Effects of Intra-Uterine Fluid Accumulation after Artificial Insemination on Luteal Function in Mares
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Mares
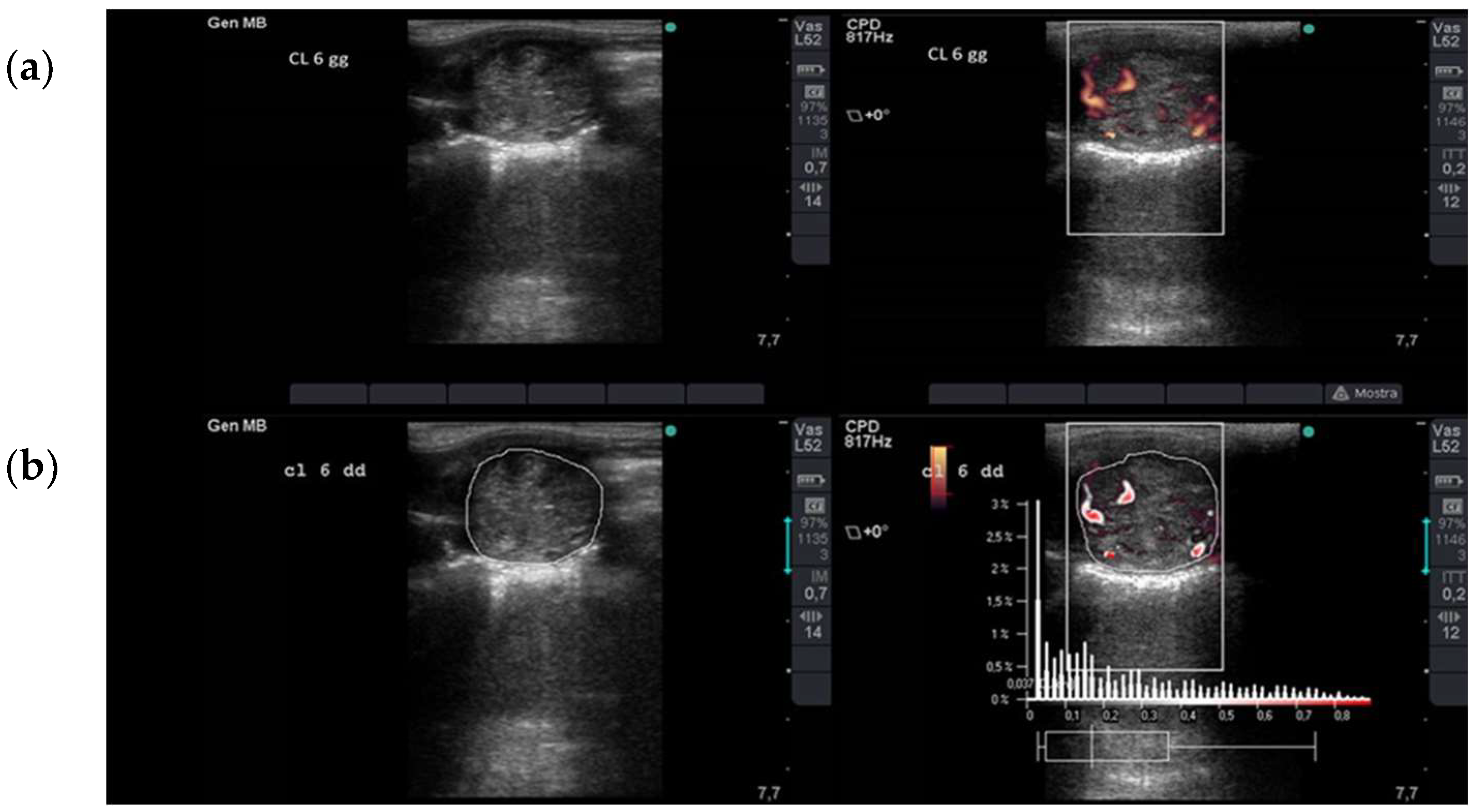
2.2. Ultrasonographic Assessment of Luteal Blood Flow
2.3. Blood Sample and Progesterone Assay
2.4. Statistical Analysis
3. Results
3.1. IUFA
3.2. Per Cycle Pregnancy Rate–Pregnancy
3.3. Fluid Grade
3.4. Age of the Mares and per Cycle Pregnancy Rate
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kotilainen, T.; Huhtinen, M.; Katila, T. Sperm-induced leucocytosis in the equine uterus. Theriogenology 1994, 41, 629–636. [Google Scholar] [CrossRef] [PubMed]
- Katila, T. Evaluation of diagnostic methods in equine endometritis. Reprod. Biol. 2016, 16, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Troedsson, M.H.T.; Loset, K.; Alghamdi, A.M.; Dahms, B.; Crabo, B.G. Interaction between equine semen and the endometrium: The inflammatory response to semen. Anim. Reprod. Sci. 2001, 68, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Woodward, E.M.; Troedsson, M.H.T. Inflammatory mechanism of endometritis. Equine Vet. J. 2015, 47, 384–389. [Google Scholar] [CrossRef]
- Troedsson, M.H.T.; Woodward, L.M. Our current understanding of the pathophysiology of equine endometritis with an emphasis of breeding-induced endometritis. Reprod. Biol. 2016, 16, 8–22. [Google Scholar] [CrossRef]
- Guvenc, K.; Reilas, T.; Katila, T. Effect of insemination dose and site on uterine inflammatory response of mares. Theriogenology 2005, 63, 2504–2512. [Google Scholar] [CrossRef]
