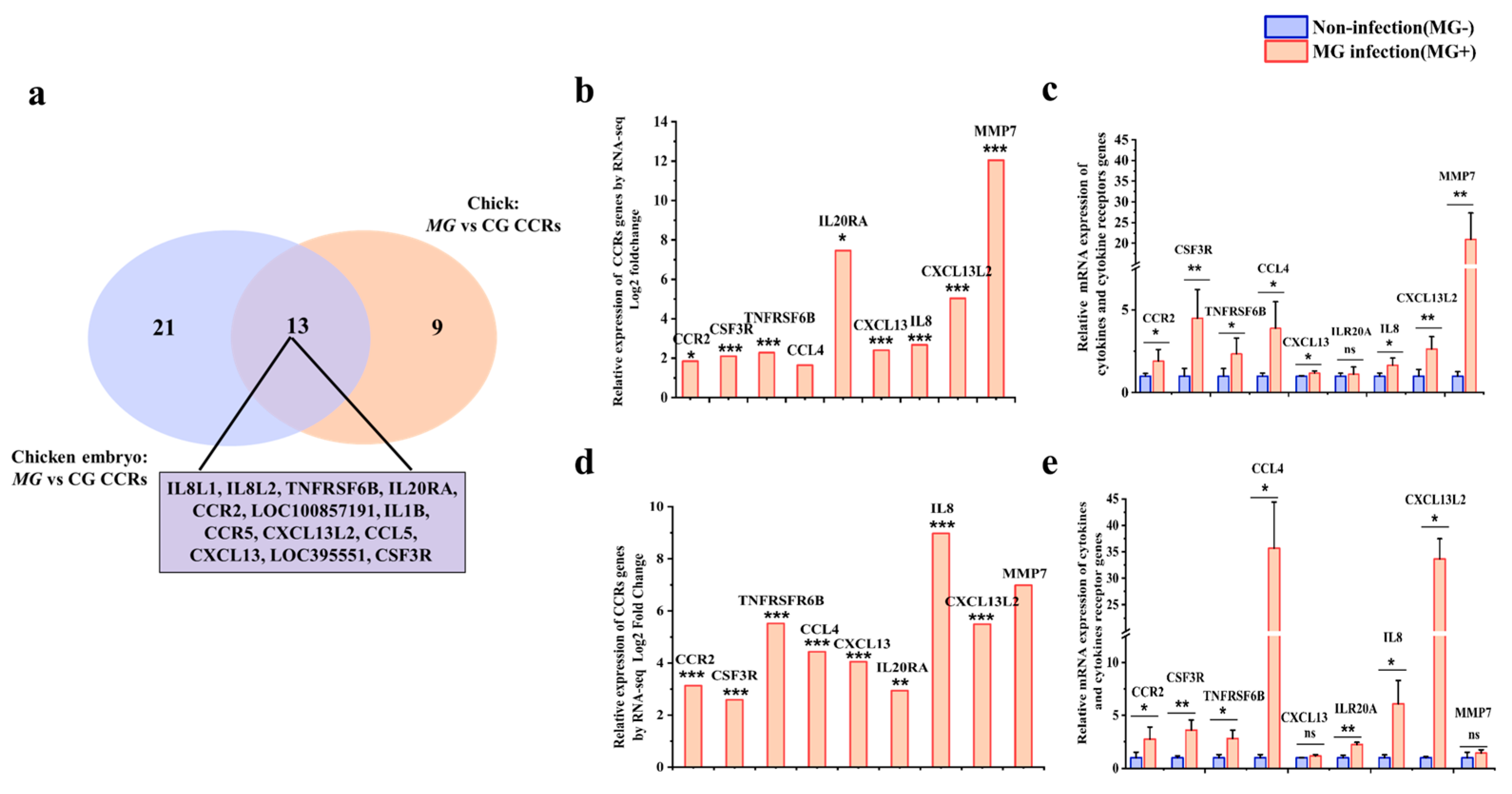

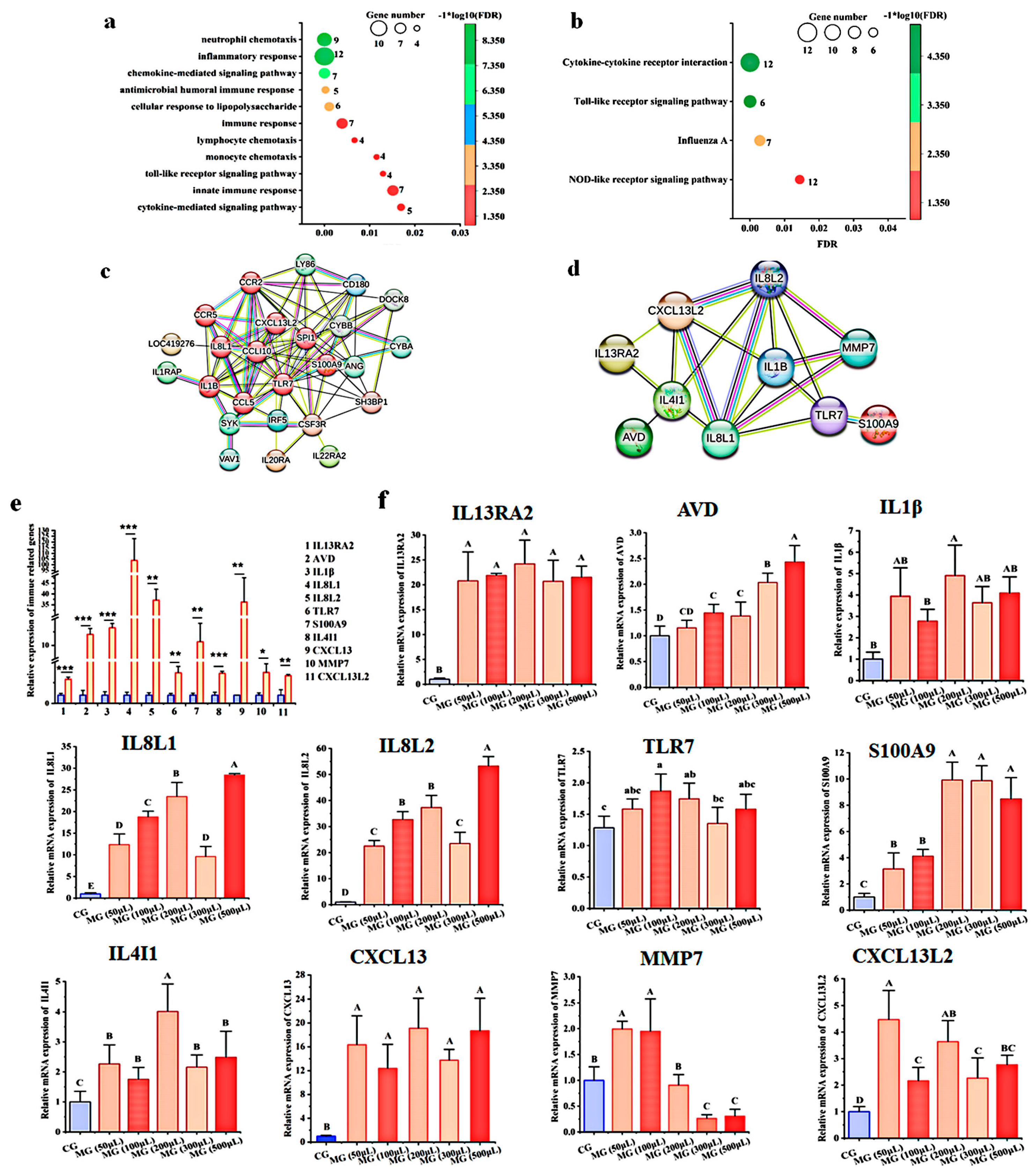
| Name | Primers | Accession No. |
|---|---|---|
| TLR1B-F | GCCGTTGCCTTCTAAAAGAGG | NM_001081709.4 |
| TLR1B-R | CCAGGACACTCTGACAGGGA | NM_001081709.4 |
| TLR2A-F | AAATCAGCGGGATGCACCTA | NM_001396827.1 |
| TLR2A-R | TTGGCATCGGATCACAGGTC | NM_001396827.1 |
| TLR7-F | TCTTTCAGAGGTGGCTGCAC | XM_046908011.1 |
| TLR7-R | GATCCCTCTGGGGACTTCCT | XM_046908011.1 |
| TLR4 -F | ATCTTTCAAGGTGCCACATC | NM_001030693.2 |
| TLR4 -R | GGATATGCTTGTTTCCACCA | NM_001030693.2 |
| TLR3-F | CTGCGGAATCTGACTGTCCT | NM_001011691.4 |
| TLR3-R | TCCTCCTGGGTTTGCACATTT | NM_001011691.4 |
| TLR5-F | CACCTGTGCCAAAGGACACT | NM_001398059.1 |
| TLR5-R | AAACGTTGCAAACCCGCAAA | NM_001398059.1 |
| TLR15-F | GGCTGTGGTATGTGAGAATG | NM_001398238.1 |
| TLR15-R | ATCGTGCTCGCTGTATGA | NM_001398238.1 |
| TLR21-F | CAGGGTATGCAGCTGTGTCA | NM_001030558.3 |
| TLR21-R | CAGGGTATGCAGCTGTGTCA | NM_001030558.3 |
| CSF3-F | ACCACGACTTCCAGCTCTTC | NM_205279.2 |
| CSF3-R | CTGGAAGGTGTCACACACGG | NM_205279.2 |
| CSF3R-F | TCCTCACACTCCCTCTTTGC | NM_001030898.2 |
| CSF3R-R | ATGGGATGGTGCCTTCTGC | NM_001030898.2 |
| TNFRSF6B-F | AGTGCCTCTACTGCAACGTC | XM_417434.6 |
| TNFRSF6B-R | CAGAGGGGGAACCCAACTTC | XM_417434.6 |
| IL-1β-F | TGCCTGCAGAAGAAGCCTCG | NM_204524.1 |
| IL-1β-R | CTCCGCAGCAGTTTGGTCAT | NM_204524.1 |
| CCL4-F | AGCGTAGGAACTCCACTCTC | XM_015295666.2 |
| CCL4-R | CCTTCTTTGTGATGAGATGATGGC | XM_015295666.2 |
| CXCL13L2-F | CAACTGCCTTCATTCCCTTGC | NM_001348656.1 |
| CXCL13L2-R | GCAGCTTTCTTCTTCAGCTTCG | NM_001348656.1 |
| CXCL13-F | TGTCAAAGTGACTGCCCAGA | NM_001348657.1 |
| CXCL13-R | CCTCTTCAGGGTGAGGATGATCT | NM_001348657.1 |
