Simple Summary
The multiple impacts of polycylic aromatic hydrocarbon on the aquatic invertebrates were rarely assessed in a chronic way and multiple-species experiments, despite the clear advantage of better mimicking natural conditions compared to traditional acute and single-species-focused toxicological experiments. The application of such an approach is essential to lower the health risks for populations that regularly consume seafood. The data presented herein supported the use of Mytilus galloprovincyalis and Ruditapes decussatus as bioindicators of phenanthrene in water and/or sediment and proved the efficacy of the biomarkers’ assessment and molecular modelling in determining environmental thresholds and policies for governments.
Abstract
The aim of the current study was to assess the multifaceted effects of the polycylic aromatic hydrocarbon phenanthrene, mainly used in the colouring, explosive, and pharmaceutical industries, on the physiology of two bivalve species with economic value as seafood, namely, the Mediterranean mussel Mytilus galloprovincyalis and the European clam Ruditapes decussatus. The current study assessed how the phenanthrene affected several biomarkers and biometric endpoints in both bivalves, based on an in vivo experiment in silico approach. The bivalves were exposed during four time slots (i.e., 7, 15, 21, and 28 days) to two concentrations of phenanthrene in water (50 µg/L and 100 µg/L). For the clam R. decussatus, an additional contamination of sediment was applied due their typical benthic lifestyle (50 µg/kg and 100 µg/kg). The phenanthrene significantly reduced the ability of bivalves to tolerate desiccation and their Median Lethal Time, and also inhibited the activity of the enzyme acetylcholinesterase in a time-dependent manner. The activity of catalase indicated that bivalves also experienced oxidative stress during the first 21 days of the experiment. The significant decline in catalase activity observed during the last week of the experiment for the mussel M. galloprovincyalis supported a depletion of enzymes caused by the phenanthrene. The phenanthrene has also toxicokinetic and toxicodynamic properties, as assessed by the in silico approach. Overall, the results obtained suggest that the bivalves Ruditapes decussatus and M. galloprovincyalis can be used as a sentinel species in monitoring studies to assess the environmental impact of phenanthene in marine ecosystems. The significance of our findings is based on the fact that in ecotoxicology, little is known about the chronic effects, the simultaneous use of multiple species as bioindicators, and the interactions molecular modelling.
1. Introduction
Marine ecosystems are increasingly polluted by a wide variety of substances with deleterious effects for fauna, including humans [1]. Polycyclic Aromatic Hydrocarbons (hereafter PAHs) are considered among the most toxic marine organic pollutants [2]. More than half of the PAHs that reach the marine habitats have their origins in human activities, such as civil combustion and industrial sources, maritime traffic, and petroleum accidents (6%) [3], as well as from the discharge of oil hydrocarbons in coastal waters [3]. According to the American list of priority pollutants [4], phenanthrene (three cycles), widely used in the colouring, explosive, and pharmaceutical industries [5], is of high concern given its high concentrations and persistence in marine habitats and bioaccumulation potential in marine fauna, increasing its toxicity. Phenanthrene is partially degradable in aquatic habitats; in just four weeks, up to 54% of the initial phenanthrene can be naturally degraded (OECDE301C method) [6], and its half-lives range from 64 to 800 days [7]. The degradation of phenanthrene appears to be slower in marine habitats compared to fresh waters because of its higher resistance to native bacteria [8]. Therefore, phenanthrene is considered a stable compound, with a high rate of bioaccumulation in consumers, and an affinity for substrates given its increased octanol/water partition coefficient (log Kow = 4.53) [9,10]. This PAH was proved to induce both chronic and acute toxic effects on wildlife, including marine biota [11,12].The toxicity of this PAH was previously investigated by experiments carried on specific sentinel species covering all trophic levels in marine food webs, such as the microalgae Chlorella salina (1–3 mg/L, 96 h, [13]), the copepod Schizopera knabeni (7.24 µmol/L, 96 h, [14]), the polychaete worm Nereis (Neanthes) arenaceodentata (100–1000 µg/L, 14 days, [15]), and the medaka Oryzias melastigma (100–800 µg/L, 25 days, [16]). In humans, the extent to which the phenanthrene is absorbed through the skin is less known, but traces of this substance were found in blood two days after its application on skin [17].
Similar to other marine species, the phenanthrene is suspected to induce detrimental effects on bivalves as well. Bivalves are particularly prone to bioaccumulation compared to other organisms, given their filter-feeding habit which leads to high concentrations accumulated in their tissues, with far-reaching hazardous effects on humans, given their consumption on large scale as seafood [18]. For these reasons the bivalves are considered efficient ecological indicators for the detection of early signs of stress induced by emerging pollutants [19,20]. Their global distribution, sedentary lifestyle, and low acquisition costs make the clams and mussels routinely used species in ecotoxicologic experiments [21,22,23,24].
