Staphylococcus microti Strains Isolated from an Italian Mediterranean Buffalo Herd
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Statement
2.2. Collection of Samples and Somatic Cell Count
2.3. Bacterial Isolation and Identification
2.4. Sequencing of 16S rRNA Gene
2.5. Antimicrobial Susceptibility Testing of S. microti Isolates
2.6. Genotypic Characterization of Tetracycline Resistance
2.7. Data Analysis
3. Results
3.1. Somatic Cell Count (SCC) Results
3.2. S. microti and Somatic Cell Count (SCC) Correlation
3.3. Sequencing of 16S rRNA Gene
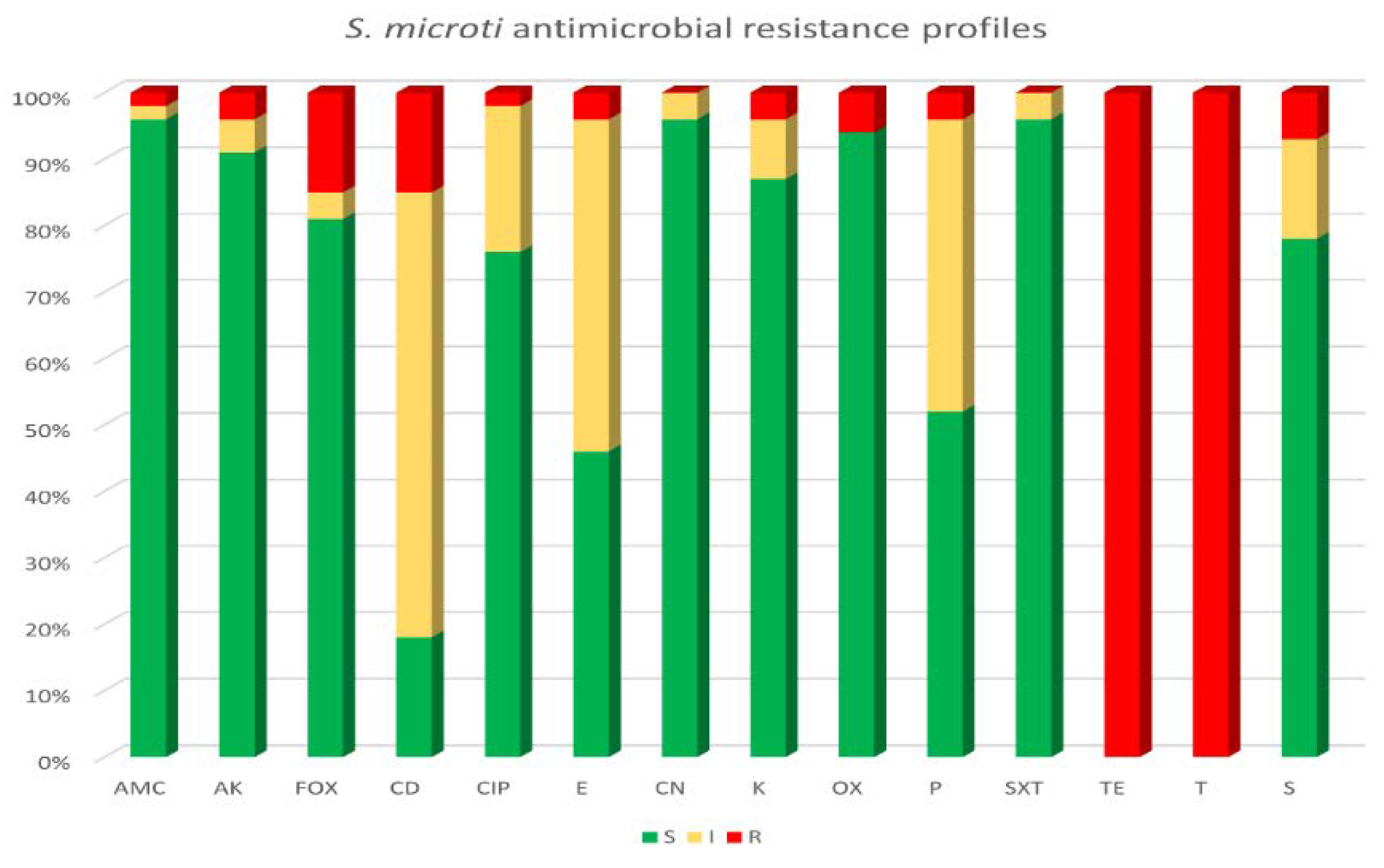
3.4. Antimicrobial Susceptibility Testing and Genotyping Characterization of Tetracycline Resistance in S. microti Strains
3.5. Analysis of the Major Constituents of Milk
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nagasawa, Y.; Kiku, Y.; Sugawara, K.; Yabusaki, T.; Oono, K.; Fujii, K.; Suzuki, T.; Maehana, K.; Hayashi, T. The bacterial load in milk is associated with clinical severity in cases of bovine coliform mastitis. J. Vet. Med. Sci. 2019, 81, 107–112. [Google Scholar] [CrossRef]
- Middleton, J.R.; Fox, L.K.; Pighetti, G. Laboratory Handbook on Bovine Mastitis (National Mastitis Council), 3rd ed.; National Mastitis Council: New Prague, MN, USA, 2017. [Google Scholar]
- Puggioni, G.M.G.; Tedde, V.; Uzzau, S.; Guccione, J.; Ciaramella, P.; Pollera, C.; Moroni, P.; Bronzo, V.; Addis, M.F. Evaluation of a bovine cathelicidin ELISA for detecting mastitis in the dairy buffalo: Comparison with milk somatic cell count and bacteriological culture. Res. Vet. Sci. 2020, 128, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Lange, C.C.; Brito, M.A.; Reis, D.R.; Machado, M.A.; Guimarães, A.S.; Azevedo, A.L.; Salles, B.; Alvim, M.C.; Silva, F.S.; Meurer, I.R. Species-level identification of staphylococci isolated from bovine mastitis in Brazil using partial 16S rRNA sequencing. Vet. Microbiol. 2015, 176, 382–388. [Google Scholar] [CrossRef] [PubMed]
- Moroni, P.; Rossi, C.S.; Pisoni, G.; Bronzo, V.; Castiglioni, B.; Boettcher, P.J. Relationships between Somatic Cell Count and Intramammary Infection in Buffaloes. J. Dairy Sci. 2006, 89, 998–1003. [Google Scholar] [CrossRef] [PubMed]
- Nováková, D.; Pantůček, R.; Hubálek, Z.; Falsen, E.; Busse, H.-J.; Schumann, P.; Sedláček, I. Staphylococcus microti sp. nov., isolated from the common vole (Microtus arvalis). Int. J. Syst. Evol. Microbiol. 2010, 60, 566–573. [Google Scholar] [CrossRef] [PubMed]
