Simple Summary
There is a global focus on the search for identifying species of macroalgae that can be fed to livestock as a feedstock, or as a methane mitigant. This study evaluated the effect of three red seaweeds to assess their potential for decreasing ruminant enteric methane production. While only Asparagopsis taxiformis was effective at decreasing methane production, this study provides new information on the requirements for adapting animals to seaweed feeding.
Abstract
Several red seaweeds have been shown to inhibit enteric CH4 production; however, the adaptation of fermentation parameters to their presence is not well understood. The objective of this study was to examine the effect of three red seaweeds (Asparargopsis taxiformis, Mazzaella japonica, and Palmaria mollis) on in vitro fermentation, CH4 production, and adaptation using the rumen simulation technique (RUSITEC). The experiment was conducted as a completely randomized design with four treatments, duplicated in two identical RUSITEC apparatus equipped with eight fermenter vessels each. The four treatments included the control and the three red seaweeds added to the control diet at 2% diet DM. The experimental period was divided into four phases including a baseline phase (d 0–7; no seaweed included), an adaptation phase (d 8–11; seaweed included in treatment vessels), an intermediate phase (d 12–16), and a stable phase (d 17–21). The degradability of organic matter (p = 0.04) and neutral detergent fibre (p = 0.05) was decreased by A. taxiformis during the adaptation phase, but returned to control levels in the stable phase. A. taxiformis supplementation resulted in a decrease (p < 0.001) in the molar proportions of acetate, propionate, and total volatile fatty acid (VFA) production, with an increase in the molar proportions of butyrate, caproate, and valerate; the other seaweeds had no effect (p > 0.05) on the molar proportions or production of individual VFA. A. taxiformis was the only seaweed to suppress CH4 production (p < 0.001), with the suppressive effect increasing (p < 0.001) across phases. Similarly, A. taxiformis increased (p < 0.001) the production of hydrogen (H2, %, mL/d) across the adaptation, intermediate, and stable phases, with the intermediate and stable phases having greater H2 production than the adaptation phase. In conclusion, M. japonica and P. mollis did not impact rumen fermentation or inhibit CH4 production within the RUSITEC. In contrast, we conclude that A. taxiformis is an effective CH4 inhibitor and its introduction to the ruminal environment requires a period of adaptation; however, the large magnitude of CH4 suppression by A. taxiformis inhibits VFA synthesis, which may restrict the production performance in vivo.
1. Introduction
Increased greenhouse gas (GHG) concentrations in the atmosphere have resulted in alterations to the ozone layer, consequently raising the global surface temperature [1]. The agricultural sector contributes to 26.0% of anthropogenic global GHG, mainly CO2, N2O, and CH4 [2]. Enteric CH4 is produced from the natural fermentation of carbohydrates within the rumen, and contributes to approximately 6.0% of the global anthropogenic GHG [3]. While CH4 has a comparatively shorter half-life in the atmosphere (~10 years) than CO2 which can persist in the atmosphere for hundreds of years, CH4 has 28 times the global warming potential of CO2, making it an attractive target for abatement [4].
There has been a growing interest in the use of macroalgae or seaweed and their associated by-products to reduce enteric CH4 emissions from ruminants [5]. Macroalgae are rich in complex carbohydrates and polysaccharides, including two groups of compounds that are known CH4 inhibitors. Specific to red seaweeds are the presence of halogenated low molecular weight compounds, including bromoforms and haloforms [6,7]. These compounds have been shown to be extremely effective at mitigating enteric CH4 production with reports of greater than 67% reduction observed with the feeding of the Asparagopsis species to various ruminants [6,7]. Secondly, seaweeds contain a variety of phlorotannins that are similar to their terrestrial counterparts [5]. Terrestrial tannins have been shown to decrease enteric CH4 production by as much as 30%, with the extent varying dramatically based on tannin profiles [8]. It is hypothesised that seaweeds with high concentrations of phlorotannins may exhibit a similar antimethanogenic property to terrestrial tannins.
Rumen adaptation to feed additives such as tannins, fats, and chemical inhibitors has been well studied; however, knowledge about the ruminal adaptation dynamics to seaweed supplementation is still required. Therefore, the objective of this study was to examine the effect of the three red seaweeds Asparagopsis taxiformis, Mazzaella japonica, and Palmaria mollis on in vitro fermentation and gas production, and evaluate the adaptation response of fermentation characteristics to seaweed supplementation within a rumen simulation technique (RUSITEC) system fed a barley straw and silage diet.
2. Materials and Methods
The experiment was conducted at Agriculture and Agri-Food Canada in Lethbridge, AB, Canada. Donor heifers used in this experiment were cared for in accordance with the guidelines of the Canadian Council on Animal Care (2009).
