First Study on Gyrodactylus (Monogenea: Gyrodactylidae) in Morocco, with Description of a New Species from Luciobarbus pallaryi and Luciobarbus ksibi (Actinopterygii: Cyprinidae)
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
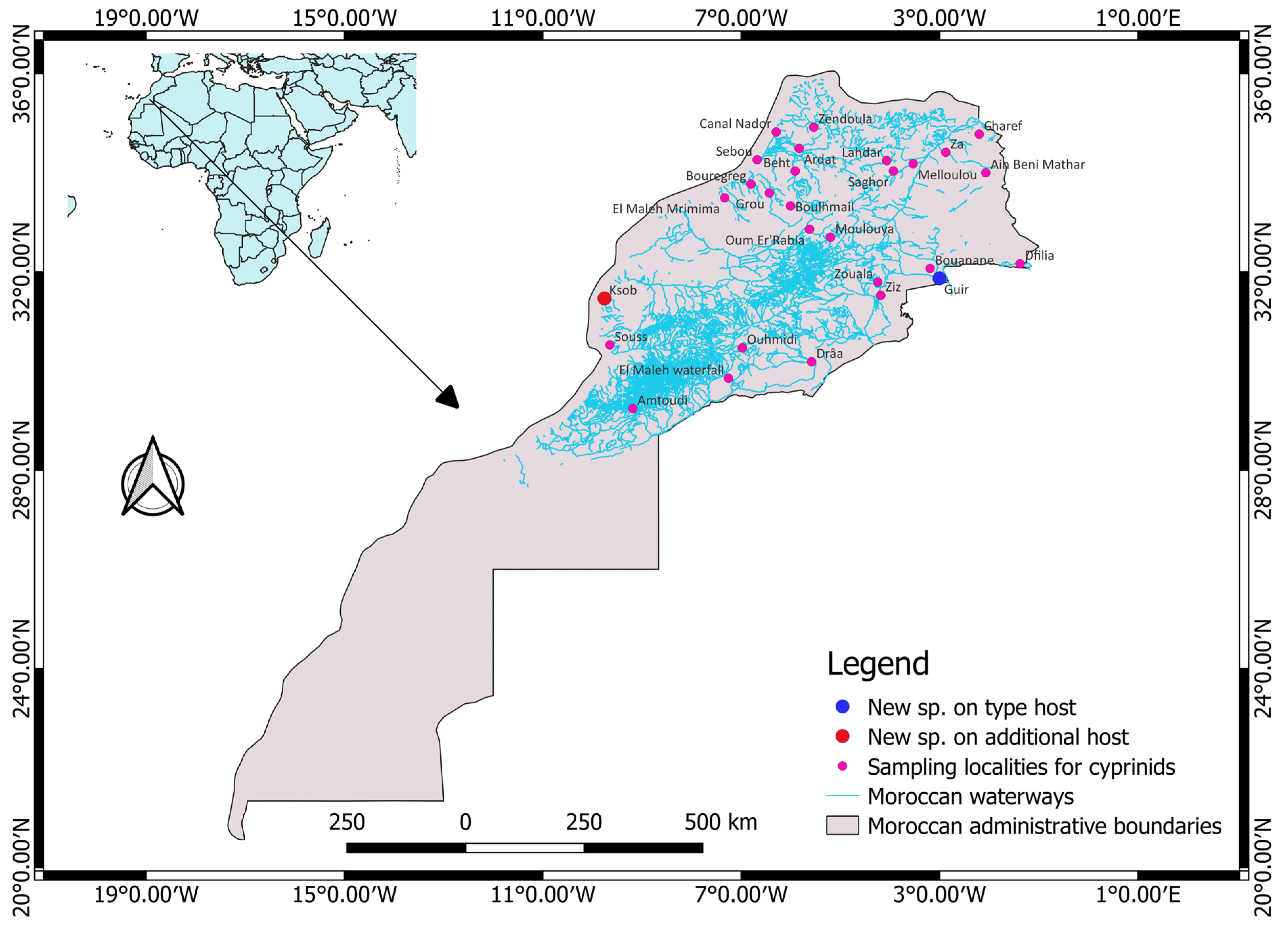
2.1. Sample Collection
2.2. Parasitological Examination
2.3. Identification of Representatives of Gyrodactylus
2.4. Infection Parameters
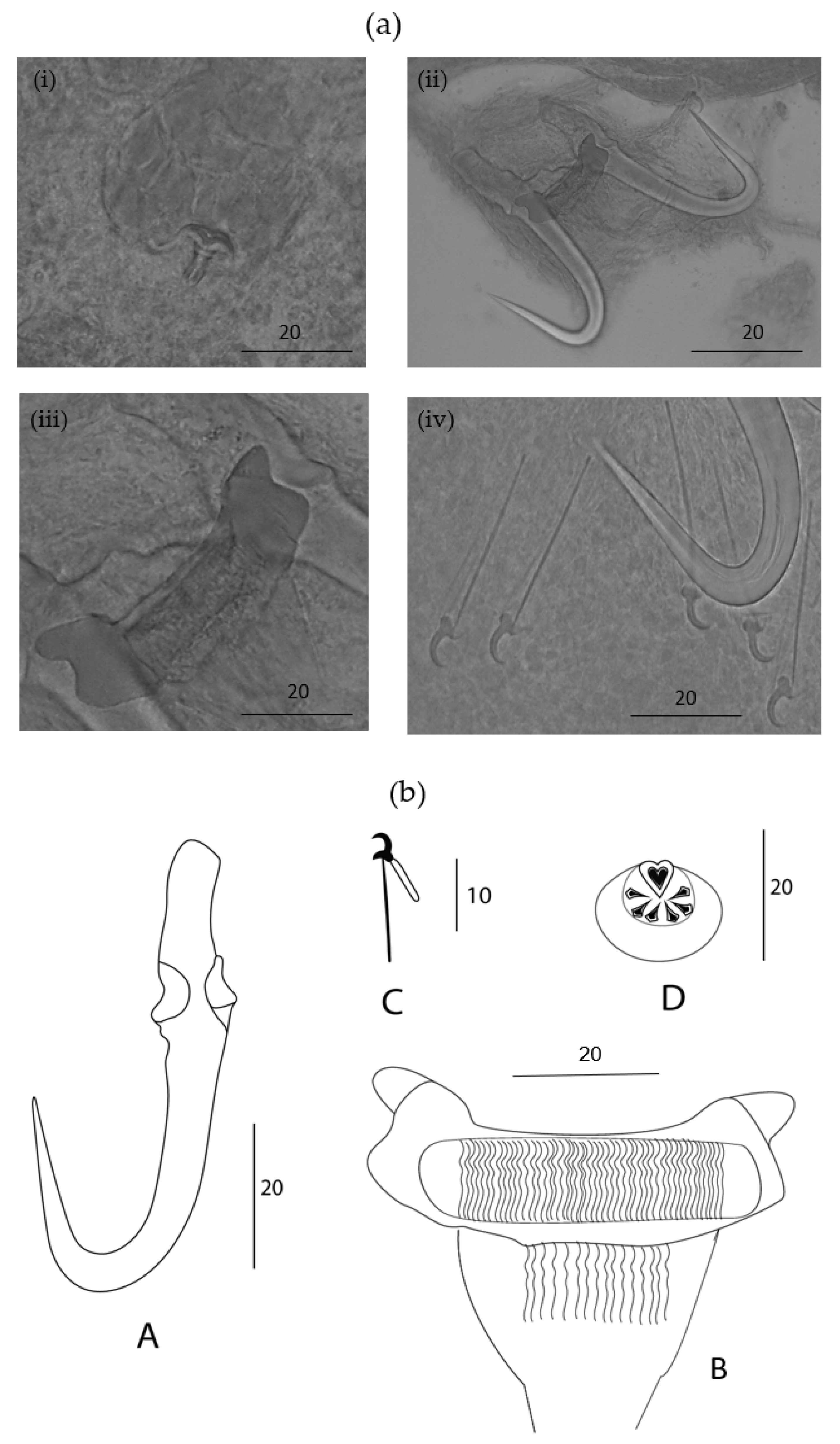
2.5. Morphological Characterization of Members of Gyrodactylus
2.6. Statistical Analyses
3. Results
3.1. Specimens Examined and Individuals of Gyrodactylus Isolated
3.2. Infection Parameters
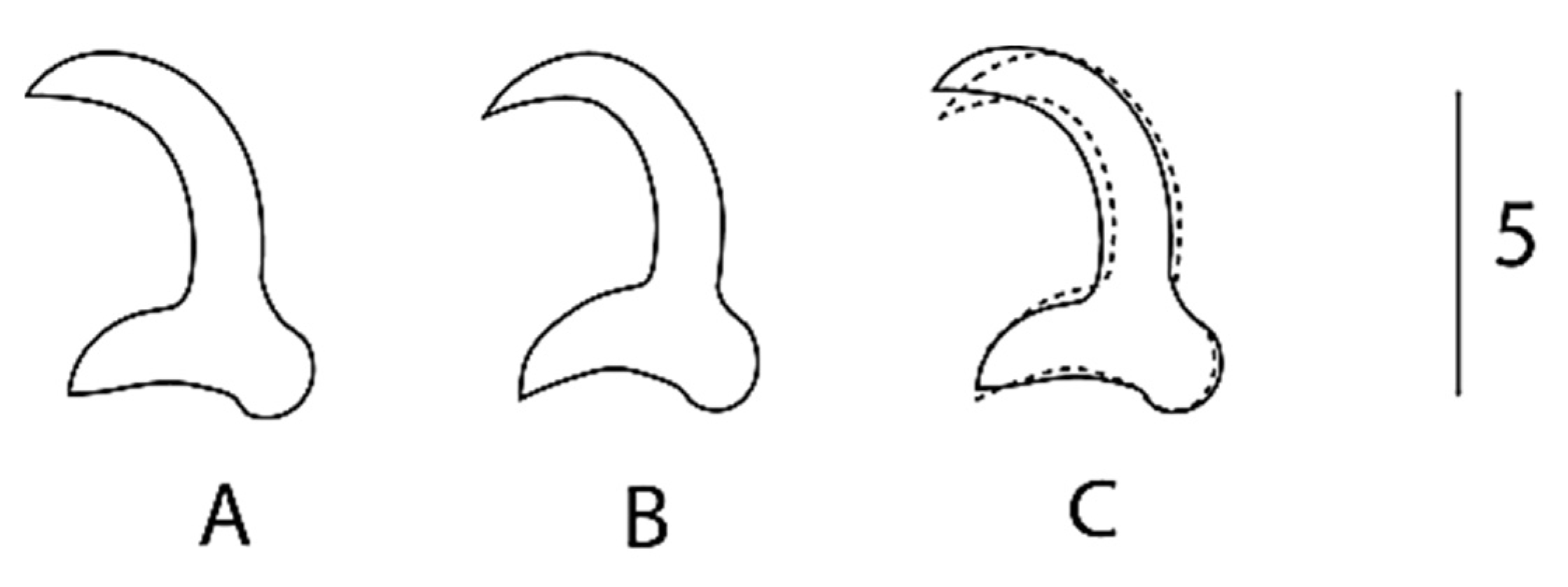
3.3. Characterization of a New Species of Gyrodactylus
Remarks
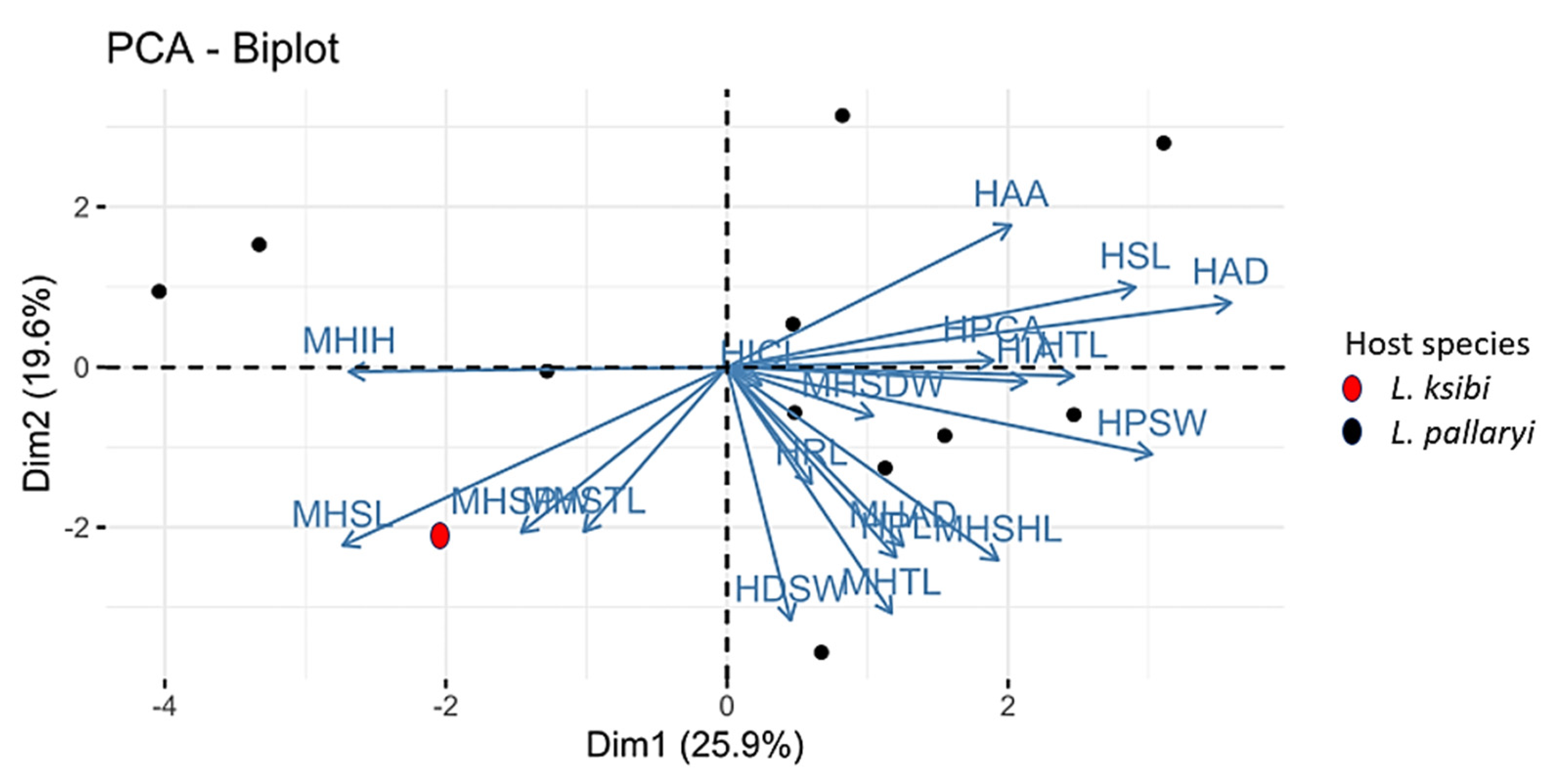
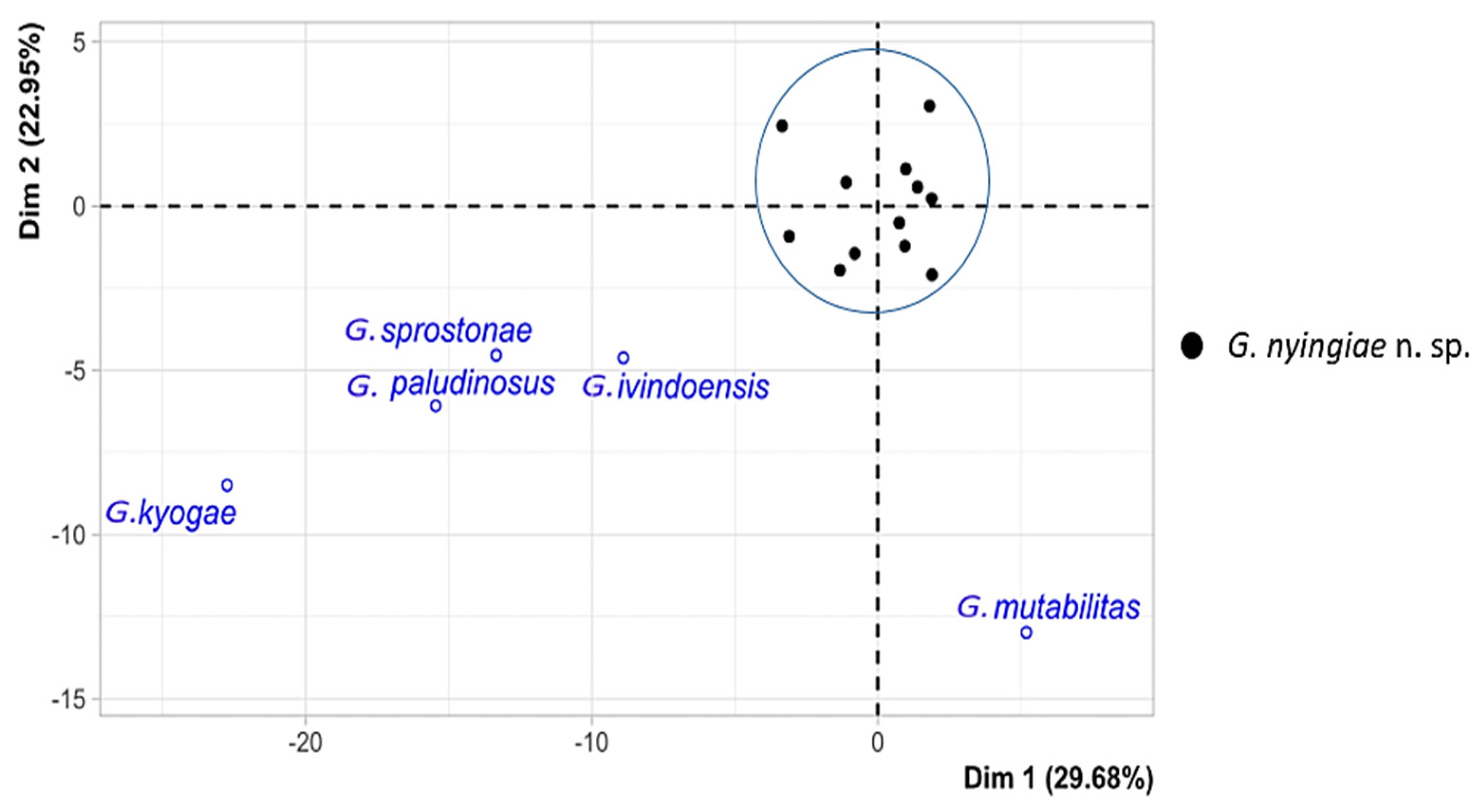
3.4. Multivariate Statistics
4. Discussion
5. Conclusion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO. The State of World Fisheries and Aquaculture 2018. In Meeting the Sustainable Development Goals; FAO: Rome, Italy, 2018. [Google Scholar]
- Dahani, S.; Bouchriti, N.; Benabbes, I.; Boudakkou, A.; Chiaar, A. Occurrence Des Parasites Dans Les Poissons Collectés Au Niveau Du Littoral Marocain. Eur. Sci. J. ESJ 2019, 15, 497–506. [Google Scholar] [CrossRef]
- Mohammed, B.; Salwa, O.; Yahyaoui, A. Perceived Barriers to Consumption of Freshwater Fish in Morocco. Br. Food J. 2015, 117, 274–285. [Google Scholar] [CrossRef]
- Doadrio, I. Freshwater Fish Fauna of North Africa and Its Biogeography. Ann. Mus. Afr. Centr. Zool. 1994, 275, 21–34. [Google Scholar]
- Rahmouni, I.; Řehulková, E.; Pariselle, A.; Rkhami, O.B.; Šimková, A. Four New Species of Dactylogyrus Diesing, 1850 (Monogenea: Dactylogyridae) Parasitising the Gills of Northern Moroccan Luciobarbus Heckel (Cyprinidae): Morphological and Molecular Characterisation. Syst. Parasitol. 2017, 94, 575–591. [Google Scholar] [CrossRef] [PubMed]
- Cowling, R.M.; Rundel, P.W.; Lamont, B.B.; Arroyo, M.K.; Arianoutsou, M. Plant Diversity in Mediterranean-Climate Regions. Trends Ecol. Evol. 1996, 11, 362–366. [Google Scholar] [CrossRef] [PubMed]
- Sauquet, H.; Weston, P.H.; Anderson, C.L.; Barker, N.P.; Cantrill, D.J.; Mast, A.R.; Savolainen, V. Contrasted Patterns of Hyperdiversification in Mediterranean Hotspots. Proc. Natl. Acad. Sci. USA 2009, 106, 221–225. [Google Scholar] [CrossRef]
- Scholz, T.; Smit, N.; Jayasundera, Z.; Gelnar, M. A Guide to the Parasites of African Freshwater Fishes; Royal Belgian Institute of Natural Sciences: Brussels, Belgium, 2018; Volume 18, ISBN 9789073242388. [Google Scholar]
- FAO. The State of World Fisheries and Aquaculture 2020. In Sustainability in Action; FAO: Rome, Italy, 2020. [Google Scholar]