- Hauschild, T.; Śliżewski, P.; Masiewicz, P. Species distribution of staphylococci from small wild mammals. Syst. Appl. Microbiol. 2010, 33, 457–460. [Google Scholar] [CrossRef] [PubMed]
- Riesen, A.; Perreten, V. Staphylococcus rostri sp. nov., a haemolytic bacterium isolated from the noses of healthy pigs. Int. J. Syst. Evol. Microbiol. 2010, 60, 2042–2047. [Google Scholar] [CrossRef][Green Version]
- Król, J.; Wanecka, A.; Twardoń, J.; Mrowiec, J.; Dropińska, A.; Bania, J.; Podkowik, M.; Korzeniowska-Kowal, A.; Paściak, M. Isolation of Staphylococcus microti from milk of dairy cows with mastitis. Vet. Microbiol. 2016, 182, 163–169. [Google Scholar] [CrossRef]
- Hu, X.; Shang, Y.; Guo, J.; Zhang, H.; Liang, Y.; Sun, J.; Yue, F. Draft genome sequence of Staphylococcus microti DSM 22147, isolated from the common vole. Genome Announc. 2018, 6, e00420-18. [Google Scholar] [CrossRef]
- Addis, M.F.; Maffioli, E.M.; Penati, M.; Albertini, M.; Bronzo, V.; Piccinini, R.; Tangorra, F.; Tedeschi, G.; Cappelli, G.; Di Vuolo, G.; et al. Peptidomic changes in the milk of water buffaloes (Bubalus bubalis) with intramammary infection by non-aureus staphylococci. Sci. Rep. 2022, 12, 8371. [Google Scholar] [CrossRef]
- Bannoehr, J.; Ben Zakour, N.L.; Waller, A.S.; Guardabassi, L.; Thoday, K.L.; van den Broek, A.H.M.; Fitzgerald, J.R. Population genetic structure of the Staphylococcus intermedius group: Insights into agr diversification and the emergence of methicillin-resistant strains. J. Bacteriol. 2007, 189, 8685–8692. [Google Scholar] [CrossRef] [PubMed]
- Hongoh, Y.; Ohkuma, M.; Kudo, T. Molecular analysis of bacterial microbiota in the gut of the termite Reticulitermes speratus (Isoptera; Rhinotermitidae). FEMS Microbiol. Ecol. 2003, 44, 231–242. [Google Scholar] [CrossRef] [PubMed]
- McAllister, S.M.; Davis, R.E.; McBeth, J.M.; Tebo, B.M.; Emerson, D.; Moyer, C.L. Biodiversity and emerging biogeography of the neutrophilic iron-oxidizing Zetaproteobacteria. Appl. Environ. Microbiol. 2011, 77, 5445–5457. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Disk and Diluition Susceptibility Tests for Bacteria Isolated From Animals. VET01S; Clinical and Laboratory Standards Institute: Wayne, IL, USA, 2015. [Google Scholar]
- European Committee on Antimicrobial Susceptibility Testing. Breakpoint Tables for Interpretation of MICs and Zone Diameters; European Committee on Antimicrobial Susceptibility Testing: Växjö, Sweden, 2018; Available online: http://www.eucast.org. (accessed on 20 March 2018).