2.1. Seaweed
The three seaweeds used in this experiment included A. taxiformis, M. japonica, and P. mollis. The seaweeds were chosen based on their biomass availability, harvest potential, and biochemical composition. A. taxiformis was chosen based on its previously observed characterisation to manipulate rumen fermentation and CH4 production and was assigned as a positive control. The novel seaweeds M. japonica and P. mollis are wild harvested off the coast of British Columbia, Canada; M. japonica mainly for its carrageenan content and P. mollis for human consumption.
2.2. Experimental Design and Treatments
The experiment was conducted as a completely randomized design with four treatments, duplicated in two identical RUSITEC apparatus equipped with eight fermenter vessels each. The four treatments included the control (no added seaweed), and three red seaweeds (A. taxiformis, M. japonica, P. mollis) included at 2% diet DM. The substrate consisted of a 50:50 barley straw and barley silage diet (DM basis). The chemical compositions of the substrates and seaweeds are shown in Table 1. An elemental analysis of the seaweeds are shown in Table 2.

Table 1.
Chemical composition of ingredients used in a rumen simulation technique (RUSITEC) to examine the effect of seaweeds on in vitro fermentation and methane production.

Table 2.
Elemental analysis of the seaweeds used in a rumen simulation technique (RUSITEC) to examine the effect of seaweeds on in vitro fermentation and methane production.
The experimental period was divided into four phases: a baseline phase (d 0–7) where fermenters were only fed the diet substrate and measurements were only recorded on d 5–7; the adaptation phase in which seaweed was introduced into the allocated diets and measurements were recorded from d 8–11; the intermediate phase (d 12–16); and the stable phase (d 17–21) to assess the changes in rumen fermentation and the microbial populations in response to feeding on seaweed (Figure 1).
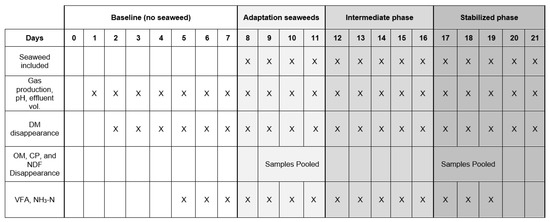
Figure 1.
Outline of experiment dosing and sampling.
2.3. Substrate Processing
Barley straw, barley silage, and seaweeds were ground through a 4 mm screen using a Wiley mill (standard model 4; Arthur H. Thomas Co., Philadelphia, PA, USA). A total of 10 g diet DM was fed to each fermenter daily in bags (10 × 20 cm; 50 ± 10 µ porosity; R1020, ANKOM Technology, Macedon, NY, USA). For the control and during the baseline phase, 5 g DM each of barley straw and barley silage were included in the bags. For the seaweed treatments, 0.2 g DM of seaweed replaced equal proportions of barley straw and barley silage.
2.4. Inoculum Sampling and Incubation Procedure
Two RUSITEC apparatuses each fitted with eight fermenters (920 mL) were used for the in vitro incubation, so that each treatment was randomly allocated to two fermenters within each apparatus (n = 4 vessels/treatment). Each fermenter was fitted with a site for artificial saliva infusion and effluent output. Rumen inoculum was obtained from three ruminally cannulated beef heifers previously adapted for two weeks to a barley straw and barley silage diet which included a mineral supplement. Rumen fluid and solid contents were pooled from the three heifers, filtered through four layers of cheesecloth, and transported to the laboratory in an insulated thermos. The fermenters were maintained at 39 °C by immersion in a water bath. Each fermenter was pre-filled with 180 mL of pre-warmed McDougall’s buffer (pH = 8.2; [9])and 720 mL of strained rumen fluid.
One R1020 bag (ANKOM Technology) containing 20 g of mixed solid rumen digesta, and one bag containing 10 g DM of the diet were allocated to each fermenter. After 24 h, the bag containing rumen digesta was replaced by a bag containing the diet. Thereafter, one bag was replaced daily so that each bag remained in the fermenter for 48 h. Bags containing the seaweed treatments were introduced into the fermenters on d 8.
The artificial saliva was continually infused into the fermenters using a peristaltic pump set to achieve a dilution rate of 2.9%/h. The effluent was collected in a 1 L flask, and gas was collected in a 2 L gas tight bag (Curity®; Conviden Ltd., Mansfield, MA, USA) attached to the effluent flask. Feed bag exchange, fermenter pH, gas production, and effluent volume were measured every 24 h at 10 am.