- Cable, J.; Harris, P.D. Gyrodactylid Developmental Biology: Historical Review, Current Status and Future Trends. Int. J. Parasitol. 2002, 32, 255–280. [Google Scholar] [CrossRef]
- Bakke, T.A.; Cable, J.; Harris, P.D. The Biology of Gyrodactylid Monogeneans: The “Russian-Doll Killers”. Adv. Parasitol. 2007, 64, 162–220. [Google Scholar] [CrossRef]
- Allalgua, A.; Guerfi, S.; Kaouachi, N.; Boualleg, C.; Boucenna, I.; Barour, C. L’infestation de Cyprinus carpio (Cyprinides) Peuplant Le Barrage Foum El-Khanga (Souk Ahras, Algerie) Par Les Monogenes Parasites. Bull. Soc. Zool. Fr. 2015, 140, 217–232. [Google Scholar]
- Bakke, T.A.; Harris, P.D.; Cable, J. Host Specificity Dynamics: Observations on Gyrodactylid Monogeneans. Int. J. Parasitol. 2002, 32, 281–308. [Google Scholar] [CrossRef]
- Truter, M.; Smit, N.J.; Malherbe, W.; Přikrylová, I. Description of Gyrodactylus paludinosus sp. nov. (Monogenea: Gyrodactylidae) from the Straightfin Barb, Enteromius paludinosus (Peters, 1852), in South Africa. Acta Parasitol. 2022, 67, 446–453. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, Q.M.; Maina, J.N.; Avenant-Oldewage, A. Gyrodactylus magadiensis n. sp. (Monogenea, Gyrodactylidae), Parasitising the Gills of Alcolapia grahami (Perciformes, Cichlidae), a Fish Inhabiting the Extreme Environment of Lake Magadi, Kenya. Parasite 2019, 26, 76. [Google Scholar] [CrossRef] [PubMed]
- Rubio-Godoy, M.; Muñoz-Córdova, G.; Garduño-Lugo, M.; Salazar-Ulloa, M.; Mercado-Vidal, G. Microhabitat Use, Not Temperature, Regulates Intensity of Gyrodactylus cichlidarum Long-Term Infection on Farmed Tilapia-Are Parasites Evading Competition or Immunity? Vet. Parasitol. 2012, 183, 305–316. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhi, T.; Xu, X.; Zhang, S.; Zheng, Y.; Yang, T. Molecular Characterization and Dynamic Expressions of Three Nile Tilapia (Oreochromis niloticus) Complement Genes after Gyrodactylus cichlidarum (Monogenea) Infection. Aquaculture 2019, 502, 176–188. [Google Scholar] [CrossRef]
- Buchmann, K.; Bresciani, J. Microenvironment of Gyrodactylus derjavini on Rainbow Trout Oncorhynchus mykiss: Association between Mucous Cell Density in Skin and Site Selection. Parasitol. Res. 1997, 84, 17–24. [Google Scholar] [CrossRef]
- Bakke, T.A.; Harris, P.D.; Hansen, H.; Cable, J.; Hansen, L.P. Susceptibility of Baltic and East Atlantic Salmon Salmo salar Stocks to Gyrodactylus salaris (Monogenea). Dis. Aquat. Organ. 2004, 58, 171–177. [Google Scholar] [CrossRef]
- Kerr, S.J.; Grant, R.E. Ecological Impacts of Fish Introductions: Evaluating the Risk; Fish and Wildlife Branch, Ontario Ministry of Natural Resources: Peterborough, ON, Canada, 2000; ISBN 0777893169. [Google Scholar]