- Magiorakos, A.-P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef]
- Schwarz, S.; Chaslus-Dancla, E. Use of antimicrobials in veterinary medicine and mechanisms of resistance. Vet. Res. 2001, 32, 201–225. [Google Scholar] [CrossRef] [PubMed]
- Qu, Y.; Zhao, H.; Nobrega, D.B.; Cobo, E.R.; Han, B.; Zhao, Z.; Li, S.; Li, M.; Barkema, H.W.; Gao, J. Molecular epidemiology and distribution of antimicrobial resistance genes of Staphylococcus species isolated from Chinese dairy cows with clinical mastitis. J. Dairy Sci. 2019, 102, 1571–1583. [Google Scholar] [CrossRef] [PubMed]
- Ullah, F.; Malik, S.A.; Ahmed, J.; Ullah, F.; Shah, S.M.; Ayaz, M.; Hussain, S.; Khatoon, L. Investigation of the genetic basis of tetracycline resistance in Staphylococcus aureus from Pakistan. Trop. J. Pharm. Res. 2012, 11, 925–931. [Google Scholar] [CrossRef][Green Version]
- Dhakal, I.P.; Neupane, M.; Nagahata, H. Evaluation of direct and indirect measures of quarter milk from crossbred buffaloes. Anim. Sci. J. 2008, 79, 628–633. [Google Scholar] [CrossRef]
- Vásquez-Garcia, A.; dos Santos Silva, T.; de Alemida-Queiroz, S.R.; Godoy, S.H.S.; Fernandes, A.M.; Sousa, R.L.M.; Franzolin, R. Species identification and antimicrobial susceptibility profile of bacteria causing subclinical mastitis in buffalo. Pesq. Vet. Bras. 2017, 37, 447–452. [Google Scholar] [CrossRef]
- Pizauro, L.J.L.; Silva, D.G.; Santana, A.M.; Clemente, V.; Lara, G.H.B.; Listoni, F.J.P.; Vaz, A.C.N.; Vidal-Martins, A.M.C.; Ribeiro, M.G.; Fagliari, J.J. Prevalence and etiology of buffalo mastitis and milk somatic cell count in dry and rainy seasons in a buffalo herd from Analândia, São Paulo State, Brazil. Arq. Bras. Med. Vet. Zootec. 2014, 66, 1703–1710. [Google Scholar] [CrossRef]
- Condas, L.A.Z.; De Buck, J.; Nobrega, D.B.; Carson, D.A.; Naushad, S.; De Vliegher, S.; Zadoks, R.N.; Middleton, J.R.; Dufour, S.; Kastelic, J.P.; et al. Prevalence of non-aureus staphylococci species causing intramammary infections in Canadian dairy herds. J. Dairy Sci. 2017, 100, 5592–5612. [Google Scholar] [CrossRef]
- Thorberg, B.-M.; Danielsson-Tham, M.-L.; Emanuelson, U.; Persson Waller, K. Bovine subclinical mastitis caused by different types of coagulase-negative staphylococci. J. Dairy Sci. 2009, 92, 4962–4970. [Google Scholar] [CrossRef] [PubMed]
- Supré, K.; Haesebrouck, F.; Zadoks, R.N.; Vaneechoutte, M.; Piepers, S.; De Vliegher, S. Some coagulase-negative Staphylococcus species affect udder health more than others. J. Dairy Sci. 2011, 94, 2329–2340. [Google Scholar] [CrossRef]