| IL20RA-F | CGGCGCACACAGGAATGTT | XM_015284185.2 |
| IL20RA-R | GCTCTTCATGGTCAGCAGTCT | XM_015284185.2 |
| IL8L1-F | ATGTGAACCTCACCCCTAGC | NM_205018.1 |
| IL8L1-R | TGAATGGCGTTGTCTCCCAC | NM_205018.1 |
| IL13RA2-F | GGAGGTCCAGAGTGAATGGC | NM_001048078.1 |
| IL13RA2-R | AGCATGCAGCCCATGTTTAC | NM_001048078.1 |
| IL1R2-F | ATTTCCGCTGTGCCTTGAGT | XM_015277810.2 |
| IL1R2-R | CAGTAGGAGTTGTTCCTGTGCT | XM_015277810.2 |
| MMP7-F | CAGAGCCTTCCGTTTCCAGT | NM_001006278.1 |
| MMP7-R | GGGATTCCACATCTGGGCTG | NM_001006278.1 |
| OLFM4-F | AGAAGACTACCAGCCAGCAC | NM_001040463.2 |
| OLFM4-R | AAAGGTGGTATCCGGCAAGT | NM_001040463.2 |
| AVD-F | CCCACCTTTGGCTTCACTG | NM_205320.2 |
| AVD-R | ACCTCCTTCCCGTTCCTGT | NM_205320.2 |
| IL8L2-F | TTCAGCTGCTCTGTCGCAA | NM_205498.2 |
| IL8L2-R | GCACACCTCTCTTCCATCCTT | NM_205498.2 |
| S100A9-F | CCACTTCTGTGAGGACCACC | NM_001305151.2 |
| S100A9-R | GGCAGCAAAACCATCAAACCT | NM_001305151.2 |
| Gene Name | Chicken Embryos CG vs. MG | Newly Hatched Chicks CG vs. MG | ||
|---|---|---|---|---|
| Log2 FC | p Value-Adj | Log2 FC | p Value-Adj | |
| LOC770026 | 7.871279 | 5.93 × 10−7 | 5.628906 | 9.02 × 10−5 |
| IL4I1 | 8.292192 | 9.01 × 10−24 | 4.34217 | 0.001511 |
| IL8L2 | 8.973782 | 4.22 × 10−10 | 2.677474 | 0.000107 |
| AVD | 8.112968 | 1.10 × 10−229 | 3.530443 | 0.001406 |
| CXCL13 | 4.050071 | 2.39 × 10−4 | 7.463658 | 4.76 × 10−5 |
| IL8L1 | 6.075514 | 5.63 × 10−5 | 4.837175 | 0.01823 |
| CXCL13L2 | 5.491891 | 1.62 × 10−41 | 5.034946 | 0.000766 |
| CLEC5A | 6.426947 | 1.13 × 10−5 | 3.781138 | 0.000599 |
| DCSTAMP | 5.106985 | 8.03 × 10−11 | 4.602756 | 3.74 × 10−7 |