Traditionally, the ecotoxicological studies relating pollutants and bivalves focused on acute (i.e., effects of short-term exposure, 24–96 h) and single-bivalve species. These classic studies also used a predefined set of endpoints, such as accumulation rate, shell allometry, mortality rate, and enzymatic activities. However, it is known that exposure to PAHs has multiple, often interlinking, effects on marine species and a comprehensive understanding of the overall process is still missing [19,20,21,22,23]. Further, the effects of chronic (i.e., longer-term) exposure are even less understood [25]. Therefore, the aim of the current study was to provide a better understanding of the multiple effects of phenanthrene on marine fauna by using three novel approaches. First, we simultaneously exposed two species of bivalves, the mussel Mytilus galloprovincialis and the clam Ruditapes decussatus, to increasing concentrations of phenanthrene. The second approach was to assess the toxic impact on both species by measuring the changes in time of various morphometric and mass end-points, as well as through neurotoxic and oxidative stress biomarkers, during a chronic exposure experiment (i.e., 7, 15, 21, and 28 days). In the third approach, bioaccumulation and high toxicity were linked to the toxicokinetics and toxicodynamics of phenanthrene, as well as its bioavailability. Such attributes mainly depend on the physico-chemical parameters of the pollutant itself, similar to several toxicants including PAHs using combined in silico and in vivo/in vitro approaches. Overall, the novelty and strength of the current study is twofold: it focused on the chronic rather than acute exposure and combined in vivo experiments with in silico tools, which is a relatively new investigation pathway in current ecotoxicological studies.
2. Material and Methods
A graphical representation that shows the timeline and design of the experiment is given in Figure 1. Details of all schematic steps are presented below.
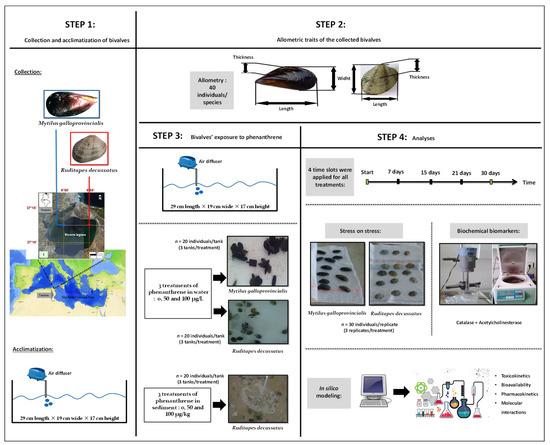
Figure 1.
Graphical summary of steps and methodology adopted.
2.1. Bivalve Sampling and Experimental Set-Up
The bivalves M. galloprovincialis and R. decussatus were collected on the April 5, 2015 from Bizerte Lagoon (Tunisia), next to the Cities of Menzel Abderrahmane (37°13′43.7″ N, 9°51′45.7″ E) and Menzel Jemil (37°13′31.6″ N 9°55′40.9″ E), respectively. The bivalves were transferred to a cooling box containing lagoon water and transported to the laboratory (Figure 1). Afterwards, individuals were chosen randomly, regardless of sex (male or female) and size (M. galloprovincialis: 4.9 ± 0.5 cm, R. decussatus: 2.9 ± 0.2 cm) and placed in bins filled with water from their native habitat.
The bivalves were acclimatized for three days in tanks (29 cm length × 19 cm wide × 17 cm height) in an air-conditioned room. The water salinity, temperature, dissolved oxygen, and pH were maintained close to the values recorded in their natural habitat and measured on a daily basis with a thermo-salinity meter (LF 196; WTW, Weilheim, Germany), an oxymeter (OXI330/SET, WTW, Weilheim, Germany), and a pH meter (pH 330/SET-1, WTW, Weilheim, Germany), respectively. The tanks were constantly ventured with air-diffusers, allowing an even distribution of oxygen. A light/dark cycle was set at 12 h/12 h and the seawater was renewed every 24 h throughout the experiment. The temperature was kept at 18.03 ± 0.71 °C, the salinity at 37.16 ± 0.25 PSU, the pH at 8.2 ± 0.22, and the dissolved oxygen at saturation (9.54 ± 0.89 mg/L).
2.2. Contamination
Following the acclimatization period, a trial experiment was performed to better understand the response of bivalves to phenanthrene as a function of time and concentration. For each type of treatment, three tanks were considered, each comprising with 20 individuals. Stock solutions were prepared by dissolving phenanthrene (CAS Number 85-01-8 (Sigma-Aldrich, Co, St. Louis, MO, USA) in dimethyl sulfoxide (DMSO) (CAS Number 67-68-5, Sigma-Aldrich, Co, St. Louis, MO, USA). This procedure was performed before by dilution in lagoonal water, since the DMSO has no discernible effect on biomarkers [26]. The concentration-effect responses of mussels and clams were assessed by using two dosages of phenanthrene in water: 50 μg/L (hereafter WC1) and 100 μg/L (hereafter WC2) and paralleled by an Untreated Control where only the DMSO was involved (WUt). For clams only, a second experiment was set, with Untreated controls (SUt) and two sediment treatments: 50 µg/kg (hereafter SC1) and 100 µg/kg (hereafter SC2). The time-effect response was observed during four time slots (i.e., 7, 15, 21, and 28 days). The highest concentration in water (i.e., 100 μg/L) was chosen as such as to be approximately two thirds of the Lethal Concentration 50 (LC50) established for the mussel M. edulis, which is 148 µg/L [27]. The low concentration (i.e., 50 μg/L) of phenanthrene corresponded to approximately a third of the LC50 (50 µg/L). The phenanthrene concentrations used to contaminate sediments (i.e., 50 and 100 µg/kg) were within the range of Threshold Effect Levels (TEL) reported by the Canadian Council of Ministers of the Environment (CCME) [28] and MacDonald et al. [29] equal to 41.9 and 86.7 µg/kg, respectively.