- Hosseinzadeh, S.; Saei, H.D. Staphylococcal species associated with bovine mastitis in the North West of Iran: Emerging of coagulase-negative staphylococci. Int. J. Vet. Sci. Med. 2014, 2, 27–34. [Google Scholar] [CrossRef]
- Srinivasan, P.; Jagadeswaran, D.; Manoharan, R.; Giri, T.; Balasubramaniam, G.A.; Balachandran, P. Prevalence and etiology of subclinical mastitis among buffaloes (Bubalus bubalus) in Namakkal, India. Pak. J. Biol. Sci. 2013, 16, 1776–1780. [Google Scholar] [CrossRef] [PubMed]
- Hoque, M.N.; Talukder, A.K.; Saha, O.; Hasan, M.M.; Sultana, M.; Rahman, A.A.; Das, Z.C. Antibiogram and virulence profiling reveals multidrug resistant Staphylococcus aureus as the predominant aetiology of subclinical mastitis in riverine buffaloes. Vet. Med. Sci. 2022, 8, 2631–2645. [Google Scholar] [CrossRef] [PubMed]
- Krishnamoorthy, P.; Goudar, A.L.; Suresh, K.P.; Roy, P. Global and countrywide prevalence of subclinical and clinical mastitis in dairy cattle and buffaloes by systematic review and meta-analysis. Res. Vet. Sci. 2021, 136, 561–586. [Google Scholar] [CrossRef] [PubMed]
- Koivula, M.; Pitkälä, A.; Pyörälä, S.; Mäntysaari, E.A. Distribution of bacteria and seasonal and regional effects in a new database for mastitis pathogens in Finland. Acta Agric. Scand. Sect. A Anim. Sci. 2007, 57, 89–96. [Google Scholar] [CrossRef]
- Bradley, A.J.; Leach, K.A.; Breen, J.E.; Green, L.E.; Green, M.J. Survey of the incidence and aetiology of mastitis on dairy farms in England and Wales. Vet. Rec. 2007, 160, 253–258. [Google Scholar] [CrossRef]
- Joshi, S.; Gokhale, S. Status of mastitis as an emerging disease in improved and periurban dairy farms in India. Ann. N. Y. Acad. Sci. 2006, 1081, 74–83. [Google Scholar] [CrossRef]
- Singha, S.; Ericsson, C.D.; Chowdhury, S.; Nath, S.C.; Paul, O.B.; Hoque, M.A.; Boqvist, S.; Persson, Y.; Rahman, M.M. Occurrence and aetiology of subclinical mastitis in water buffalo in Bangladesh. J. Dairy Res. 2021, 88, 314–320. [Google Scholar] [CrossRef] [PubMed]
- Catozzi, C.; Sanchez Bonastre, A.; Francino, O.; Lecchi, C.; De Carlo, E.; Vecchio, D.; Martucciello, A.; Fraulo, P.; Bronzo, V.; Cuscó, A.; et al. The microbiota of water buffalo milk during mastitis. PLoS ONE 2017, 12, e0184710. [Google Scholar] [CrossRef] [PubMed]
- Hamel, J.; Zhang, Y.; Wente, N.; Krömker, V. Non-S. aureus staphylococci (NAS) in milk samples: Infection or contamination? Vet. Microbiol. 2020, 242, 108594. [Google Scholar] [CrossRef] [PubMed]