| CD72L1 | 5.84905 | 7.68 × 10−14 | 3.81117 | 1.56 × 10−5 |
| MLKL | 6.022089 | 2.49 × 10−10 | 3.513237 | 0.002472 |
| CD72 | 4.639555 | 5.09 × 10−3 | 4.272585 | 4.67 × 10−7 |
| MRGPRH | 4.359756 | 4.33 × 10−73 | 4.054451 | 0.010629 |
| S100A9 | 4.645942 | 2.61 × 10−76 | 3.256855 | 0.001827 |
| LOC419276 | 4.625564 | 3.41 × 10−3 | 2.765353 | 0.025887 |
| MX1 | 4.17664 | 5.01 × 10−19 | 2.5511 | 0.00012 |
| LOC101748032 | 3.504725 | 4.38 × 10−14 | 3.160072 | 0.001962 |
| LPAR5 | 3.489633 | 0.023681 | 2.833963 | 0.014503 |
| CCL5 | 3.601561 | 9.63 × 10−7 | 2.597259 | 0.018252 |
| CYBB | 3.470943 | 1.64 × 10−43 | 2.539925 | 0.005234 |
| Feature | Pathological Changes/Enriched GO and KEGG Term | Affected Genes |
|---|---|---|
| Similarities |
| No |
| No | |
| No | |
| Upregulation of genes for TLR6, TLR7, and TLR1A in lung tissues; up-regulation of genes for TLR6 in spleen, thymus, and bursa of Fabricius; | |
| Upregulation of genes for IL8L1, IL8L2, TNFRSF6B, IL20RA, CCR2, LOC100857191, IL1B, CCR5, CXCL13L2, CCL5, CXCL13, LOC395551, and CSF3R; | |
| RNASE6, S100A9, RSFR, and LBP. | |
| IL1B, HBE1, RNASE6, S100A9, BST1, ADGRG5, RSFR, LBP, and CXCL13L2; | |
| IL8L1, CSF3R, IL8L2, SYK, CCL5, LOC100857191, CXCL13L2, CXCL13, CCL26; | |
| Differences |
| Upregulations of genes for MAP3K8, SPP1, TLR4, TLR2A, TLR15, CD88, FOS, CCL4, TLR6, TLR3, LY96, and IRF7 were observed in chicken embryos, but not chicks |
| Upregulations of genes for CSF3, IL18RAP, CSF2RB, IL16, IL18R1, IL10RA, CCL20, CSF1R, IL18, IL13RA2, TNFRSF4, IL1R2, XCR1, IL2RA, CCL4, CX3CR1, CXCR1, CRCBL, IL2RG, CSF2RA, and LOC101747500 in were observed in chicken embryos, but not in chicks | |