- Hickley, P.; Muchiri, S.M. Management and Ecological Note Discovery of Carp, Cyprinus carpio, in the Already Stressed Fishery of Lake Naivasha, Kenya. Mar. Freshw. Res. 2004, 11, 139–142. [Google Scholar]
- Otachi, E.O.; Magana, A.E.M.; Jirsa, F.; Fellner-Frank, C. Parasites of Commercially Important Fish from Lake Naivasha, Rift Valley, Kenya. Parasitol. Res. 2014, 113, 1057–1067. [Google Scholar] [CrossRef]
- Ormerod, S.J. Current Issues with Fish and Fisheries: Editor’s Overview and Introduction. J. Appl. Ecol. 2003, 40, 204–213. [Google Scholar] [CrossRef]
- Gherardi, F.; Robert Britton, J.; Mavuti, K.M.; Pacini, N.; Grey, J.; Tricarico, E.; Harper, D.M. A Review of Allodiversity in Lake Naivasha, Kenya: Developing Conservation Actions to Protect East African Lakes from the Negative Impacts of Alien Species. Biol. Conserv. 2011, 144, 2585–2596. [Google Scholar] [CrossRef]
- Pellegrin, J. Poissons Du Gribingui Par M. Baudon. Description de Sept Especes Nouvelles. Bull. Société Zool. Fr. 1919, 44, 201–214. [Google Scholar]
- Justine, J.L.; Briand, M.J.; Bray, R.A. A Quick and Simple Method, Usable in the Field, for Collecting Parasites in Suitable Condition for Both Morphological and Molecular Studies. Parasitol. Res. 2012, 111, 341–351. [Google Scholar] [CrossRef] [PubMed]
- Fricke, R.; Eschmeyer, W.; Van der Laan, R. Eschmeyer’s Catalog of Fishes: Genera, Species, References. Available online: https://researcharchive.calacademy.org/research/ichthyology/catalog/fishcatmain.asp (accessed on 23 July 2021).
- Pariselle, A.; Euzet, L. Three New Species of Cichlidogyrus Paperna, 1960 (Monogenea, Ancyrocephalidae) Parasitic on Tylochromis jentinki (Steindachner, 1895) (Pisces, Cichlidae) in West Africa. Syst. Parasitol. 1994, 29, 229–234. [Google Scholar] [CrossRef]
- Humason, G.L. Animal Tissue Techniques; Beadle, G.W., Emerson, R., Whitaker, D.M., Eds.; W. H. Freeman and Company: New York, NY, USA, 1979. [Google Scholar]
- Malmberg, G. The Excretory Systems and the Marginal Hooks as a Basis for the Systematics of Gyrodactylus (Trematoda, Monogenea). Ark. Zool. 1970, 23, 1–235. [Google Scholar]
- Bates, J.W. The Slide-Sealing Compound “Glyceel”. J. Nematol. 1997, 29, 565–566. [Google Scholar] [PubMed]
- Pugachev, O.N.; Gerasev, P.; Gussev, A.; Ergens, R.; Khotenowsky, I. Guide to Monogenoidea of Freshwater Fish of Palaeartic and Amur Regions; Ledizoni-Ledipublishing: Lombardy, Italy, 2009. [Google Scholar]
- Bush, A.O.; Lattertyf, K.D.; Lotz, J.M.; Shostakll, A.W.; Brandon, Z.; Lafferty, K.D.; Lotz, J.M.; Shostak, A.W. Parasitology Meets Ecology on Its Own Terms: Margolis et Al. Revisited. J. Parasitol. 1997, 83, 575–583. [Google Scholar] [CrossRef]