- Rajendhran, J.; Gunasekaran, P. Microbial phylogeny and diversity: Small subunit ribosomal RNA sequence analysis and beyond. Microbiol. Res. 2011, 166, 99–110. [Google Scholar] [CrossRef]
- Yutin, N.; Puigbò, P.; Koonin, E.V.; Wolf, Y.I. Phylogenomics of prokaryotic ribosomal proteins. PLoS ONE 2012, 7, e36972. [Google Scholar] [CrossRef]
- Cameron, M.; Barkema, H.W.; De Buck, J.; De Vliegher, S.; Chaffer, M.; Lewis, J.; Keefe, G.P. Identification of bovine-associated coagulase-negative staphylococci by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry using a direct transfer protocol. J. Dairy Sci. 2017, 100, 2137–2147. [Google Scholar] [CrossRef] [PubMed]
- Ruegg, P.L.; Oliveira, L.; Jin, W.; Okwumabua, O. Phenotypic antimicrobial susceptibility and occurrence of selected resistance genes in gram-positive mastitis pathogens isolated from Wisconsin dairy cows. J. Dairy Sci. 2015, 98, 4521–4534. [Google Scholar] [CrossRef] [PubMed]
- Mbindyo, C.M.; Gitao, G.C.; Plummer, P.J.; Kulohoma, B.W.; Mulei, C.M.; Bett, R. Antimicrobial Resistance Profiles and Genes of Staphylococci Isolated from Mastitic Cow’s Milk in Kenya. Antibiotics 2021, 10, 772. [Google Scholar] [CrossRef] [PubMed]
- Guzmán-Rodríguez, J.J.; León-Galván, M.F.; Barboza-Corona, J.E.; Valencia-Posadas, M.; Loeza-Lara, P.D.; Sánchez-Ceja, M.; Ochoa-Zarzosa, A.; López-Meza, J.E.; Gutiérrez-Chávez, A.J. Analysis of virulence traits of Staphylococcus aureus isolated from bovine mastitis in semi-intensive and family dairy farms. J. Vet. Sci. 2020, 21, e77. [Google Scholar] [CrossRef]
- Erdem, H.; Okuyucu, İ.C. Influence of hygiene status of cows on somatic cell count and milk components during summer season. Large Anim. Rev. 2019, 25, 7–10. [Google Scholar]
| Primer | Sequence | Reference |
|---|---|---|
| Smi16-11F | GGCGGCGTGCCTAATACATG | This study |
| Smi16-750F | GTGGGGATCAAACAGGAT | This study |
| Smi16F | CCTCTTCGGAGGACAAAGTGA | [12] |
| Smi16Fdown | GAATACGTTCCCGGGTCTTG | This study |
| Smi16-337R | CTGCTGCCTCCCGTAGG | This study |
| Smi16Rup | ATCCTGTTTGATCCCCAC | This study |
| Smi16-1027R | TCACTTTGTCCTCCGAAGAGG | This study |
| Smi16R | GACCCGGGAACGTATTCACC | [12] |
| Smi16-1527R | TAGAAAGGAGGTGATCCAGC | This study |
| B27F | AGAGTTTGATCMTGGCTCAG | [13] |
| B1492R | TACCTTGTTACGACTT | [14] |
| Antibiotics | Disk Content | Antibiotic Class | Reference for Breakpoints |
|---|---|---|---|
| Amoxicillin–clavulanate (AMC) | 20/10 µg | Penicillins | [15] |
| Penicillin (P) | 10 IU | ||