2.5. Nutrient Disappearance
Dry matter degradability (DMD) was determined from d 3 to 21 after 48 h of fermentation. The feed bags were removed, washed in cold running water for 2 min, and dried at 55 °C for 48 h ([10]; method 930.15) for the determination of DMD. After drying, the residues were pooled from d 9 to 11, and d 17 to 19, ground through a 1 mm screen (Wiley mill, standard model 4; Arthur H. Thomas Co., Philadelphia, PA, USA), and analysed for organic matter (OM), NDF, CP, and ether extract (EE) concentrations. This generated samples representing the adaptation and stable phase. Insufficient samples were available for the baseline and intermediate phases, as the samples were used for a microbial profiling analysis, published elsewhere [11].
The samples were dried at 550 °C for 5 h and OM was calculated as 100—ash ([10]; method 942.05). The NDF content was determined using an ANKOM200 Fibre Analyser based on the procedure described by Van Soest, et al. [12] using sodium sulphite and α-amylase as reagents and expressed exclusive of residual ash. The total N concentration was quantified by flash combustion with gas chromatography and thermal conductivity detection (Carlo Erba Instruments, Milan, Italy [13]; method 990.03). The CP content was calculated as the N concentration × 6.25. Fat was determined according to AOAC (2006; method 2003.05) using ether extraction (Extraction Unit E-816 HE; Büchi Labortechnik AG, Flawil, Switzerland).
2.6. Gas Production
The total gas production was determined daily using a gas meter (Model DM3A, Alexander-Wright, London, England, UK). A 20 mL sample was collected from the septum of the collection bag using a 26-gauge needle and transferred to a pre-evacuated exetainer (6.8 mL; Labco Ltd., Wycombe, Buckinghamshire, UK). Concentrations of CH4, O2, H2, and CO2 were determined using a gas chromatograph equipped with a GS-Carbon-PLOT (30 m × 0.32 mm × 3 mm) column and thermal conductivity detector (Agilent Technologies Canada, Inc., Mississauga, ON, Canada) at an isothermal oven temperature of 35 °C, with He as the carrier gas (27 cm/s).
2.7. Fermentation Variables
Effluent volume and fermenter pH was recorded daily at the time of feed bag exchange. Two 5 mL effluent samples were placed in vials prefilled with 1 mL of 25% (wt/vol) metaphosphoric acid and 1 mL of 1% (wt/vol) sulphuric acid for an analysis of volatile fatty acid (VFA) composition and NH3 concentration, respectively. Sample vials were kept at −20 °C until analysis.
The concentration of VFA was determined by gas chromatography (5890A Series Plus II, Hewlett Packard Co., Palo Alto, CA, USA) equipped with a 30 m Zebron free fatty acid phase fused silica capillary (0.32 mm i.d., 1.0 μm film thickness; Phenomenex, Torrance, CA, USA). The concentration of NH3 was determined using the phenol-hypochlorite method as described by Broderick and Kang [14].
2.8. Elemental Analysis
The elemental analysis of seaweeds was conducted by a commercial laboratory (Cumberland Valley Analytical Services, Waynesboro, PA, USA). Phosphorus, Ca, Mg, K, Na, Fe, Mn, Zn, and Cu were determined using the AOAC method 985.1 [15] with modifications where seaweeds were ashed for 1 h at 535 °C, digested in open crucibles for 20 min in 15% HNO3 on a hotplate, diluted to 50 mL, and then analysed using inductively coupled plasma. The samples used for the Mo analysis were ashed at 480 °C for 4 h, digested in an open crucible for 20 min in 15% HNO3 on a hotplate, diluted to 50 mL, and then analysed on axial view inductively coupled plasma. The selenium was analysed using the AOAC method 996.16 [15]. The bromoform concentration of the seaweed was conducted by a commercial laboratory (Bigelow Analytical Services, East Boothbay, ME, USA) using methanol extraction with samples analysed by GC/MS.
2.9. Calculations and Statistical Analysis
The disappearance of DM, OM, NDF, and CP (DMD, OMD, NDFD, and CPD) was calculated as the difference between the nutrient content before and after incubation, and expressed as a percentage. Methane production was expressed as mg per g of DM digested (DMd) and incubated (DMi).
DMd = Total Dmi − Total DM remaining after incubation
Data were analysed using the MIXED model procedure of SAS (SAS Inc., Cary, NC, USA). Individual fermenter was considered the experimental unit with the day of sampling treated as a repeated measure. Treatment, phase, and treatment × phase were considered as fixed effects while fermenter within the vessel was considered as a random effect. Minimum values of Akaike’s information criterion were used to select the covariance structure. Data were tested for normality of variance. Significance was declared at p ≤ 0.05.
3. Results
There was a treatment × phase effect (p ≤ 0.05) on OMD, NDFD, propionate, branched-chain VFA (BCVFA), caproate, valerate, acetate:propionate molar proportions, and total VFA production (Table 3). When averaged across all phases, DMD was greatest (p < 0.01) for A. taxiformis and lowest for P. mollis; however, neither were different from the control (p > 0.05). There was no effect (p > 0.05) of treatment on CPD, pH, or NH3 production. Effluent output was greater (p = 0.03) in P. mollis than A. taxiformis, although neither were different from the control (p > 0.05). Both OMD (p = 0.04) and NDFD (p = 0.05) were decreased by A. taxiformis during the adaptation phase, but returned to control levels in the stable phase (Figure 2).