- Watson, E.D.; Barbacini, S.; Berrocal, B.; Sheerin, O.; Marchi, V.; Zavaglia, G.; Necchi, D. Effect of insemination time of frozen semen on incidence of uterine fluid in mares. Theriogenology 2001, 56, 123–131. [Google Scholar] [CrossRef]
- Barbacini, S.; Necchi, D.; Zavaglia, G.; Squires, E. Retrospective study on the incidence of post- insemination uterine fluid in mares inseminated with frozen/thawed semen. J. Equine Vet. Sci. 2003, 23, 493–496. [Google Scholar] [CrossRef]
- Brinsko, S.P.; Rigby, S.L.; Varner, D.D.; Blanchard, T.L. A practical method for recognizing mares susceptible to post-breeding endometritis. AAEP Proc. 2003, 49, 363–365. [Google Scholar]
- Vandervall, D.K.; Schmidt, A.R.; Boston, R.C. Incidence, severity and factors associated with intrauterine fluid accumulation in mares after insemination with cooled or frozen semen. Clin. Theriogenol. 2012, 4, 565–566. [Google Scholar]
- Hughes, J.P.; Loy, R.G. Investigations on the effect of inoculation of Streptococcus zooepidemicus in mares. AAEP Proc Am. Assoc. Equine Pract. 1969, 15, 289–292. [Google Scholar]
- Watson, E. Post-breeding endometritis in the mare. Anim. Reprod. Sci. 2000, 61, 221–232. [Google Scholar] [CrossRef]
- Canisso, I.F.; Stewart, J.; da Silva, M.C. Endometritis-Managing Persistent Post-Breeding Endometritis. Vet. Clin. N. Am. Equine 2016, 32, 465–480. [Google Scholar] [CrossRef] [PubMed]
- Christoffersen, M.; Troedsson, M.H.T. Inflammation and fertility in the mare. Reprod. Domest. Anim. 2017, 52 (Suppl. S3), 14–20. [Google Scholar] [CrossRef]
- Morris, L.H.A.; McCue, P.M.; Aurich, C. Equine Endometritis: A review of challenges and new approaches. Reproduction 2020, 160, R95–R110. [Google Scholar] [CrossRef]