| Oxacillin (OX) | 1 µg | ||
| Amikacin (AK) | 30 µg | Aminoglycosides | [15] |
| Kanamycin (K) | 30 µg | ||
| Gentamicin (CN) | 10 µg | ||
| Streptomycin (S) | 10 µg | ||
| Cefoxitin (FOX) | 30 µg | Cephalosporins | [16] |
| Ciprofloxacin (CIP) | 5 µg | Quinolones | [15] |
| Clindamycin (DA) | 2 µg | Lincosamides | [15] |
| Erythromycin (E) | 15 µg | Macrolides | [15] |
| Tetracycline (TE) | 30 µg | Tetracyclines | [15] |
| Oxytetracycline (T) | 30 µg | ||
| Sulfamethoxazole–trimethoprim (SXT) | 25 µg | Sulfonamides | [16] |
| Gene | Primer Sequences (5’-3’ Sense and Antisense) | Amplicon Size (bp) | Amplifications Program |
|---|---|---|---|
| tetM | F: AGTTTTAGCTCATGTTGATG R: TCCGACTATTTAGACGACGG | 1862 | 94 °C 15 s; 94 °C 1 min, 52 °C 1 min, 72 °C 90 s, for 30 cycles; 72 °C 5 min |
| tetK | F: GTAGCGACAATAGGTAATAGT R: GTAGTGACAATAAACCTCCTA | 360 |
| Bacterial Culture | SCC Values (cell/mL) | Status * | No. of Samples/ 200 Samples | % | Fisher’s Two-Tailed |
|---|---|---|---|---|---|
| No bacterial growth | SCC ≤ 200.000 | H | 54 | 27.0% | p < 0.05 |
| SCC > 200.000 | SCM | 0 | 0% | ||
| Bacterial growth | SCC ≤ 200.000 | IMI | 124 | 62% | p < 0.05 |
| SCC > 200.000 | SCM | 22 | 11% |
| Sample | SCC/mL | Identified Bacterial Strains (Colony Forming Unit, CFU) | MALDI-TOF Score |
|---|---|---|---|
| 1 | 2 × 105 | Aeromonas hydrophila (3000 CFU/mL) | 2.11 |
| 2 | 2.9 × 105 | Acinetobacter johnsonii (5000 CFU/mL) Staphylococcus simulans (1000 CFU/mL) | 2.26 2.20 |
| 3 | 2.5 × 105 | Staphylococcus microti (10,000 CFU/mL) Citrobacter freundii (10,000 CFU/mL) | 2.07 1.96 |
| 4 | 2.8 × 105 | Escherichia coli (5000 CFU/mL) Staphylococcus simulans (1000 CFU/mL) Staphylococcus sciuri (500 CFU/mL) | 2.09 2.20 2.09 |
| 5 | 2 × 106 | Streptococcus agalactiae (6000 CFU/mL) Lactococcus lactis (1100 CFU/mL) Aeromonas hydrophila (5000 CFU/mL) | 2.10 2.17 1.88 |
| 6 | 2.7 × 105 | Aeromonas hydrophila (3000 CFU/mL) | 2.12 |
| 7 | 5.3 × 105 | Streptococcus agalactiae (10,000 CFU/mL) Corynebacterium xerosis (1000 CFU/mL) | 2.27 2.04 |
| 8 | 3.6 × 105 | Staphylococcus microti (3000 CFU/mL) Aerococcus viridans (2000 CFU/mL) Rothia endophytica (200 CFU/mL) | 2.04 1.79 1.91 |
| 9 | 2.9 × 105 | Aerococcus viridans (2000 CFU/mL) | 1.81 |
| 10 | 2.7 × 105 | Staphylococcus microti (4000 CFU/mL) | 2.04 |
| 11 | 2.3 × 105 | Escherichia coli (4000 CFU/mL) | 2.16 |
| 12 | 2.9 × 105 | Pseudomonas aeruginosa (4000 CFU/mL) | 2.34 |
| 13 | 2.2 × 105 | Streptococcus agalactiae (3000 CFU/mL) | 2.43 |