Table 3.
Effect of seaweed on in vitro nutrient disappearance, pH, volatile fatty acid, and ammonia production in a RUSITEC.
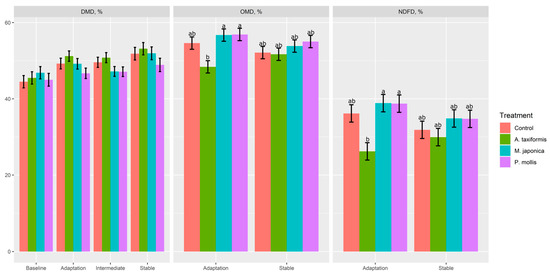
Figure 2.
The effect of seaweed and phase on dry matter disappearance (DMD), organic matter disappearance (OMD), and neutral detergent fibre disappearance (NDFD) in a RUSITEC (n = 4). a,b Within variable, means without a common superscript differ (p ≤ 0.05); variables without letters do not have a significant (p > 0.05) treatment × phase effect.
The molar proportions of acetate were decreased (p < 0.001) and butyrate increased (p < 0.001) by A. taxiformis compared with all other treatments. A. taxiformis decreased the molar proportions of propionate (p < 0.001) during the adaptation, intermediate, and stable phases with the decreases being largest in the intermediate and stable phases (Figure 3a). There was no effect of M. japonica or P. mollis on total VFA across any phase; however, A. taxiformis had less (p = 0.01) total VFA during the intermediate and stable phases compared with the other treatments. The acetate:propionate was higher in A. taxiformis than the M. japonica and P. mollis treatments during adaptation, although it was not different from the control during this phase (Figure 3b). A. taxiformis increased (p < 0.001) molar proportions of caproate, valerate, and BCVFA during the intermediate and stable phase, with no difference detected during the adaptation phase (Figure 3b).
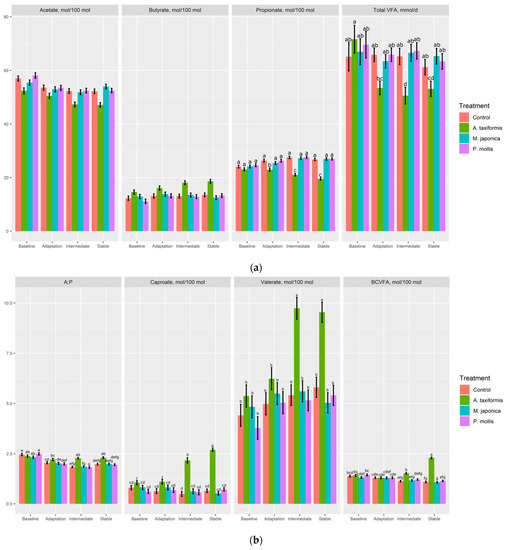
Figure 3.
The effect of seaweed and phase on the (a) molar portions of acetate, propionate, butyrate, and total volatile fatty acids; and (b) acetate:propionate ratio, and molar proportions of caproate, branched-chain volatile fatty acids (BCVFA), and valerate (n = 4). a–g Within variable, means without a common superscript differ (p ≤ 0.05); variables without letters do not have a significant (p > 0.05) treatment × phase effect.
There was no effect (p ≥ 0.34) of treatment on gas or O2 production (% or mL; Table 4). A. taxiformis decreased (p < 0.05) CO2 (%, mL/d) production compared to P. mollis, but neither was different from the control (p > 0.05). There was a treatment × phase effect (p < 0.001) for CH4 (%, mL/d, mg/d, mg/g DMd, mg/g DMi) and H2 (%, mL/d). The control, M. japonica, and P. mollis had similar CH4 production across all phases (Figure 4a,b), whereas A. taxiformis decreased (p < 0.001) CH4 production across the adaptation, intermediate, and stable phases compared with other treatments. The production of H2 (%, mL/d) was increased (p < 0.001) across the adaptation, intermediate, and stable phases by A. taxiformis, with the intermediate and stable phases having greater H2 production than the adaptation phase (Figure 5).

Table 4.
Effect of seaweed on in vitro gas production in a RUSITEC.