- Zent, W.; Troedsson, M.H.T.; Xue, J. Post-breeding uterine fluid accumulation in a normal population of thoroughbred mares: A field study. Proc. Am. Assoc. Equine Pract. 1998, 44, 64–65. [Google Scholar]
- Pycock, J.F. The relationship between intraluminal fluid, endometritis and pregnancy rate in the mare. Equine Pract. 1996, 18, 19–22. [Google Scholar]
- Katila, T.; Dias, G.F. Evolution of the Concepts of Endometrosis, Post-Breeding Endometritis, and Susceptibility in Mares. Animals 2022, 12, 779. [Google Scholar] [CrossRef]
- Woodward, E.M.; Christoffersen, M.; Campos, J.; Squires, E.L.; Troedsson, M.H.T. Susceptibility to persistent breeding-induced endometritis in the mare: Relationship to endometrial biopsy score and age, and variations between seasons. Theriogenology 2012, 78, 495–501. [Google Scholar] [CrossRef]
- Bollwein, H.; Sowade, C.; Stolla, R. The effect of semen extender, seminal plasma and raw semen on uterine and ovarian blood flow in mares. Theriogenology 2003, 60, 607–616. [Google Scholar] [CrossRef] [PubMed]
- Lüttgenau, J.; Imboden, I.; Wellnitz, O.; Romer, R.; Scaravaggi, I.; Neves, A.P.; Borel, N.; Bruckmaier, R.M.; Janett, F.; Bollwein, H. Intrauterine infusion of killed semen adversely affects uterine dlood flow and endometrial gene expression of inflammatory cytokines in mares susceptible to persistent breeding-induced endometritis. Theriogenology 2021, 163, 18–30. [Google Scholar] [CrossRef] [PubMed]
- Bollwein, H.; Mayer, R.; Weber, F.; Stolla, R. Luteal blood-flow during the estrous cycle in mares. Theriogenology 2002, 65, 2043–2051. [Google Scholar] [CrossRef]