| 14 | 2.5 × 105 | Rothia amarae (6000 CFU/mL) | 2.05 |
| 15 | 5.1 × 105 | Escherichia coli (2800 CFU/mL) Rothia amarae (500 CFU/mL) Aerococcus viridans (700 CFU/mL) | 2.22 1.94 1.82 |
| 16 | 2.9 × 105 | Escherichia coli (400 CFU/mL) Streptococcus agalactiae (5400 CFU/mL) Aerococcus viridans (200 CFU/mL) | 2.14 2.29 1.99 |
| 17 | 1.5 × 106 | Escherichia coli (2000 CFU/mL) Aerococcus viridans (1100 CFU/mL) | 2.17 2.14 |
| 18 | 8.3 × 105 | Streptococcus agalactiae (5000 CFU/mL) | 2.19 |
| 19 | 2.1 × 105 | Staphylococcus microti (200 CFU/mL) Aerococcus viridans (100 CFU/mL) Escherichia coli (500 CFU/mL) | 2.06 1.89 2.31 |
| 20 | 4.5 × 105 | Aerococcus viridans (400 CFU/mL) | 1.90 |
| 21 | 8.8 × 105 | Streptococcus agalactiae (4700 CFU/mL) | 2.04 |
| 22 | 2.3 × 105 | Aerococcus viridans (4800 CFU/mL) | 1.99 |
| Penicillins | Aminoglycosides | Cephalosporins | Quinolones | Lincosamides | Macrolides | Tetracyclines | Sulfonamides | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S. microti Strains | AMC | P | OX | AK | K | CN | S | FOX | CIP | DA | E | TE | T | SXT |
| 7 | R | R | R | R | ||||||||||
| 11 | R | R | R | R | R | |||||||||
| 12 | R | R | R | R | ||||||||||
| 13 | R | R | R | R | R | |||||||||
| 14 | R | R | R | R | ||||||||||
| 15 | R | R | R | R | ||||||||||
| 16 | R | R | R | R | ||||||||||
| 17 | R | R | R | R | ||||||||||
| 19 | R | R | R | R | ||||||||||
| 20 | R | R | R | R | ||||||||||
| 22 | R | R | R | R | R | |||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ambrosio, M.; Nocera, F.P.; Garofalo, F.; De Luca, P.; Grinberg, A.; De Martino, L. Staphylococcus microti Strains Isolated from an Italian Mediterranean Buffalo Herd. Animals 2023, 13, 182. https://doi.org/10.3390/ani13010182
Ambrosio M, Nocera FP, Garofalo F, De Luca P, Grinberg A, De Martino L. Staphylococcus microti Strains Isolated from an Italian Mediterranean Buffalo Herd. Animals. 2023; 13(1):182. https://doi.org/10.3390/ani13010182
Chicago/Turabian StyleAmbrosio, Monica, Francesca Paola Nocera, Francesca Garofalo, Pasquale De Luca, Alex Grinberg, and Luisa De Martino. 2023. "Staphylococcus microti Strains Isolated from an Italian Mediterranean Buffalo Herd" Animals 13, no. 1: 182. https://doi.org/10.3390/ani13010182
APA StyleAmbrosio, M., Nocera, F. P., Garofalo, F., De Luca, P., Grinberg, A., & De Martino, L. (2023). Staphylococcus microti Strains Isolated from an Italian Mediterranean Buffalo Herd. Animals, 13(1), 182. https://doi.org/10.3390/ani13010182