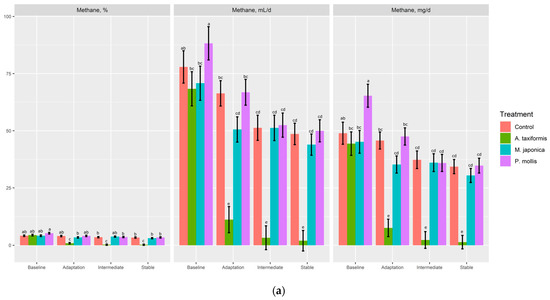
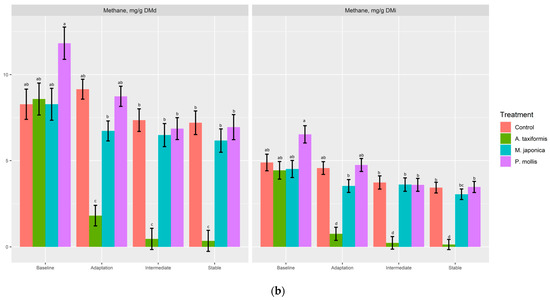
Figure 4.
The effect of seaweed and phase on the (a) percentage of CH4, and CH4 production (mL/d, mg/d), and (b) CH4 production on a dry matter disappearance (mg/g DMd) and dry matter incubated basis (mg/g DMi) (n = 4). a–d Within variable, means without a common superscript differ (p ≤ 0.05).
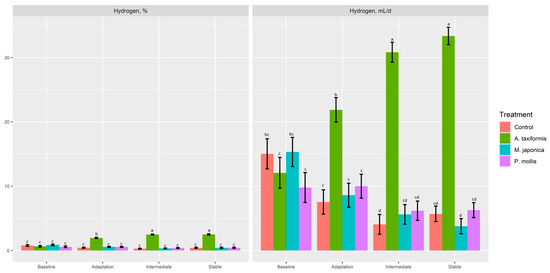
Figure 5.
The effect of seaweed and phase on the percentage and production of H2 within a RUSITEC (n = 4). a–d Within variable, means without a common superscript differ (p ≤ 0.05).
4. Discussion
This study examined the effect of three red seaweeds on fermentation, nutrient degradability, and gas production within a RUSITEC fed a roughage-based diet. The novelty in this study was the examination of the adaptation of the fermentation system to the introduction of three different seaweeds (A. taxiformis, M. japonica, and P. palmate). A 7 d period was allocated for system adaptation (baseline) as well as examining the fermentation parameters before the introduction of seaweeds. Thereafter, the measurements were divided into the adaptation (d 8–11), intermediate (d 12–16), and stable phases (d 17–21) to evaluate the changes that occurred after administrating the seaweeds until the system and the microbial population became more stabilised.
The lack of difference in DMD between the control and each seaweed demonstrates the ability of the microbial population to effectively degrade seaweed carbohydrates, with all treatments improving in DMD across the different phases indicating that the microbial population became more efficient over time [11]. Compared with DMD, OMD and NDFD were more sensitive to the addition of A. taxiformis when introduced to the fermenters, given the initial decrease in both variables during the adaptation phase. The disruption to the fermentation of feed due to the introduction of A. taxiformis may have been caused by the radical suppression of CH4, the increase in H2, and an overall shift in the metabolome requiring the adaptation of the microbiome. Conversely, the recovery of OMD and NDFD in the stable phase indicated that only a relatively short period of time was required for the rumen microbial community to adapt to the presence of A. taxiformis. This short disruption in feed degradation was unique to A. taxiformis and was not observed with either M. japonica or P. mollis.
The suppression of CH4 production by A. taxiformis has been previously documented by both in vitro and in vivo studies [5,7,16,17,18,19]. The present study also observed a rapid drop in CH4 production with A. taxiformis inclusion throughout the adaptation, intermediate, and stable phases compared to the control. The drop in CH4 production (mL/g DMd) compared to the control was 80.2, 93.7, and 95.1% over the adaptation, intermediate and stable phases, respectively. Although not significantly different between these phases, the results suggest that there was an adaptation period to the introduction of A. taxiformis, with the CH4 suppressing effect increasing over time with continued A. taxiformis addition. However, within a RUSITEC system, it is frequent to observe reductions in certain populations of the microbiota as the length of the experiment increases. For example, Mateos, et al. [20] found that in solid associated samples, protozoal DNA concentration and an abundance of Fibrobacter succinogenes, Ruminococcus albus, and fungi decreased, and the abundance of methanogenic archaea increased over a 14 d period within a RUSITEC, despite relatively stable fermentation variables [20]. The changes in microbiota over time may also explain the differences observed within the same treatment over time, because although there were significant decreases in CH4 metrics observed throughout the phases for M. japonica and P. mollis, within each phase they were not different from the control [11]. The lack of effect of these two species on CH4 production is reinforced by consistent H2 production observed across all phases. Similarly, in a companion study in our laboratory using a batch culture technique (unpublished data), we observed that neither M. japonica nor P. mollis had an effect on in vitro fermentation or CH4 production in a barley straw diet. The ineffectiveness of these two seaweeds on reducing CH4 production is likely due to the lack of concentrations of bromoforms in these species. Bromoform has been verified as the effective component in some seaweeds that inhibit enteric CH4 production [7].