| Upregulations of AVBD1, 3, 4, 5, 6, 7, and 8 and DEFB4A, CATH1, CATH2, and CATH3 were observed in chicken embryos only | |
| Upregulations of C1R, NCF2, BLB1, NCF4, ITGB2, TUBA4AL, TLR1A, TAP1, NCF1C, CYBB, TUB4A, TLR2A, BF1, MMR1L3, LOC771876, MMR1L4, MARCO, DMA, DMB2, LOC420160, ATP6V1G3, ATP6V0D2, TLR4, LOC428421 were observed in chicken embryos only | |
| Upregulations of FEN1, RFC3, MCM3, MCM4, MCM5, DNA2, MCM2, BUB1B, CDK1, BUB1 were observed in chicks only |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zou, M.; Wang, T.; Wang, Y.; Luo, R.; Sun, Y.; Peng, X. Comparative Transcriptome Analysis Reveals the Innate Immune Response to Mycoplasma gallisepticum Infection in Chicken Embryos and Newly Hatched Chicks. Animals 2023, 13, 1667. https://doi.org/10.3390/ani13101667
Zou M, Wang T, Wang Y, Luo R, Sun Y, Peng X. Comparative Transcriptome Analysis Reveals the Innate Immune Response to Mycoplasma gallisepticum Infection in Chicken Embryos and Newly Hatched Chicks. Animals. 2023; 13(10):1667. https://doi.org/10.3390/ani13101667
Chicago/Turabian StyleZou, Mengyun, Tengfei Wang, Yingjie Wang, Ronglong Luo, Yingfei Sun, and Xiuli Peng. 2023. "Comparative Transcriptome Analysis Reveals the Innate Immune Response to Mycoplasma gallisepticum Infection in Chicken Embryos and Newly Hatched Chicks" Animals 13, no. 10: 1667. https://doi.org/10.3390/ani13101667
APA StyleZou, M., Wang, T., Wang, Y., Luo, R., Sun, Y., & Peng, X. (2023). Comparative Transcriptome Analysis Reveals the Innate Immune Response to Mycoplasma gallisepticum Infection in Chicken Embryos and Newly Hatched Chicks. Animals, 13(10), 1667. https://doi.org/10.3390/ani13101667