2.3. Biometric Study
The mass and other allometric relations were measured for 40 individuals comprising mussels and clams. For each individual, the following parameters were measured:
- -
- Length (L): the longest dimension from the dorsal to the ventral edge;
- -
- Width (W): the longest dimension from the anterior to the posterior edge;
- -
- Thickness (T): the longest dimension given by the convexity of both valves when gathered;
- -
- Total Fresh Mass (TFM): the mass of a living individual with the shell cleaned of mud and water;
- -
- Fresh Chair Mass (FCM): the mass of drained fresh visceral mass on filter paper;
- -
- The Fresh Shell Mass (FSM): the mass of fresh wiped shell.
2.4. Stress on Stress
The Stress on stress concept was first described by [30] and is based on the rationale of measuring any given endpoint of molluscs exposed to two different types of stressors. As such, the bivalves were first exposed to anoxic conditions by placing them out of water. The molluscs are usually tolerant organisms to desiccation; however, their resistance to such stress is reduced if they were exposed already to desiccation in their natural habitats. Given this double type of disturbance, the most sensitive bivalves will die, whereas their strongest congeners will endure the treatments for longer periods of time. The stress on stress method is very simple and requires only a thermostatic room to store the animals in open air. By employing this method, the Median Lethal Time or the time until death of 50% of individuals (hereafter LT50) following exposure of a given population to a toxic substance or stressful condition can be assessed [31,32].
At the beginning of the stress on stress study, the mussels were divided into 36 groups, each comprising 30 individuals: 4 time slots (i.e., 7, 15, 21, and 30 days) × 3 treatments (i.e., control and two phenanthrene concentrations) × 3 replicates. For clams, the number of groups was doubled (72), due to the two different environments where the experiment took place: water and sediment. The bivalves were immediately exposed afterwards to anoxia in an air-conditioned room (18 °C, light-dark cycle 12 h/12 h) until death of all individuals was recorded. The dead specimens were removed every 24 h. We mention that the bivalves used in this experiment differed to those used to measure the biometry and in the evaluation of the enzymatic activities of biomarkers (Figure 1).
2.5. Enzymatic Biomarkers
2.5.1. Sample Preparation for Total Protein Dosages
The control animals and those exposed to phenanthrene were opened by removing their valves, following the incision of the adductive muscles with a scalpel. After dissection, the animals were kept on ice at 4 °C to prevent protein denaturation and grounded afterwards in TBS buffer (Tris 50 mM, NaCl 150 mM) (pH 7.4) using a T25 Ultra-Turrax tissue homogenizer. The homogenate was centrifuged at 9000× g for 30 min at 4 °C. The supernatant (S9), comprising the post-mitochondrial fraction (i.e., cytosol, endoplasmic reticulum, and lysosomes), was stored in Eppendorf tubes at −80 °C until further biochemical analysis. The dosage of Total Protein was prepared according to the Bradford protocol [18]. The reagent Coomassy blue was used to interact with S9 proteins to produce a complex that absorbs light at 595 nm; the colour intensity is proportional to the amount of protein. The protein content was quantified using bovine serum albumin (BSA) as standard and equal loading was confirmed by electrophoresis by directly diluting the sample to a volume of 20 μL into sample buffer [33].
2.5.2. Biochemical Analyses
The changes in optical density were quantified with a Beckman Du® 520 type spectrophotometer. The activity of acetylcholinesterase was measured according to the colorimetric protocol described by [34]. This method evaluates the production rates of thiocholine from the acetylcholine by the cholinesterase into a buffer Na2PO4/NaHPO4 (0.05 M, pH 7.0) solution, 8 mM DTNB, and 45 mM acetylcholine. The released thiocholine reacts with 5-5-dithio-bis (2-nitrobenzoate (DTNB) to form 5-thio-bis-2-nitrobenzoate (TNB), a yellow product that absorbs light at 412 nm. The colour intensity is proportional to the amount of acetylcholinesterase in the sample. Following incubation of samples at 25 °C, the optical density was measured at 412 nm every 5 s for 30 min. Results are given as µmol/min/mg of hydrolysed substrate/total protein content, according to [35], which used this technique for microplate readers. The results are given as μmol/min/mg protein.