The impact of including A. taxiformis within a RUSITEC has been previously evaluated; however, measurements were only taken from the immediate addition of the seaweed and at 4, 12, and 24 h intervals each day over a period of 4 days in that study [18]. Roque, Brooke, Ladau, Polley, Marsh, Najafi, Pandey, Singh, Kinley, Salwen, Eloe-Fadrosh, Kebreab and Hess [18] observed that the 5% OM inclusion rate of A. taxiformis decreased CH4 production by 95%, with the suppression of CH4 production observed at the first sampling that was conducted 28 h after seaweed introduction into the system. Methane production in that study was almost zero after 76 h of incubation, although no further measurements were observed past this time. In contrast, our experiment showed that the ability of A. taxiformis to decrease CH4 improved over time with some CH4 production still observed (~1.35 mg/d) during the final phase. The decrease in CH4 production from A. taxiformis in the present study is consistent with an increase in H2 production over the phases, with the greatest CH4 suppression observed in the intermediate and stable phases resulting in more H2 production than in the adaptation phase. This is further verified by a companion study that found that the inclusion of A. taxiformis had a significant impact on the microbiome, including a large reduction in all major archaeal species [11].
Although the total gas production was not affected by seaweed treatment, A. taxiformis caused a reduction in total VFA production in the adaptation, intermediate, and stable phases, demonstrating a reduction in the microbial degradation of nutrients. Organic matter is degraded by the rumen consortium, generating VFA, the main source of energy provided to ruminants. Iso-acids are products from the degradation of valine, isoleucine, leucine, and proline which are used in the biosynthesis of higher BCVFA [21]. The BCVFA are required for optimal fibre degradation and efficiency of ruminal fermentation [22]. The increase in these intermediates (butyrate, caproate, BCVFA, and valerate) corresponds with the decreased production of acetate and propionate indicating that A. taxiformis inhibited major VFA synthesis. This conclusion is verified by O’Hara [11], who demonstrated that A. taxiformis inhibited major VFA producing bacteria including species of Fibrobacter and Ruminococcus. Other chemical CH4 inhibitors that have resulted in large decreases (7–29%) of CH4 have also been found to result in the increased production of valerate and isovalerate [23,24], consistent with the hypothesis that increased rumen H2 favours the fermentation pathways that consume H2, including valerate and caproate [25,26].
Inhibiting CH4 production can theoretically increase the availability of H2 for incorporation into VFA, thereby increasing energy availability to the animal [26,27]. Yet, despite the increase in H2 production that accompanied the decrease in CH4 production for the A. taxiformis treatment in the present study, the total VFA was not increased. Furthermore, an in vitro study found that at 1, 2, and 5% OM inclusion of A. taxiformis, total VFA was decreased by 16.6, 25.0, and 39.5%, respectively [17]. Machado, Magnusson, Paul, Kinley, de Nys and Tomkins [17] also observed alterations in the molar proportions of VFA with propionate, butyrate, valerate, and isovalerate increasing and acetate and isobutyrate decreasing compared with the control. In contrast, Roque, Brooke, Ladau, Polley, Marsh, Najafi, Pandey, Singh, Kinley, Salwen, Eloe-Fadrosh, Kebreab and Hess [18] did not observe a significant decrease in total VFA with A. taxiformis, but did find a decrease in the acetate:propionate ratio and valerate production, in comparison with the current study where valerate production increased with the inclusion of A. taxiformis. Valerate along with caproate are intermediary VFA and the increase with A. taxiformis is related to its lack of incorporation into the three main VFA, indicating an inefficiency of fermentation possibly brought by the decrease in H2 incorporation into CH4 [21]. The increase in BCVFA, valerate, and caproate may also indicate that ruminal microbial growth is not optimised in the presence of A. taxiformis, as these VFA are essential for cellulolytic bacteria growth, which may contribute towards the reduced NDFD during adaptation.
5. Conclusions
In conclusion, this study found that M. japonica and P. palamata did not impact rumen fermentation or exhibit a CH4-suppressing capacity. In contrast, A. taxiformis was shown to be an effective CH4 suppressant with its immediate addition causing negative alterations to fermentation variables, with the large magnitude of CH4 suppression inhibiting VFA synthesis. These findings may indicate that feeding A. taxiformis to ruminants at a dose rate that results in a large decrease in CH4 production may alter rumen metabolism in a manner that restricts production performance through reduced VFA synthesis.