- Shinn, A.P.; Hansen, H.; Olstad, K.; Bachmann, L.; Bakke, T.A. The Use of Morphometric Characters to Discriminate Specimens of Laboratory-Reared and Wild Populations of Gyrodactylus salaris and G. thymalli (Monogenea). Folia Parasitol. 2004, 51, 239–252. [Google Scholar] [CrossRef]
- Paperna, I. New Species of Monogenea (Vermes) from African Freshwater Fish. A Preliminary Report. Rev. Zool. Bot. Africaines 1973, 87, 505–518. [Google Scholar]
- Benovics, M.; Nejat, F.; Abdoli, A.; Šimková, A. Molecular and Morphological Phylogeny of Host-Specific Dactylogyrus Parasites (Monogenea) Sheds New Light on the Puzzling Middle Eastern Origin of European and African Lineages. Parasites and Vectors 2021, 14, 372. [Google Scholar] [CrossRef]
- Jalali, B.; Shamsi, S.; Barzegar, M. Occurrence of Gyrodactylus spp. (Monogenea: Gyrodactylidae) from Iranian Freshwater Fishes. Iran. J. Fish. Sci. 2005, 4, 19–30. [Google Scholar]
- Barzegar, M.; Ebrahimzadeh Mousavi, H.; Rahmati-holasoo, H.; Taheri Mirghaed, A.; Bozorgnia, A. Gyrodactylus (Monogenea, Gyrodactylidae) Parasite Fauna of Fishes in Some Rivers of the Southern Caspian Sea Basin in Mazandaran Province. Iran. J. Vet. Med. 2017, 12, 35–44. [Google Scholar] [CrossRef]
- Younis, S.A.; Shamall, M.A.A. Monogenean Infections on Fishes from Darbandikhan Lake in Kurdistan Region, Iraq. Basrah J. Agric. Sci. 2013, 26, 117–131. [Google Scholar] [CrossRef]
- Roohi, J.D.; Dalimi Asl, A.; Pourkazemi, M.; Shamsi, S. Occurrence of Dactylogyrid and Gyrodactylid Monogenea on Common Carp, Cyprinus carpio, in the Southern Caspian Sea Basin. Parasitol. Int. 2019, 73, 101977. [Google Scholar] [CrossRef] [PubMed]
- Roohi, J.D.; Asl, A.D.; Mohammad, P.; Ghasemi, M.; Shamsi, S. Morphometric and Molecular Identification of Gyrodactylus sprostonae in Guilan Province Warm Water Fishes with an Attitude of Intensity and Prevalence in Selected Farms. Iran. Vet. J. 2018, 15, 50–59. [Google Scholar] [CrossRef]
- Tu, X.; Ling, F.; Huang, A.; Wang, G. An Infection of Gyrodactylus kobayashii Hukuda, 1940 (Monogenea) Associated with the Mortality of Goldfish (Carassius auratus) from Central China. Parasitol. Res. 2015, 114, 737–745. [Google Scholar] [CrossRef]
- Derbel, H.; Châari, M.; Neifar, L. Checklist of the Monogenea (Platyhelminthes) Parasitic in Tunisian Aquatic Vertebrates. Helminthologia 2022, 59, 179–199. [Google Scholar] [CrossRef]
- Pinacho-Pinacho, C.D.; Calixto-Rojas, M.; García-Vásquez, A.; Guzmán-Valdivieso, I.; Barrios-Gutiérrez, J.J.; Rubio-Godoy, M. Species Delimitation of Gyrodactylus (Monogenea: Gyrodactylidae) Infecting the Southernmost Cyprinids (Actinopterygii: Cyprinidae) in the New World. Parasitol. Res. 2021, 120, 831–848. [Google Scholar] [CrossRef]
