The Spritztube: A New Device for the Extraglottic Intubation of Rabbits
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
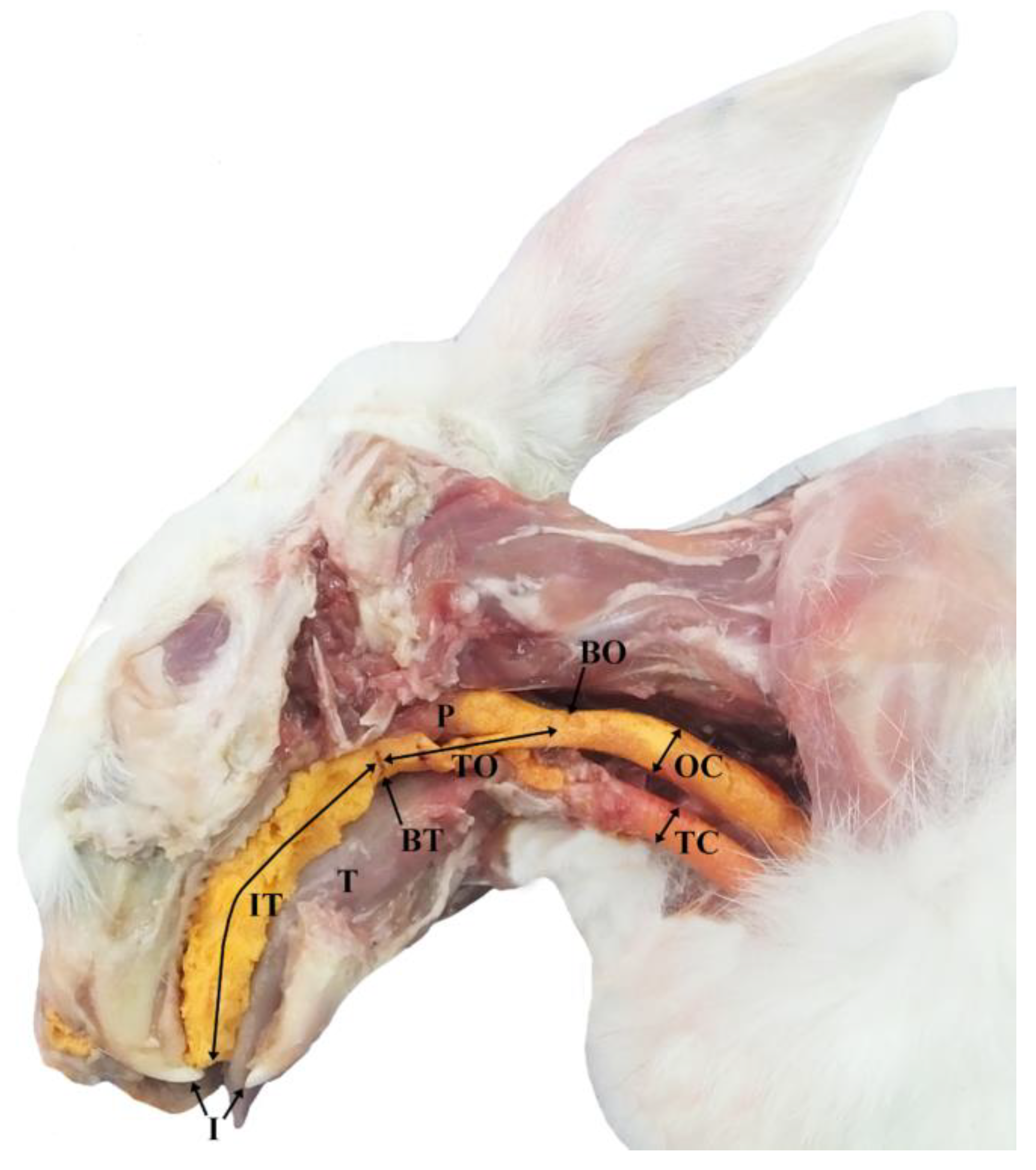
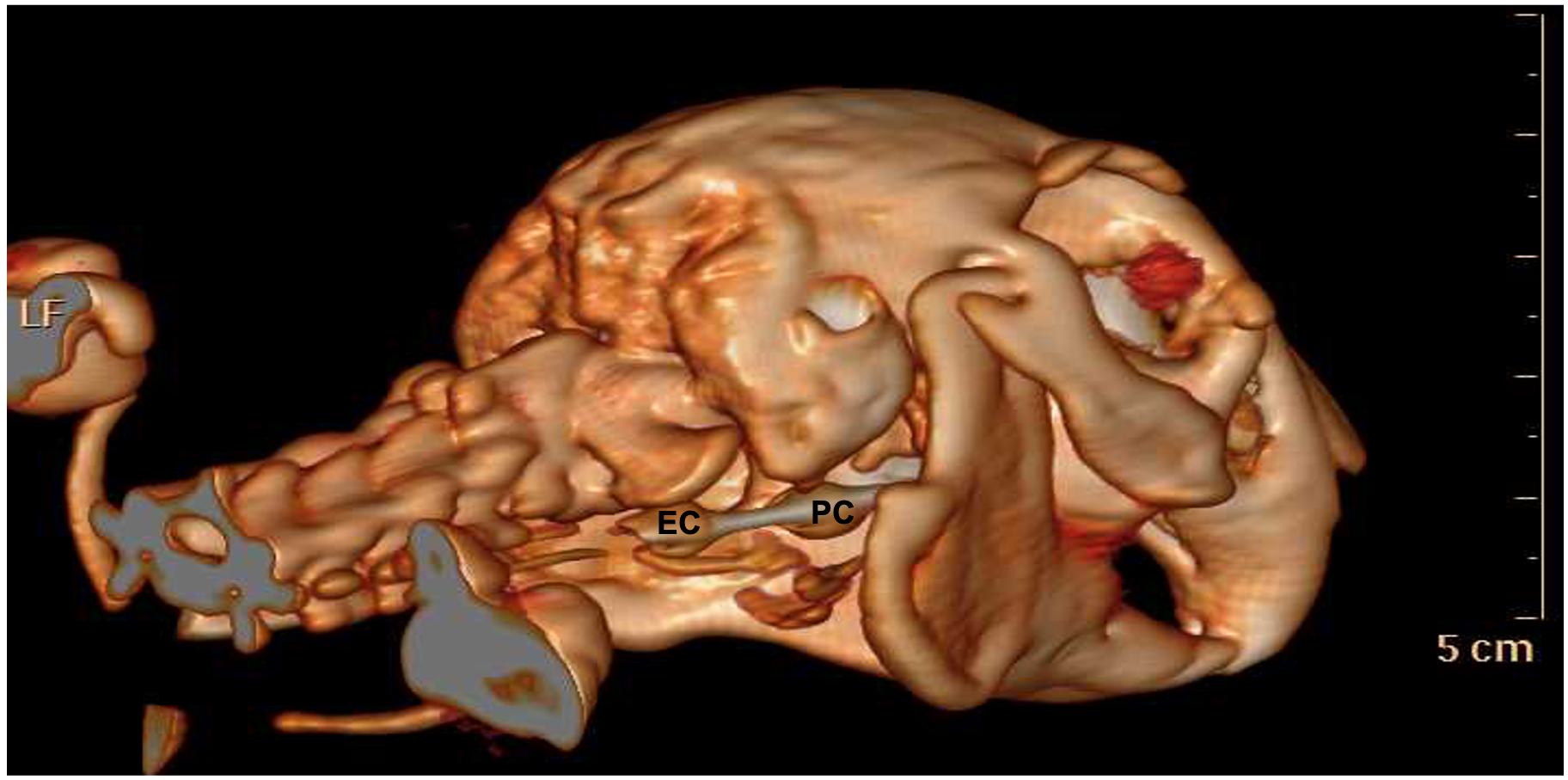
2.1. Phase I
2.2. Phase II
Anaesthetic Protocol
2.3. Statistical Analysis
3. Results
3.1. Phase I
3.2. Phase II
4. Discussion
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brodbelt, D.C.; Blissitt, K.J.; Hammond, R.A.; Neath, P.J.; Young, L.E.; Pfeiffer, D.U.; Wood, J.L. The risk of death: The confidential enquiry into perioperative small animal fatalities. Vet. Anaesth. Analg. 2008, 35, 365–373. [Google Scholar] [CrossRef] [PubMed]
- Nowland, M.H.; Brammer, D.W.; Garcia, A.; Rush, H.G. Biology and Diseases of Rabbits. In Laboratory Animal Medicine, 3rd ed.; Anferson, L., Otto, G., Pritchett-Corning, K., Whary, M., Eds.; Academic Press: Amsterdam, The Netherlands, 2015; pp. 411–461. [Google Scholar]
- Phaneuf, L.R.; Barker, S.; Groleau, M.A.; Turner, P.V. Tracheal injury after endotracheal intubation and anesthesia in rabbits. J. Am. Assoc. Lab Anim. Sci. 2006, 45, 67–72. [Google Scholar] [PubMed]
- Imai, A.; Eisele, P.H.; Steffey, E.P. A new airway device for small laboratory animals. Lab. Anim. 2005, 39, 111–115. [Google Scholar] [CrossRef] [PubMed]
- Uzun, M.; Kiraz, H.A.; Ovali, M.A.; Sahin, H.; Erbas, M.; Toman, H. The investigation of airway management capacity of v-gel and cobra-PLA in anaesthetised rabbits. Acta Cir. Bras. 2015, 30, 80–86. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Engbers, S.; Larkin, A.; Rousset, N.; Prebble, M.; Jonnalagadda, M.; Knight, C.G.; Pang, D. Comparison of a Supraglottic Airway Device (v-gel®) with Blind Orotracheal Intubation in Rabbits. Front. Vet. Sci. 2017, 4, 49. [Google Scholar] [CrossRef] [PubMed]