Catalase (hereafter CAT) is an enzyme that intervenes in the cell defence mechanisms against the oxidative stress. The activity of CAT (EC 1.11.1) was measured according to [36] and [37], with the aid of a Beckman Du® 520 type spectrophotometer, bitec0, Minnesota, USA. The optical density was measured to assess the CAT activity at 240 nm, by the depletion rate of H2O2 in a reaction buffer solution Na2PO4/NaHPO4 (0.05 M, pH 7.0), 10 mM H2O2 [38]. The CAT activities are given as μmol/min/mg protein.
2.6. Dosage of Phenanthrene in Sediment and Water
Methods for extracting hydrocarbons from sediments were described by [39] and [40]. Soxhlet extraction with chloroform (1:2 w/v) for 8 h (40 °C) was performed on 50 g of dried sediment. The extract was then concentrated by rotary evaporation and separated into Non-Aromatic-(hereinafter referred to as NAH) and Total Aromatic (hereinafter referred to as TArom) hydrocarbons by liquid adsorption chromatography, using an alumina column and silica gel. The chemicals n-Hexane or n-Hexane/Chloroform (2:1) are considered solvents for NAH and TArom fractions, respectively. After solvent evaporation, TArom fractions containing all types of PAHs were analysed using a Hewlett-Packard 5890 gas chromatograph equipped with a temperature-controlled injector, flame ionization detector (GC/FID), and capillary column HP5: 5% diphenyl, 95% Dimethicone (25 m × 0.32 mm × 0.52 µm). For seawater, the separation and quantification of phenanthrene was performed by gas chromatography (GC) according to [41] and based on comparison with known standards injected under the same conditions using a certified standard reference (National Institute of Standards and Technology (NIST, Gaithersburg, MD, USA)). All analyses were performed in triplicate.
2.7. In Silico Analyses Analyses: Bioavailability, Toxicokinetics and Toxicodynamics
The toxicokinetics and inhibition of cytochrome P450 (CYP) isoforms of phenanthrene were assessed based on the ADME/Tox (for Absorption, distribution, metabolism, elimination and toxicity) attributes (i.e., absorption, distribution, metabolism, excretion, and toxicity) as previously described [42,43,44]. The bioavailability and toxicodynamics were also assessed based on the physico-chemical parameters [45,46,47].
2.8. Statistical Analyses
The biometric studies measure the relative growth of individuals. Hence, the following allometric equation was used: Y = a Xb, where b is the allometric coefficient and a is the slope. After loge-transformations [48], the equation became loge Y = loge a + b loge X, where a was the slope and b the intercept of the allometric regression. The biometric parameters were compared with Student t-tests. The intensity of the allometric growth was evaluated by comparing the values of b with the theoretical value of 1 for dimensions and with 3 for mass. If b = 1, the two parameters studied evolve in the same manner (i.e., isometric growth) with no significant differences (Student t-test). When b ≠ 1, the growth rate becomes allometric, with two potential scenarios: for b > 1, the growth rate of individuals is faster compared to that of reference populations (Student t-test: p-value < 0.05), whereas for b < 1, the growth rate is slower.
The changes in enzymatic activities were compared with one-way analysis of variance (1-ANOVA), followed by in-between multiple comparisons with Tukey’s HSD (Honestly Significant Difference) tests. To fulfil the normal distribution requirements for parametric tests, normality (Kolmogorov–Smirnov) and homogeneity of variances (Levene) tests were used. The statistical analyses were performed in STATISTICA v.8.
3. Results
3.1. Concentrations of Phenanthrene
The phenanthrene concentrations in the water and sediment collected on 5 April 2015 from Menzel Abderrahmane, in Bizerte Lagoon, Tunisia, were equal to 10.2 ± 0.14 µg/L and 8.8 ± 0.09 µg/kg, respectively. At the end of the bioassay, no significant changes in phenanthrene concentrations were registered with time (i.e., 7, 15, 21, and 28 days) for each type of treatment (df = 3, 1-ANOVA: p-value ˃ 0.05, Table 1). At each time slot, the phenanthrene concentrations in water or sediment showed a gradual significant increase as follows: Ut → C1 → C2 (df = 6, Tukey’s HSD comparisons: p-values < 0.0001, Table 1).

Table 1.
Targeted and actual concentrations of phenanthrene measured in water and sediment where two bivalve species (Mytilus galloprovincialis and Ruditapes decussatus) were reared and exposed to an Untreated control (Ut) and two concentrations of phenanthrene in water [50 µg/L (WC1) and 100 µg/L (WC2)] and sediment [50 µg/kg (SC1) and 100 µg/kg (SC2)] for 28 days. Different letters (a, b, c, A, B, and C) indicate significant differences with the correspondent untreated controls, represented by ‘a’ for Mytilus galloprovincialis and ‘A’ for Ruditapes decussatus (n = 3, log-transformed data, Tukey’s HSD test: p < 0.05).
3.2. Biometric Study
All bivalves used in the current experiments for allometric measurements and mass were collected from the field and were not exposed to phenanthrene in the laboratory. Their allometric characterization was needed to assess a priori if they comprised morphometrically normal individuals compared to previous findings in Bizerte lagoon and for the appropriateness of the study.