- Razo-Mendivil, U.; García-Vásquez, A.; Rubio-Godoy, M. Spot the Difference: Two Cryptic Species of Gyrodactylus von Nordmann, 1832 (Platyhelminthes: Monogenea) Infecting Astyanax aeneus (Actinopterygii, Characidae) in Mexico. Parasitol. Int. 2016, 65, 389–400. [Google Scholar] [CrossRef]
- Louizi, H.; Vanhove, M.P.M.; Rahmouni, I.; Berrada Rkhami, O.; Benhoussa, A.; Van Steenberge, M.; Pariselle, A. Species Depauperate Communities and Low Abundances of Monogenean Gill Parasites at the Edge of the Natural Distribution Range of Their Cichlid Hosts in Northern Africa. Hydrobiologia 2022. [Google Scholar] [CrossRef]
- Gharbi, S.E.; Birgi, E.; Lambert, A. Monogènes Dactylogyridae Parasites de Cyprinidae Du Genre Barbus d’Afrique Du Nord. Syst. Parasitol. 1994, 27, 45–70. [Google Scholar] [CrossRef]
- Přikrylová, I.; Blažek, R.; Vanhove, M.P.M. An Overview of the Gyrodactylus (Monogenea: Gyrodactylidae) Species Parasitizing African Catfishes, and Their Morphological and Molecular Diversity. Parasitol. Res. 2012, 110, 1185–1200. [Google Scholar] [CrossRef]
- Vanhove, M.P.M.; Snoeks, J.; Volckaert, F.A.M.; Huyse, T. First Description of Monogenean Parasites in Lake Tanganyika: The Cichlid Simochromis diagramma (Teleostei, Cichlidae) Harbours a High Diversity of Gyrodactylus Species (Platyhelminthes, Monogenea). Parasitology 2011, 138, 364–380. [Google Scholar] [CrossRef]

| Coordinates | ||||||
|---|---|---|---|---|---|---|
| Host | Locality | Watershed | Latitude | Longitude | No. of Hosts Sampled | No. of Specimens of Gyrodactylus Isolated from the Hosts |
| Luciobarbus pallaryi (Pellegrin, 1919) | Oued Guir | Ziz | 31°52′12″ N | 003°0′00″ W | 157 | 14 |
| Oued Bouanane | Ziz | 32°04′04″ N | 003°11′23.9″ W | |||
| Oued Dfilia | Ziz | 32°9′48.892″ N | 001°22′37.4″ W | |||
| Luciobarbus rabatensis Doadrio, Perea and Yahyaoui, 2015 | Oued Grou | Bouregreg | 33°35′28.0″ N | 006°25′49.6″ W | 24 | 3 |
| Oued Bouregreg | Bouregreg | 33°46′18.0″ N | 006°48′16.6″ W | |||
| Oued Boulhmail | Bouregreg | 33°19′49.6″ N | 006°00′15.1″ W | |||
| Luciobarbus maghrebensis Doadrio, Perea and Yahyaoui, 2015 | Oued Lahdar | Sebou | 34°14′32.7″ N | 004°03′53.9″ W | 55 | 1 |
| Oued Saghor | Sebou | 34°02′4.0″ N | 003°55′45.5′W | |||
| Oued Ardat | Sebou | 34°29′26.8″ N | 005°49′49.2″ W | |||
| Oued Beht | Sebou | 34°01′55.5″ N | 005°54′43.2″ W | |||
| Oued Sebou | Sebou | 34°15′48.0″ N | 006°40′42.0″ W | |||
| Canal Nador | Sebou | 34°49′19.7″ N | 006°17′36.7″ W | |||
| Luciobarbus rifensis Doadrio, Casal-López and Perea 2015 | Oued Zendoula | Loukkos | 34°54′57.6″ N | 005°32′17.2″ W | 19 | 3 |
| Luciobarbus guercifensis Doadrio, Perea and Yahyaoui, 2016 | Oued Melloulou | Moulouya | 34°10′51.7″ N | 003°31′59.6″ W | 4 | 0 |