- Kazakos, G.M.; Anagnostou, T.; Savvas, I.; Raptopoulos, D.; Psalla, D.; Kazakou, I.M. Use of the laryngeal mask airway in rabbits: Placement and efficacy. Lab Anim. 2007, 36, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.C.; Robertson, L.D.; Auhll, A.; March, T.J.; Derring, C.; Bolon, B. Endotracheal tubes versus laryngeal mask airways in rabbit inhalation anesthesia: Ease of use and waste gas emissions. Contemp. Top Lab. Anim. Sci. 2004, 43, 22–25. [Google Scholar] [PubMed]
- Bateman, L.; Ludders, J.W.; Gleed, R.D.; Erb, H.N. Comparison between facemask and laryngeal mask airway in rabbits during isoflurane anesthesia. Vet. Anaesth. Analg. 2005, 32, 280–288. [Google Scholar] [CrossRef] [PubMed]
- Crotaz, I.R. Initial feasibility investigation of the v-gel airway: An anatomically designed supraglottic airway device for use in companion animal veterinary anaesthesia. Vet. Anaesth. Analg. 2010, 37, 579–580. [Google Scholar] [CrossRef] [PubMed]
- Fusco, A.; Douglas, H.; Barba, A.; Hopster, K.; Stefanovski, D.; Sinder, B.; Cahill, P.J.; Snyder, B.; Schaer, T.P. V-Gel® Guided Endotracheal Intubation in Rabbits. Front. Vet. Sci. 2021, 8, 684624. [Google Scholar] [CrossRef] [PubMed]
- De Rosa, S.; Ferrari, F.; Carboni Checcacci, S.; Rigobello, A.; Gennaro, P.; De Luca, D.; Primadei, M.; Politi, F.; Pellizzari, A.; Bonato, R. Airway management with Fastrach laryngeal mask versus Spritztube: A prospective randomized manikin-based study. Minerva Anestesiol. 2018, 84, 455–462. [Google Scholar] [CrossRef] [PubMed]
- De Rosa, S.; Messina, A.; Sorbello, M.; Rigobello, A.; Colombo, D.; Piccolo, A.; Bonaldi, E.; Gennaro, P.; Urukalo, V.; Pellizzari, A.; et al. Laryngeal Mask Airway Supreme vs. the Spritztube tracheal cannula in anaesthetised adult patients: A randomised controlled trial. Eur. J. Anaesthesiol. 2019, 36, 955–962. [Google Scholar] [CrossRef] [PubMed]
- Agrò, F.E.; Pascarella, G. The Spritztube: A new revolution in extraglottic airway devices. Minerva Anestesiol. 2018, 84, 426–428. [Google Scholar] [CrossRef] [PubMed]