3.2.1. Mytilus galloprovincialis
The Pearson coefficients R2 between length, width, and thickness ranged between 0.821 and 0.92 and were significant (p-values < 0.001, see Figure 2). The regression slopes were significantly different to 1 (Student t-test ˃ 1.96, p-values < 0.05). The regressions between total length, width, and thickness (Figure 2) showed major allometry, whereas the regressions between width and thickness (Figure 2) showed minor allometry.
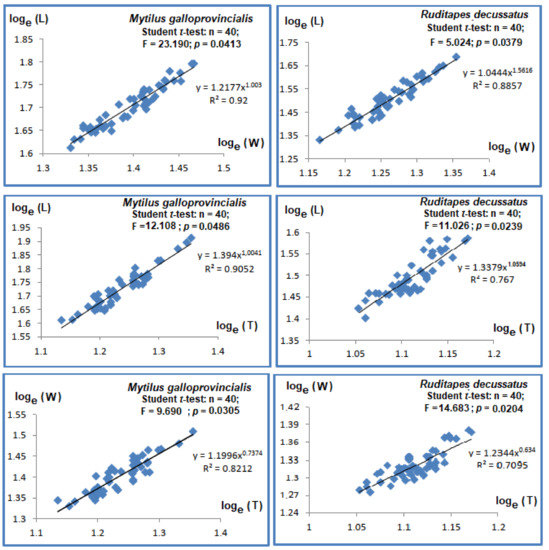
Figure 2.
Regressions relating linear parameters in bivalve species Mytilus galloprovincialis and Ruditapes decussatus. Length (L); Width (W); Thickness (T).
The relative mass growth rates were assessed through regressions between Total Fresh Mass (TFM), Fresh Chair Mass (FCM), Fresh Shell Mass (FSM), and length (L) (see Figure 3). The regressions among various types of masses to length were inter-correlated (p-values < 0.001, R2 > 0.7). The comparisons of slopes with Student t-tests to the theorical value of 3 supported significant differences between masses and length. The allometry was minor, given all slopes had values below 3.
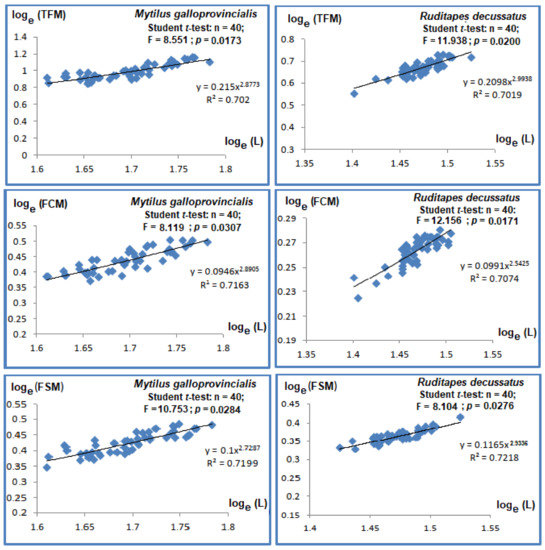
Figure 3.
Regressions relating length and masses in bivalve species Mytilus galloprovincialis and Ruditapes decussatus. Total Fresh Mass (TFM); Fresh Chair Mass (FCM); Fresh Shell Mass (FSM), Length (L).
3.2.2. Ruditapes decussatus
The Pearson correlations between length and width were strong and significant (R2 = 0.767, p-value < 0.05), as with the correlations between length and thickness (R2 = 0.767, p-value < 0.05), with slopes significantly different and higher than unity (p-values < 0.05), showing major allometry (Figure 2). Student t-test showed significant differences (p-values < 0.05) between width and thickness, with minor allometry (slope = 0.634, Figure 2).
The Pearson coefficients of regressions between length and different types of masses varied between 0.701 and 0.721 and were significant (p-values < 0.05, Figure 3). The slope comparisons via Student t-test with the theoretical value of 3 led to the conclusion that the allometries were minor among the types of measured masses (i.e., TFM, FCM, and FSM).
3.3. Stress on Stress
3.3.1. Mytilus galloprovincialis
The average LT50s ranged between 9 ± 0.21 to 8.15 ± 0.73 days in controls and were higher compared to treatments; the lowest LT50 were measured in WC2 for all time slots considered (1-ANOVA: df = 6, p < 0.01, Tukey’s HSD test: p-values < 0.01) (Figure 4). During the first 7 days of the experiment, LT50 decreased with 26%, from 8.6 ± 0.69 days for control to 6.4 ± 0.37 days when exposed to 100 µg phenanthrene/L (Figure 4). LT50 of WC2 was lower compared to WC1 for all time slots (Figure 4).