- Ginther, O.J.; Gastal, E.L.; Gastal, M.O.; Utt, M.D.; Beg, M.A. Luteal blood flow and progesterone production in mares. Anim. Reprod. Sci. 2007, 99, 213–220. [Google Scholar] [CrossRef]
- Crabtree, J.R.; Wilsher, S. The effect of equine Chorionic Gonadotrphin (eCG) on luteal vascularity in the mare. J. Equine Vet. Sci. 2014, 1, 201. [Google Scholar] [CrossRef]
- Relave, F.; Lefebvre, R.C.; Beaudoin, S.; Price, C. Accuracy of a rapid enzyme-linked immunosorbent assay to measure progesterone in mares. Can. Vet. J. 2007, 48, 823–826. [Google Scholar] [PubMed]
- Ferreira-Dias, G.; Costa, A.S.; Mateus, L.; Korzekwa, A.J.; Galvão, A.; Redmer, D.A.; Lukasik, K.; Szostek, A.Z.; Woclawek-Potocka, I.; Skarzynski, D.J. Nitric oxide stimulates progesterone and prostaglandin E2 secretion as well as angiogenic activity in the equine corpus luteum. Domest. Anim. Endocrin. 2011, 40, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.A.; Scholtz, E.L.; Chenier, T.S. The Nitric Oxide System in Equine Reproduction: Current Status ad Future Directions. J. Equine Vet. Sci. 2015, 35, 481–487. [Google Scholar] [CrossRef]
- Khan, F.A.; Chenier, T.S.; Murrant, C.L.; Foster, R.A.; Hewson, J.; Scholtz, E.L. Dose-dependent inhibition of uterine contractility by nitric oxid: A potential mechanism underlying persistent breeding-induced endometritis in the mare. Theriogenology 2017, 90, 59–64. [Google Scholar] [CrossRef]
- Alghamdi, A.S.; Foster, G.N.; Troedsson, M.H.T. Nitric oxide levels and nitric oxide synthase expression in uterine samples from mares susceptible and resistant to persistent breeding-induced endometritis. Am. J. Reprod. Immunol. 2005, 53, 230–237. [Google Scholar] [CrossRef]