Author Contributions
Conceptualization, K.A.B.; methodology, A.M.K., P.M.T.L., R.J.G., D.W.A. and K.A.B.; formal analysis, S.A.T.; data curation, A.M.K. and P.M.T.L.; writing—original draft preparation, S.A.T.; writing—review and editing, S.A.T., A.M.K., P.M.T.L., R.J.G., D.W.A. and K.A.B. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Canadian government as part of the FACCE-ERA-GAS consortium.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Acknowledgments
The authors would like to acknowledge the technical assistance of C. Sapsford and D. Vedres. Authors also acknowledge Spencer Serin and Edgar Smith for their procurement of the novel seaweeds (M. japonica, P. mollis) and assistance with the project. We also thank the São Paulo Research Foundation—FAPESP, Grant/Award Number: 2018/19580–0 for the research scholarship of P.M.T. Lima and Professor Adibe L. Abdalla from the University of São Paulo, Centre for Nuclear Energy in Agriculture (Laboratory of Animal Nutrition) for providing support for the research abroad period of A. M. Kruger and P. M. T. Lima.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Masson-Delmotte, V.; Zhai, P.; Pirani, A.; Connors, S.L.; Péan, C.; Berger, S.; Caud, N.; Chen, Y.; Goldfarb, L.; Gomis, M. Climate Change 2021: The Physical Science Basis; Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; IPCC: Geneva, Switzerland, 2021; Volume 2. [Google Scholar]
- Frank, S.; Havlík, P.; Soussana, J.-F.; Levesque, A.; Valin, H.; Wollenberg, E.; Kleinwechter, U.; Fricko, O.; Gusti, M.; Herrero, M. Reducing greenhouse gas emissions in agriculture without compromising food security? Environ. Res. Lett. 2017, 12, 105004. [Google Scholar] [CrossRef]
- Beauchemin, K.A.; Ungerfeld, E.M.; Eckard, R.J.; Wang, M. Review: Fifty years of research on rumen methanogenesis: Lessons learned and future challenges for mitigation. Animals 2020, 14, s2–s16. [Google Scholar] [CrossRef]
- IPCC. Global Warming of 1.5 °C. An IPCC Special Report on the Impacts of Global Warming of 1.5 °C above Pre-Industrial Levels and Related Global Greenhouse Gas Emission Pathways, in the Context of Strengthening the Global Response to the Threat of Climate Change, Sustainable Development, and Efforts to Eradicate Poverty; Masson-Delmotte, V., Zhai, P., Pörtner, H.-O., Roberts, D., Skea, J., Shukla, P.R., Pirani, A., Moufouma-Okia, W., Péan, C., Pidcock, R., et al., Eds.; Intergovernmental Panel on Climate Change: Geneva, Switzerland, 2018. [Google Scholar]
- Abbott, D.W.; Aasen, I.M.; Beauchemin, K.A.; Grondahl, F.; Gruninger, R.; Hayes, M.; Huws, S.; Kenny, D.A.; Krizsan, S.J.; Kirwan, S.F.; et al. Seaweed and Seaweed Bioactives for Mitigation of Enteric Methane: Challenges and Opportunities. Animals 2020, 10, 2432. [Google Scholar] [CrossRef]
- McCauley, J.; Labeeuw, L.; Jaramillo-Madrid, A.; Nguyen, L.; Nghiem, L.; Chaves, A.; Ralph, P. Management of Enteric Methanogenesis in Ruminants by Algal-Derived Feed Additives. Curr. Pollut. Rep. 2020, 6. [Google Scholar] [CrossRef]
- Kinley, R.D.; Martinez-Fernandez, G.; Matthews, M.K.; de Nys, R.; Magnusson, M.; Tomkins, N.W. Mitigating the carbon footprint and improving productivity of ruminant livestock agriculture using a red seaweed. J. Clean. Prod. 2020, 259, 120836. [Google Scholar] [CrossRef]
- Terry, S.; Romero, C.; Chaves, A.; McAllister, T. Nutritional factors affecting greenhouse gas production from ruminants; implications for enteric and manure emissions. In Improving Rumen Function; Mackie, C.M.a.R., Ed.; Burleigh Dodds Science Publishing: Cambridge, UK, 2020. [Google Scholar]
- McDougall, E.I. Studies on ruminant saliva. 1. The composition and output of sheep’s saliva. Biochem. J. 1948, 43, 99. [Google Scholar] [CrossRef] [PubMed]
- Association of Official Analytical Chemists. Official Methods of Analysis of AOAC International, 16th ed.; AOAC International: Arlington, WA, USA, 1995. [Google Scholar]