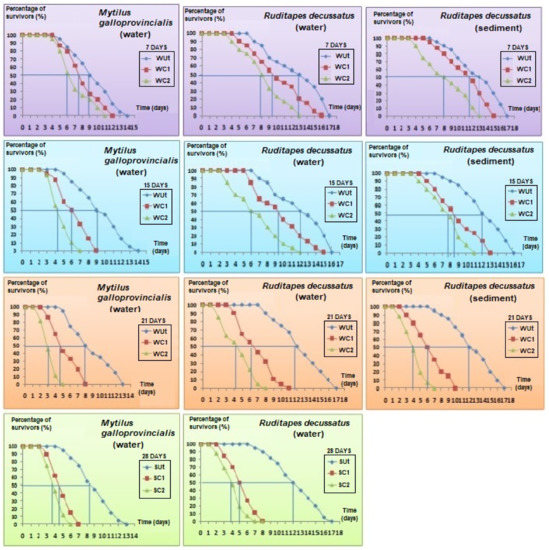
Figure 4.
Percentage of survivors in bivalves’ species Mytilus galloprovincialis and Ruditapes decussatus in open air after being exposed to an Untreated control (Ut) and two concentrations of phenanthrene in water [50 µg/L (WC1) and 100 µg/L (WC2)] and sediment [50 µg/kg (SC1) and 100 µg/kg (SC2)] over 28 days.
3.3.2. Ruditapes decussatus
The LT50 of control clams reared in water did not change in time (1-ANOVA: df = 8, p = 0.892) (Figure 4). Thus, LT50 for control bivalves varied insignificantly between 13 ± 0.74 days at the beginning of the experiment to 11.7 ± 0.38 days after 28 days of exposure, with variation not exceeding 10%. On the other hand, after one week, LT50 values decreased by 22% in WC1 (1-ANOVA: df = 6, p = 0.025, Tukey’s HSD test: p-values < 0.01) and 37% in WC2 (1-ANOVA: df = 6, p < 0.01, Tukey’s HSD test: p-values < 0.001) compared to controls.
An additional experiment was performed with sediment that was either contaminated or not contaminated with phenanthrene. Given that 100% mortality rate was reached after 28 days of exposure, the results were only considered for 3 weeks (Figure 4). LT50 of control clams varied little, between 13 ± 0.24 to 11.93 ± 0.79 days (1-ANOVA: df = 8, p = 0.766) (Figure 4). After 7 days of exposure, LT50 decreased with 14% in SC1 (1-ANOVA: df = 6, p < 0.001, Tukey’s HSD test: p-values < 0.001) to 37% in SC2 (1-ANOVA: df = 6, p < 0.001, Tukey’s HSD test: p-values < 0.001), respectively, compared to control. This trend was statistically maintained in treatments compared to control during all time slots considered (Figure 4).
3.4. Biochemical Biomarkers
3.4.1. Mytilus galloprovincialis
The AChE (Acetylcholinesterase) activity in the control was stable throughout the experiment (1-ANOVA: df = 4, F = 28.491, p = 0.844). In turn, the mussels treated with phenantrene recorded significant inhibitions of their enzymatic activity in time (1-ANOVA: df = 4, F = 50.110, p = 0.027, Tukey’s HSD test: p-values < 0.01). AChE inhibition was similar after 15 and 21 days, respectively, but dropped after 28 days of exposure.
The changes in CAT activity were measured at the same time intervals as for AChE (Figure 5). No significant changes were registered for CAT activities in the controls for all time slots considered (1-ANOVA: df = 4, F = 42.863, p-value = 0.857). In WC1 treatment, there were observed significant changes in the CAT activity after the 15th day of exposure (1-ANOVA: df = 4, F = 51.026, p = 0.045, Tukey’s HSD test: p-values < 0.01). In treatment WC2, there were significant increases in CAT activity after the 7th day of the experiment compared to controls observed, but they significantly dropped in the 28th day (1-ANOVA: df = 4, F = 104.008, p = 0.0314, Tukey’s HSD test: p-values < 0.001).
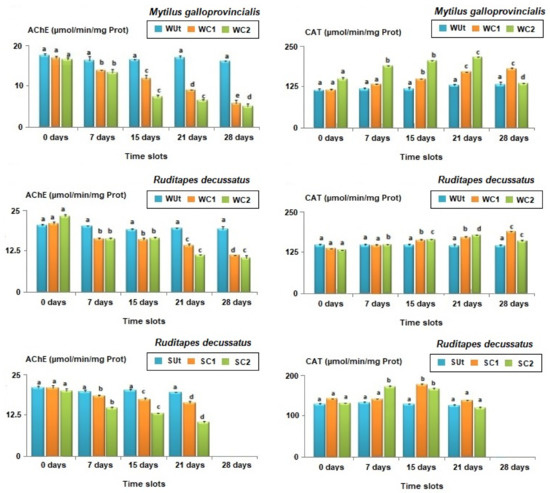
Figure 5.