- Dell’Aqua, J.A., Jr.; Papa, F.O.; Lopes, M.D.; Alvarenga, M.A.; Macedo, L.P.; Melo, C.M. Modulation of acute uterine inflammatory response after artificial insemination with equine frozen semen. Anim. Reprod. Sci. 2006, 94, 270–273. [Google Scholar]
- Bucca, S.; Carli, A.; Buckley, T.; Dolci, G.; Fogarty, U. The use of dexamethasone administered to mares at the breeding time in the modulation of persistent mating induced endometritis. Theriogenology 2008, 70, 1093–1100. [Google Scholar] [CrossRef]
- Dascanio, J.J.; Kasimanickam, R. Breeding the mare with frozen semen. Equine Vet. Educ. 2008, 20, 667–672. [Google Scholar] [CrossRef]
- Canisso, I.F.; Segabinazzi, L.G.T.M.; Fedorka, C.E. Persistent Breeding-Induced Endometritis in Mares-A Multifaced Challenge: From Clinical Aspects to Immune Pathogenesis and Pathobiology. Int. J. Mol. Sci. 2020, 21, 1432. [Google Scholar] [CrossRef] [PubMed]
- LeBlanc, M.M. Advance in the Diagnosis and Treatment of Chronic Infectious and Post-Mating Induced Endometritis in the Mare. Reprod. Domest. Anim. 2010, 45 (Suppl. S2), 21–27. [Google Scholar] [CrossRef] [PubMed]
- Woodward, E.M.; Christoffersen, M.; Campos, J.; Horohov, D.W.; Scoggins, K.E.; Squires, E.L.; Troedsson, M.H.T. An investigation of Nitric Oxid Production in Mares Susceptible and Resistant to Persistent Breeding-Induced Endometritis and the Effects of Immunomodulation. Reprod. Domest. Anim. 2013, 48, 554–561. [Google Scholar] [CrossRef] [PubMed]
- Barbacini, S.; Marchi, V.; Zavaglia, G. Equine frozen semen: Results obtained in Italy during 1994–1997 period. Equine Vet. Educ. 1999, 11, 109–112. [Google Scholar] [CrossRef]