| Oued Za | Moulouya | 34°24′38.9″ N | 002°52′28.1″ W | |||
| Luciobarbus yahyaouii Doadrio, Casal-López and Perea 2016 | Oued Za | Moulouya | 34°24′38.9″ N | 002°52′28.1″ W | 62 | 0 |
| Oued Charef | Moulouya | 34°46′44.0″ N | 002°11′56.0″ W | |||
| Oued Melloulou | Moulouya | 34°10′51.7″ N | 003°31′59.6″ W | |||
| Ain Beni Mathar | Moulouya | 34°00′00.3″ N | 002°03′58.6″ W | |||
| Luciobarbus zayanensis Doadrio, Casal-López and Yahyaoui, 2016 | Oued Oum Er’Rabia | Oum Er’Rabia | 32°51′32.8″ N | 005°37′18.9″ W | 25 | 1 |
| Oued Moulouya | Moulouya | 32°41′55.4″ N | 005°11′51.2″ W | |||
| Luciobarbus lepineyi (Pellegrin, 1939) | Oued Ziz | Ziz | 31°31′34.7″ N | 004°11′10.0″ W | 127 | 0 |
| Oued Zouala | Ziz | 31°47′31.9″ N | 004°14′43.5″ W | |||
| Oued Dfilia | Ziz | 32°9′48.892″ N | 001°22′37.4″ W | |||
| Oued Drâa | Draa | 30°11′12.24″ N | 005°34′47.34″ W | |||
| Oued Ouhmidi | Draa | 30°28′5.64″ N | 006°58′36.12″ W | |||
| Oued El Maleh Mrimima | Draa | 33°29’34.8’’N | 007°19′58.1″ W | |||
| Oued El Maleh Waterfall | Draa | 29°51′108″ N | 007°15′23″ W | |||
| Oued Amtoudi | Draa | 29°14′32.42″ N | 009°11′8.71″ W | |||
| Carasobarbus moulouyensis (Pellegrin, 1924) | Oued Moulouya | Moulouya | 32°41′55.4″ N | 005°11′51.2″ W | 44 | 1 |
| Luciobarbus ksibi (Boulenger, 1905) | Oued Oum Er’Rabia | Oum Er’Rabia | 32°51′32.8″ N | 005°37′18.9″ W | 40 | 1 |
| Oued Ksob | Ksob | 31°27′50.7″ N | 009°45′25.3″ W | |||
| Luciobarbus massaensis (Pellegrin, 1922) | Oued Souss | Souss-Massa | 30°31′33.6″ N | 009°38′53.6″ W | 21 | 1 |
| Carasobarbus fritschii (Günther, 1874) | Oued Grou | Bouregreg | 33°35′28.0″ N | 006°25′49.6″ W | 157 | 0 |
| Oued Boulhmail | Bouregreg | 33°19′49.6″ N | 006°00′15.1″ W | |||
| Oued Lahdar | Sebou | 34°14′32.7″ N | 004°03′53.9″ W | |||
| Oued Oum Er’Rabia | Oum Er’Rabia | 32°41′03.8″ N | 005°13′00.3″ W | |||
| Oued Za | Moulouya | 34°24′38.9″ N | 002°52′28.1″ W | |||
| Oued Charef | Moulouya | 34°46′44.0″ N | 002°11′56.0″ W | |||
| Oued Ksob | Ksob | 31°27′50.7″ N | 009°45′25.3″ W | |||
| Oued Ardat | Sebou | 34°29′26.8″ N | 005°49′49.2″ W | |||
| Oued Beht | Sebou | 34°01′55.5″ N | 005°54′43.2″ W | |||
| Oued Sebou | Sebou | 34°15′48.0″ N | 006°40′42.0″ W | |||
| Pterocapoeta maroccana Günther, 1902 | Oued Oum Er’Rabia | Oum Er’Rabia | 32°51′32.8″ N | 005°37′18.9″ W | 3 | 1 |
| Total | 738 | 26 | ||||
| Locality | Species | H | N | n | P = (N/H) × 100 | M.I = n/N | M.A = n/H |
|---|---|---|---|---|---|---|---|
| Oued Guir | Luciobarbus pallaryi | 157 | 1 | 14 | 0.64 | 14 | 0.09 |
| Oued Bouregreg | Luciobarbus rabatensis | 24 | 1 | 3 | 4.17 | 3 | 0.13 |
| Oued Sebou | Luciobarbus maghrebensis | 55 | 1 | 1 | 1.82 | 1 | 0.02 |