Average biomarker responses (± standard deviation) in bivalves’ species Mytilus galloprovincialis and Ruditapes decussatus after exposure to an Untreated control (Ut) and two concentrations of phenanthrene in water [50 µg/L (WC1) and 100 µg/L (WC2)] and sediment [50 µg/kg (SC1) and 100 µg/kg (SC2)] over 28 days. Catalase (CAT); Acetylcholinesterase (AChE). Different letters (a, b, c, d, and e) above bars indicate significantly differences from controls represented by ‘a’ after mutiple comparisions using Tukey’s HSD test.
3.4.2. Ruditapes decussatus
The concentrations of AChE were similar for control individuals during all time slots (i.e., 7, 15, 21, and 28 days, 1-ANOVA: df = 10, F = 44.109, p-value = 0.893, Figure 5). The AChE activities in WC1 significantly decreased (1-ANOVA: df = 10, F = 87.520, p = 0.037, Tukey’s HSD test: p-values < 0.05) after the 7th day of exposure, being reduced by 21.3%; the lowest levels were reached at the end of the experiment, with a 46.2% reduction (Figure 5). The AChE activities were also significantly lower in WC2 compared to WUt for the same time slot (7 days: 1-ANOVA: df = 6, F = 116.008, p = 0.0389/15 days: 1-ANOVA: df = 6, F = 92.145, p < 0.0257/21 days: 1-ANOVA: df = 6, F = 138.211, p < 0.01/28 days: 1-ANOVA: df = 6, F = 77.946, p < 0.01) (Tukey’s HSD test, p-values < 0.01, Figure 5).
No significant temporal variations were observed for AChE activity in control clams reared in sediment (1-ANOVA: df = 10, F = 107.241, p-value = 0.911, Figure 5). In contrast, the AChE activity in SC1 (7 days: 1-ANOVA: df = 6, F = 81.229, p = 0.0425/15 days: 1-ANOVA: df = 6, F = 135.104, p = 0.0146/21 days: 1-ANOVA: df = 6, F = 64.125, p < 0.01) and SC2 (7 days: 1-ANOVA: df = 6, F = 103.150, p = 0.0351/15 days: 1-ANOVA: df = 6, F = 78.134, p = 0.0221/21 days: 1-ANOVA: df = 6, F = 115.457, p < 0.01) significantly decreased compared to controls for all the time slots (Tukey’s HSD test, p-values < 0.01, Figure 5). Moreover, significant differences were also observed when the magnitude of AChE activity inhibition was compared in SC1 and SC2 (Student t-test: df = 4, F = 138.211, p-value = 0.0275).
The CAT activity in control clams was similar throughout the experiment (1-ANOVA: df = 6, F = 35.291, p-value = 0.816). By changing the exposure time for WC1 (1-ANOVA: df = 10, F = 218.443, p-value = 0.0127), significant changes in CAT activities were noticed. Thus, the bivalves from WC1 had first similar CAT levels with those from control following one week of exposure (Tukey’s HSD test: df = 4, p-value = 0.769). However, after the 15th day, significant differences were registered in CAT activities under WC1 compared to control (Tukey’s HSD test: df = 4, p-value = 0.0310, Figure 5). In WC2, the CAT reduction was significant compared to WC1 starting from the 7th day of exposure and continued until the 21st day (Student t-test: df = 4, p-values < 0.01, Figure 5). By the end of the experiment (i.e., 28 days) the activity of CAT under WC2 was significantly lower compared to WC1 (Student t-test: df = 4, p-values < 0.001, Figure 5).
Following the in vivo exposure of R. decussatus to phenanthrene in sediment, the CAT activity showed similar levels in control individuals no matter the exposure duration (1-ANOVA: df = 8, F = 64.527, p-value = 0.928). After 7 days of exposure (1-ANOVA: df = 6, F = 35.1291, p < 0.01), no significant effect was observed for SC1 (Tukey’s HSD: p-value = 0.928, Figure 5) but the increase was significant in SC2 for the same time slot (Tukey’s HSD: p-value < 0.001, Figure 5). After the 15th day, the CAT induction was significant (Tukey’s HSD test, p-values < 0.001 Figure 5) but thereafter, CAT activities dropped surprisingly compared to those found before at 7 and 15 days and no significant differences were found after 21st days of exposure compared to controls (1-ANOVA: df = 6, F = 201.846, p-value = 0.769).
3.5. In Silico Findings: Bioavailability and Toxicokinetics
The lipophilicity, bioavailability, and toxicokinetics data are given in Table 2. Both lipophilicity and bioavailability scores (0.55) confirm that phenanthrene is toxicologically active. The data are supported by the bioavailability radar (Figure 6). It was showed that phenanthrene is highly absorbable by the gastro-intestinal (GI) tract. It is also blood-brain barrier (BBB) permeable, but presented low skin permeability, as assessed using the Log Kp parameter. The possible inhibition of the major cytochrome P450 (CYP) isoforms was assessed. While phenanthrene was predicted to not inhibit cytochrome P450 (CYP) variants: CYP2C19, CYP2C9, CYP2D6, it instead inhibited CYP1A2 and CYP3A4, as well as the cytochrome P450 (CYP) variant, the CYP1A2. Both Tox21 stress response pathways (nrf2/ARE, HSE, MMP, TS-p53 and ATAD5) and Tox21 nuclear receptor signalling pathways (AhR, AR, AR-LBD, Aromatase, ER, ER-LBD and PPAR-γ) were inhibited following exposure to phananthrene. As showed in Figure 6, it is reported that these parameters presented significantly higher percentages, except for ER-LBD. Its toxico-dynamics revealed specifically, mutagenicity and carcinogenicity as endpoints.