- Scoggin, C.F. Not just a number: Effect of age on fertility, pregnancy and offspring vigour in Thoroughbred broodmare. Reprod. Fert. Develop. 2015, 27, 872–879. [Google Scholar] [CrossRef]
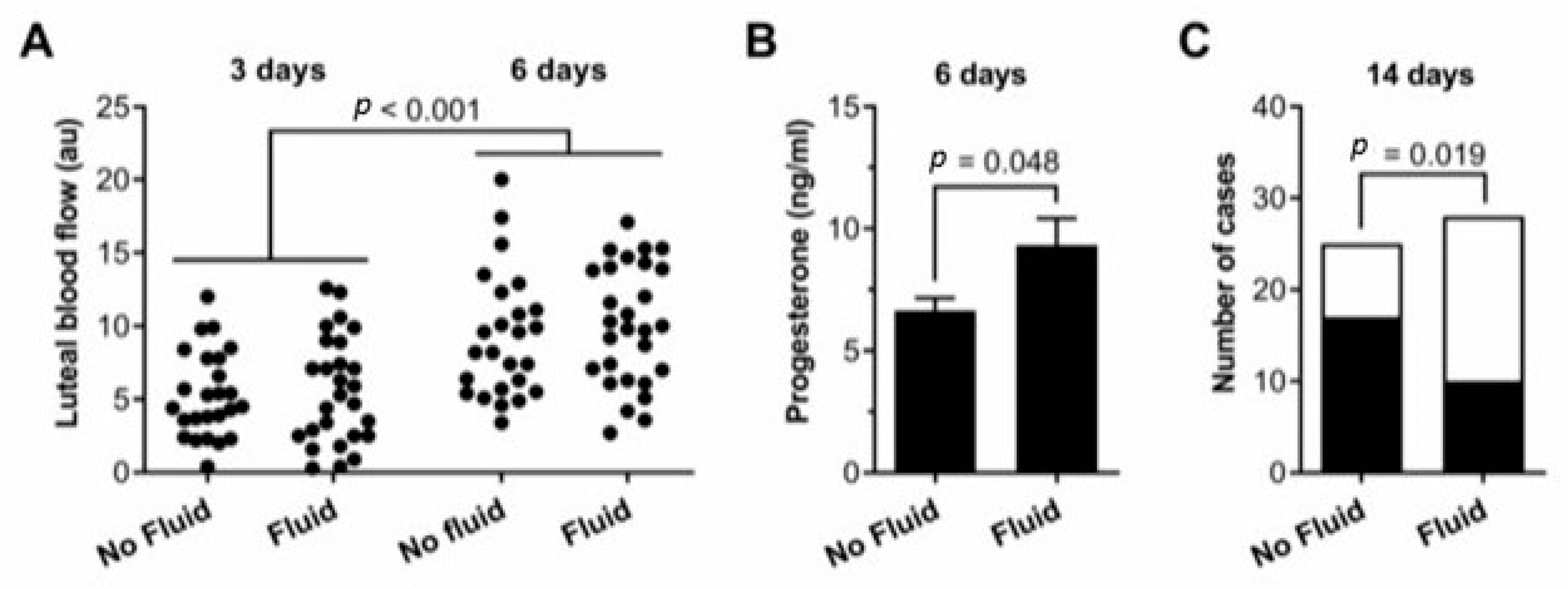
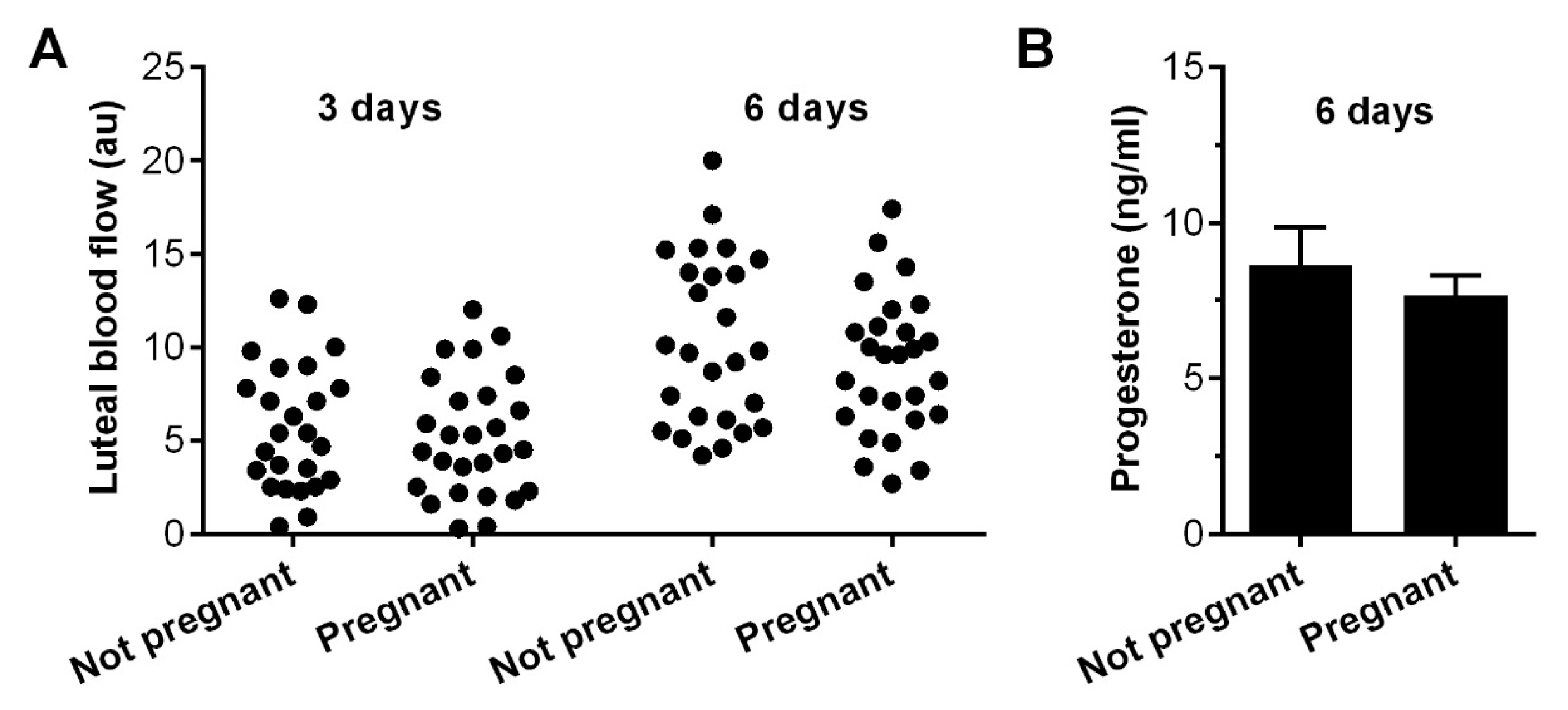
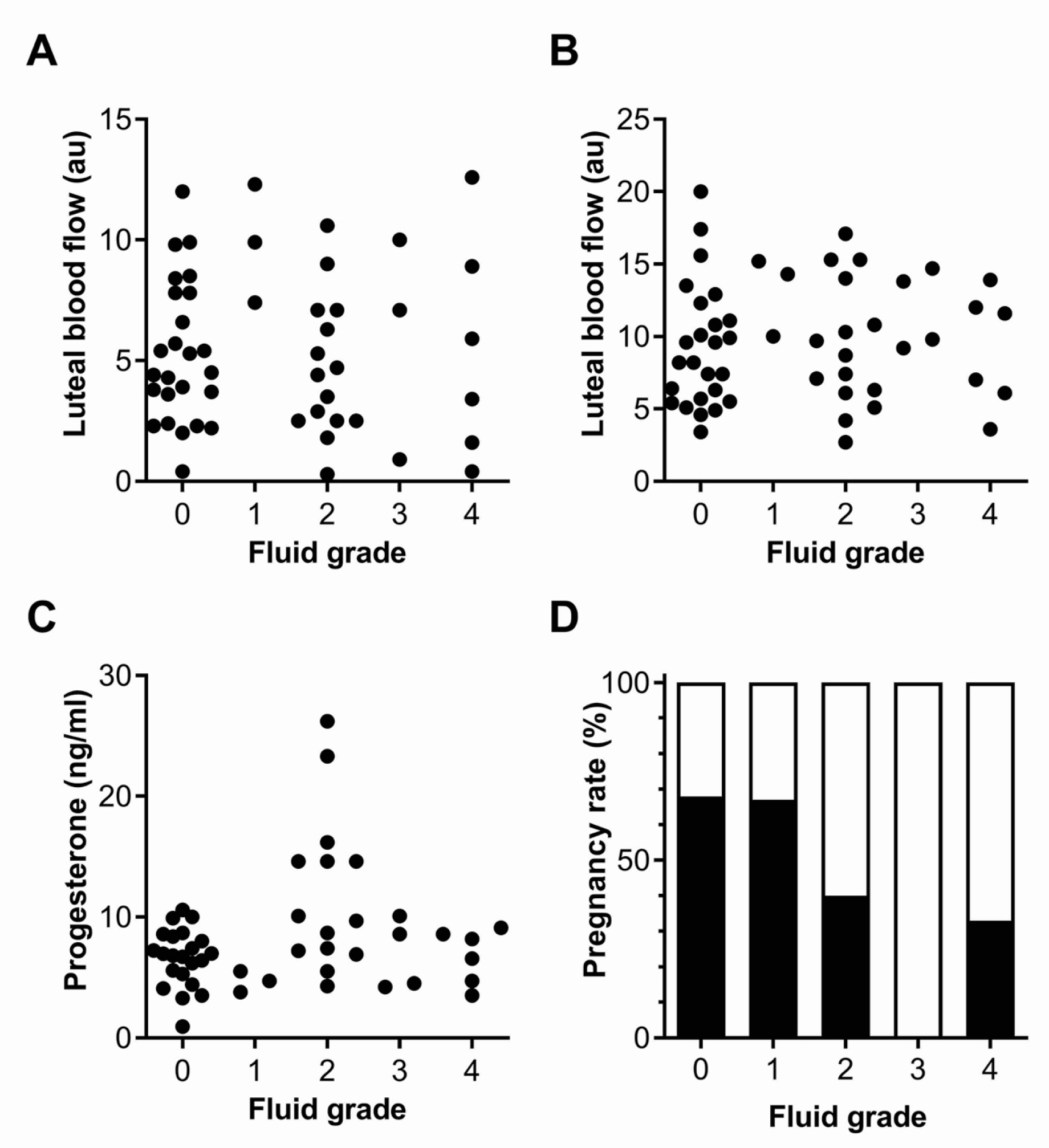
| Group (Mares n °) | LBF (a.u.) 3 d | LBF (a.u.) 6 d | P4 (ng/mL) 6 d |
|---|---|---|---|
| F (22) | 6.13 ± 4.58 * | 10.05 ± 4.08 * | 9.31 ± 5.71 ° |
| NF (18) | 5.30 ± 2.91 * | 9.25 ± 4.23 * | 6.64 ± 2.39 ° |
| All (40) | 5.74 ± 3.87 * | 9.67 ± 4.13 * | 8.11 ± 4.69 |
| Age (n) | Pregnant n (%) | Non-Pregnant n (%) |
|---|---|---|
| ≤10 (22) | 14 (53.8) | 12 (46.2) |
| 10–15 (11) | 8 (50) | 8 (50) |
| >15 (7) | 6 (54.5) | 5 (45.5) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Freccero, F.; Mislei, B.; Bucci, D.; Dondi, F.; Mari, G. Effects of Intra-Uterine Fluid Accumulation after Artificial Insemination on Luteal Function in Mares. Animals 2023, 13, 67. https://doi.org/10.3390/ani13010067
Freccero F, Mislei B, Bucci D, Dondi F, Mari G. Effects of Intra-Uterine Fluid Accumulation after Artificial Insemination on Luteal Function in Mares. Animals. 2023; 13(1):67. https://doi.org/10.3390/ani13010067
Chicago/Turabian StyleFreccero, Francesca, Beatrice Mislei, Diego Bucci, Francesco Dondi, and Gaetano Mari. 2023. "Effects of Intra-Uterine Fluid Accumulation after Artificial Insemination on Luteal Function in Mares" Animals 13, no. 1: 67. https://doi.org/10.3390/ani13010067
APA StyleFreccero, F., Mislei, B., Bucci, D., Dondi, F., & Mari, G. (2023). Effects of Intra-Uterine Fluid Accumulation after Artificial Insemination on Luteal Function in Mares. Animals, 13(1), 67. https://doi.org/10.3390/ani13010067