| Oued Zendoula | Luciobarbus rifensis | 19 | 1 | 3 | 5.26 | 3 | 0.16 |
| Oued Moulouya | Luciobarbus zayanensis | 25 | 1 | 1 | 4.00 | 1 | 0.04 |
| Oued Moulouya | Carasobarbus moulouyensis | 44 | 1 | 1 | 2.27 | 1 | 0.02 |
| Oued Ksob | Luciobarbus ksibi | 40 | 1 | 1 | 2.50 | 1 | 0.03 |
| Oued Souss | Luciobarbus massaensis | 21 | 1 | 1 | 4.76 | 1 | 0.05 |
| Oued Oum Er’Rabia | Pterocapoeta maroccana | 3 | 1 | 1 | 33.33 | 1 | 0.33 |
| Host | Luciobarbus pallaryi (n = 1) | Luciobarbus ksibi (n = 1) | Both Host Species Combined |
|---|---|---|---|
| Total body length (TBL) | 386.8 (278.3–456) 5 | 443.7 | 396.3 (278.3–456) 6 |
| Total body width (TBW) | 133 (115.8–145.9) 6 | 158 | 136.6 (115.8–158.4) 7 |
| Hamulus total length (HTL) | 76.5 (65.9–88.2) 10 | 75.3 | 76.4 (65.9–88.2) 11 |
| Hamulus sickle length (HSL) | 47.6 (42.5–54.8) 8 | 45.1 | 47.4 (42.5–54.8) 9 |
| Hamulus aperture distance (HAD) | 27.5 (21.1–30.2) 9 | 22.6 | 26.7 (21.1–30.2) 10 |
| Hamulus point length (HPL) | 36.9 (31.7–41.3) 9 | 36.3 | 36.2 (31.7–41.3) 10 |
| Hamulus inner curve length (HICL) | 1.7 (1.4–2.7) 6 | 4 | 2.1 (1.4–4) 7 |
| Hamulus distal shaft width (HDSW) | 5.5 (4.6–6.7) 10 | 6 | 5.7 (4.6–7.3) 11 |
| Hamulus root length (HRL) | 26.7 (24.2–28.3) 7 | – | 26.7 (24.2–28.3) 7 |
| Hamulus aperture angle (HAA) (in degrees) | 36.9 (31.5–45.4) 7 | 32.4 | 36.4 (31.5–45.4) 8 |
| Hamulus point curve angle (HPCA) (in degrees) | 4.4 (3.4–5.4) 4 | – | 4.4 (3.4–5.4) 4 |
| Hamulus inner angle (HIA) (in degrees) | 40.4 (36–45.4) 7 | 37.2 | 40 (36–45.4) 8 |
| Hamulus proximal shaft width (HPSW) | 10.4 (8.2–12.1) 10 | 10.3 | 10.2 (8.2–12.1) 11 |
| Marginal hook total length (MHTL) | 34.4 (31.7–42.1) 8 | 35.2 | 34.8 (31.7–42.1) 9 |
| Marginal hook shaft length (MHSHL) | 28.6 (26.1–33.4) 9 | 29.2 | 28.7 (26.1–33.4) 10 |
| Marginal hook sickle length (MHSL) | 6.2 (5.5–6.5) 9 | 6.6 | 6.3 (5.5–6.6) 10 |
| Marginal hook sickle proximal width (MHSPW) | 4.6 (3.9–5) 9 | 5.5 | 4.7 (3.9–5.5) 10 |
| Marginal hook sickle distal width (MHSDW) | 4.5 (3.9–5.1) 9 | 5 | 4.5 (3.9–5.1) 10 |
| Marginal hook sickle toe length (MHSTL) | 1.9 (1.8–2.1)9 | 2.1 | 2 (1.8–2.1) 10 |
| Marginal hook aperture distance (MHAD) | 5.5 (5–5.9) 8 | 5.3 | 5.4 (5–5.9) 9 |
| Marginal hook in-step height (MHIH) | 0.6 (0.5–0.9) 8 | 0.7 | 0.6 (0.5–0.9) 9 |
| Ventral bar total length (VBTL) | 19.6 (18.6–20.5) 2 | - | 19.6 (18.6–20.5) 2 |
| Ventral bar total width (VBTW) | 25.1 (24.8–25.4) 2 | - | 25.1 (24.8–25.4) 2 |
| Ventral bar median length (VBML) | 6.1 (5.5–6.8) 3 | - | 6.1 (5.5–6.8) 3 |
| Ventral bar membrane length (VBMBL) | 13.6 (12.7–14.5) 3 | - | 13.6 (12.7–14.5) 3 |
| Ventral bar process length (VBPL) | 3.7 (3.6–3.8) 2 | - | 3.7 (3.6–3.8) 2 |