- De Sordi, N.; Bombardi, C.; Chiocchetti, R.; Clavenzani, P.; Trerè, C.; Canova, M.; Grandis, A. A new method of producing casts for anatomical studies. Anat. Sci. Int. 2014, 89, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, M.G.; Pascoe, P.J. Anesthesia, analgesia, and sedation of Small Mammals. In Ferrets, Rabbits and Rodents Clinical Medicine and Surgery, 4th ed.; Quesenberry, K.E., Orcutt, C.J., Mans, C., Carpenter, J.W., Eds.; Elsevier Saunders: St Louis, MO, USA, 2020; pp. 536–558. [Google Scholar]
- Falcão, S.C.; Pereira Junior, J.R.; Coelho, A.R. Technique of blind tracheal intubation in rabbits (Oryctolagus cuniculi) supported by previous maneuver of esophageal cannulization. Acta Cir. Bras. 2011, 26, 352–356. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Johnson, D.H. Endoscopic intubation of exotic companion mammals. Vet. Clin. N. Am. Exot. Anim. Pract. 2010, 13, 273–289. [Google Scholar] [CrossRef] [PubMed]
- Miranda, A.; Pego, J.M.; Correia-Pinto, J. Animal facility videoendoscopic intubation station: Tips and tricks from mice to rabbits. Lab Anim. 2016, 51, 204–207. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lambertini, C.; Grandis, A.; De Silva, M.; Cassano, I.A.; Checcacci Carboni, S.; Romagnoli, N. The Spritztube: A New Device for the Extraglottic Intubation of Rabbits. Animals 2023, 13, 156. https://doi.org/10.3390/ani13010156
Lambertini C, Grandis A, De Silva M, Cassano IA, Checcacci Carboni S, Romagnoli N. The Spritztube: A New Device for the Extraglottic Intubation of Rabbits. Animals. 2023; 13(1):156. https://doi.org/10.3390/ani13010156
Chicago/Turabian StyleLambertini, Carlotta, Annamaria Grandis, Margherita De Silva, Ilaria Anna Cassano, Stefano Checcacci Carboni, and Noemi Romagnoli. 2023. "The Spritztube: A New Device for the Extraglottic Intubation of Rabbits" Animals 13, no. 1: 156. https://doi.org/10.3390/ani13010156
APA StyleLambertini, C., Grandis, A., De Silva, M., Cassano, I. A., Checcacci Carboni, S., & Romagnoli, N. (2023). The Spritztube: A New Device for the Extraglottic Intubation of Rabbits. Animals, 13(1), 156. https://doi.org/10.3390/ani13010156