- O’Hara, E.; Terry, S.A.; Moote, P.; Beauchemin, K.A.; McAllister, T.A.; Abbott, D.W.; Gruninger, R.J. Comparative analysis of macroalgae supplementation on the rumen microbial community: Asparagopsis taxiformis inhibits major ruminal methanogenic, fibrolytic, and volatile fatty acid-producing microbes in vitro. Front. Microbiol 2023, 14. [Google Scholar] [CrossRef]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for Dietary Fiber, Neutral Detergent Fiber, and Nonstarch Polysaccharides in Relation to Animal Nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Association of Official Analytical Chemists. Official Methods of Analysis of AOAC International, 18th ed.; AOAC International: Gaithersburg, MD, USA, 2005. [Google Scholar]
- Broderick, G.A.; Kang, J.H. Automated Simultaneous Determination of Ammonia and Total Amino Acids in Ruminal Fluid and In Vitro Media. J. Dairy Sci. 1980, 63, 64–75. [Google Scholar] [CrossRef] [PubMed]
- Association of Official Analytical Chemists. Official Methods of Analysis of AOAC International, 17th ed.; AOAC International: Gaithersburg, MD, USA, 2000. [Google Scholar]
- Li, X.; Norman, H.C.; Kinley, R.D.; Laurence, M.; Wilmot, M.; Bender, H.; de Nys, R.; Tomkins, N. Asparagopsis taxiformis decreases enteric methane production from sheep. Anim. Prod. Sci. 2018, 58, 681–688. [Google Scholar] [CrossRef]
- Machado, L.; Magnusson, M.; Paul, N.; Kinley, R.; de Nys, R.; Tomkins, N. Dose-response effects of Asparagopsis taxiformis and Oedogonium sp. on in vitro fermentation and methane production. J. Appl. Phycol. 2015, 28, 1443–1452. [Google Scholar] [CrossRef]
- Roque, B.M.; Brooke, C.G.; Ladau, J.; Polley, T.; Marsh, L.J.; Najafi, N.; Pandey, P.; Singh, L.; Kinley, R.; Salwen, J.K.; et al. Effect of the macroalgae Asparagopsis taxiformis on methane production and rumen microbiome assemblage. Anim. Microbiome 2019, 1, 3. [Google Scholar] [CrossRef] [PubMed]
- Roque, B.M.; Salwen, J.K.; Kinley, R.; Kebreab, E. Inclusion of Asparagopsis armata in lactating dairy cows’ diet reduces enteric methane emission by over 50 percent. J. Clean. Prod. 2019, 234, 132–138. [Google Scholar] [CrossRef]
- Mateos, I.; Ranilla, M.J.; Saro, C.; Carro, M.D. Shifts in microbial populations in Rusitec fermenters as affected by the type of diet and impact of the method for estimating microbial growth (15N v. microbial DNA). Animals 2017, 11, 1939–1948. [Google Scholar] [CrossRef] [PubMed]
- Andries, J.I.; Buysse, F.X.; De Brabander, D.L.; Cottyn, B.G. Isoacids in ruminant nutrition: Their role in ruminal and intermediary metabolism and possible influences on performances—A review. Anim. Feed Sci. Technol. 1987, 18, 169–180. [Google Scholar] [CrossRef]
- Firkins, J.L. Invited Review: Advances in rumen efficiency. Appl. Anim. Sci. 2021, 37, 388–403. [Google Scholar] [CrossRef]
- Chung, Y.-H.; Walker, N.; McGinn, S.; Beauchemin, K. Differing effects of 2 active dried yeast (Saccharomyces cerevisiae) strains on ruminal acidosis and methane production in nonlactating dairy cows. J. Dairy Sci. 2011, 94, 2431–2439. [Google Scholar] [CrossRef]
- Martínez-Fernández, G.; Abecia, L.; Arco, A.; Cantalapiedra-Hijar, G.; Martín-García, A.I.; Molina-Alcaide, E.; Kindermann, M.; Duval, S.; Yáñez-Ruiz, D.R. Effects of ethyl-3-nitrooxy propionate and 3-nitrooxypropanol on ruminal fermentation, microbial abundance, and methane emissions in sheep. J. Dairy Sci. 2014, 97, 3790–3799. [Google Scholar] [CrossRef]
- Guyader, J.; Ungerfeld, E.M.; Beauchemin, K.A. Redirection of Metabolic Hydrogen by Inhibiting Methanogenesis in the Rumen Simulation Technique (RUSITEC). Front. Microbiol. 2017, 8, 393. [Google Scholar] [CrossRef]
- Ungerfeld, E.M. Shifts in metabolic hydrogen sinks in the methanogenesis-inhibited ruminal fermentation: A meta-analysis. Front. Microbiol. 2015, 6, 37. [Google Scholar] [CrossRef]
- Johnson, K.A.; Johnson, D.E. Methane emissions from cattle. J. Anim. Sci. 1995, 73, 2483–2492. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).