| Male copulatory organ diameter (MCO) | 18.4 (16.5–19.5) 4 | 21.2 | 18.9 (16.5–21.2) 5 |
| Principal spine length | 6.5 (6.3–6.6) 3 | 6.5 | 6.5 (6.3–6.6) 4 |
| Small spine length | 3.3 (3.1–3.5) 3 | 5.4 | 4.4 (3.1–5.4) 4 |
| Dorsal bar length (DBL) | 11.9 (9.9–13.4) 3 | - | 11.9 (9.9–13.4) 3 |
| Dorsal bar width (DBW) | 1.6 (1.2–1.9) 3 | - | 1.6 (1.2–1.9) 3 |
| G. paludinosus Truter, Smit, Malherbe and Přikrylová, 2021 | G. kyogae Paperna, 1973 | G. ivindoensis Price & Gery, 1968 | G. sprostonae Ling, 1962 | G. mutabilitas Bychowskii, 1957 | |
|---|---|---|---|---|---|
| Reference | [14] | [14] | [14] | [40] | [38] |
| Country | South Africa | Uganda | Gabon | Iran | Iran |
| Host | Enteromius paludinosus (Peters, 1852) | Enteromius neumayeri (Fischer, 1884) | Enteromius cf. holotaenia (Boulenger, 1904) | Cyprinus carpio Linnaeus, 1758 | Vimba vimba (Linnaeus, 1758) |
| HPL | 19.1 | 14 | 21.4 | 22.8 | 23.8 |
| HRL | 15.4 | 9.2 | 19.4 | 16.6 | - |
| HTL | 43.3 | 33.1 | 55 | 52 | 67.5 |
| HSL | 33.8 | 28.2 | 37.6 | 39.8 | 25 |
| MHTL | 18.5 | 14.8 | 22 | 23.1 | 33.7 |
| MHSHL | 14.2 | 11.3 | 24.7 | 19.4 | - |
| MHSL | 4.4 | 3.1 | 5.5 | 4.5 | 10.3 |
| MHSPW | 2.5 | 2.4 | 3.3 | 3.2 | - |
| MHSDW | 1.9 | 2.1 | 2.5 | 3.1 | - |
| MHAD | 4.1 | 3.7 | 5.3 | - | - |
| VBTL | 17.4 | 4.8 | 18 | 19.7 | 28.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shigoley, M.I.; Rahmouni, I.; Louizi, H.; Pariselle, A.; Vanhove, M.P.M. First Study on Gyrodactylus (Monogenea: Gyrodactylidae) in Morocco, with Description of a New Species from Luciobarbus pallaryi and Luciobarbus ksibi (Actinopterygii: Cyprinidae). Animals 2023, 13, 1624. https://doi.org/10.3390/ani13101624
Shigoley MI, Rahmouni I, Louizi H, Pariselle A, Vanhove MPM. First Study on Gyrodactylus (Monogenea: Gyrodactylidae) in Morocco, with Description of a New Species from Luciobarbus pallaryi and Luciobarbus ksibi (Actinopterygii: Cyprinidae). Animals. 2023; 13(10):1624. https://doi.org/10.3390/ani13101624
Chicago/Turabian StyleShigoley, Miriam Isoyi, Imane Rahmouni, Halima Louizi, Antoine Pariselle, and Maarten P. M. Vanhove. 2023. "First Study on Gyrodactylus (Monogenea: Gyrodactylidae) in Morocco, with Description of a New Species from Luciobarbus pallaryi and Luciobarbus ksibi (Actinopterygii: Cyprinidae)" Animals 13, no. 10: 1624. https://doi.org/10.3390/ani13101624
APA StyleShigoley, M. I., Rahmouni, I., Louizi, H., Pariselle, A., & Vanhove, M. P. M. (2023). First Study on Gyrodactylus (Monogenea: Gyrodactylidae) in Morocco, with Description of a New Species from Luciobarbus pallaryi and Luciobarbus ksibi (Actinopterygii: Cyprinidae). Animals, 13(10), 1624. https://doi.org/10.3390/ani13101624