Table 2.
Toxicokinetic properties of phenanthrene based on its physico-chemical and ADME/Tox (for absorption, distribution, metabolism, elimination, and toxicity) attributes.
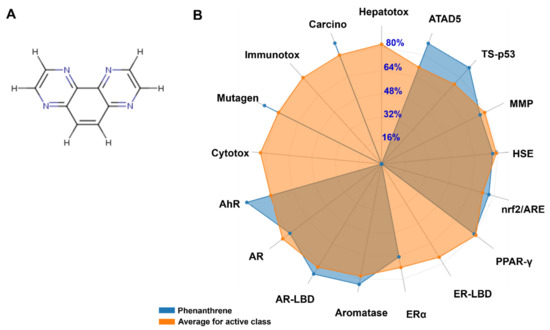
Figure 6.
The chemical structure of phananthrene (A) and the average biomarker responses related to its toxicodynamics as compared to the average of class compounds (B). Cytox: Cytotoxicity; Mutagen: Mutagenicity; Immunotox: Immunotoxicity; Carcino: Carcinogenicity; Hepatotox: Hepatotoxicity; ATAD5: ATPase family AAA domain-containing protein 5; TS-p53: Tumor Supressor (Phosphoprotein) p53; MMP: Mitochondrial Membrane Potential; HSE: Heat shock factor response element; Nrf2/ARE: Nuclear factor like 2/antioxidant; PPAR-γ: Peroxisome Ploriferator Activated Receptor Gamma; ER-LBD: Estrogen Receptor Ligand Binding Domain; ERα: Estrogen Receptor Alpha; AR-LBD: Androgen Receptor Ligand Binding Domain; AR: Androgen Receptor; AhR: Aryl hydrocarbon Receptor.
4. Discussion
4.1. Does the Addition of Contaminated Water and Sediment with Phenanthrene Modify the Quality of the Environment in Treated Experimental Units?
The phenanthrene concentrations measured at the end of the bioassay in water and sediment were close to the targeted concentrations (see Table 1). These results also support the appropriateness of the adopted experimental conditions (i.e., 18 °C, light-dark cycle 12 h/12 h) that did not influence the reactivity of phenanthrene in water and sediment. Previously, ref. [49] focused on PAHs vaporisation from sediment (up to 60 °C) and observed that 10–30% of phenanthrene was detected in vapours after 100 days. We can conclude that under the current laboratory conditions, the vaporization rate of phenanthrene is negligible, at least for sediment (~5–10%).
At the end of the experiment, the treated microcosms contained significantly higher concentrations of phenanthrene in both water and sediment compared to the control, leading to the conclusion that the observed changes for M. galloprovincialis and R. decussatus were mainly induced by phenanthrene toxicity.
4.2. What the Biometric Features of M. galloprovincialis and R. decussatus Influenced by Exposure to Phenanthrene?
The characterization of biometric traits is important since the bivalves were collected from the Bizerte lagoon, which is normally exposed to several stressors [50]. Therefore, in the first instance, the realistic estimation of exposed bivalves as being appropriate for the laboratory experiment was a crucial step. It is known that the morphology of bivalves is affected by stressors [51,52]. The linear (i.e., length, width, and thickness) and mass parameters (i.e., Total Fresh Mass, Fresh Chair Mass, and Fresh Shell Mass) were measured in the current experiment to assess if the phenantrene influenced the growth rate of bivalves. The growth rates for length and width were faster compared to thickness for the mussel M. galloprovincialis, but the growth rate of mass was lower compared to that of length. For the clam R. decussatus, the increase rate of length was the highest, for thickness intermediary, and the lowest for width. This trend may explain the triangular shape and the relative thickening of the valves, allowing the stability of individuals in sediments due their benthic lifestyle. The growth rate of the total mass was also lower compared to length. This is probably due to the fact that the tested clams were in the post-laying phase, following the release of the gonadic content. According to [53,54,55], the compression of the shell in mussels is strongly influenced by two additional factors: the relative growth rate of linear variables and the density of individuals. Thus, a lower compression of the shell is related to slower growth rates and population density. Ref. [56] showed for the bivalves M. galloprovincialis and R. decussatus, an intimate relation among linear parameters, such as lengths, width, and thickness exist, and that the relative growth of mass was proportional to that of length. The growth rate of bivalves is equally influenced by water physic-chemistry, such as temperature, pH, photoperiod, emersion time, and air temperature during tides [56,57], but also by the availability of food [58,59]. All these features seem to indicate that the collected individuals from Bizerte lagoon (Tunisia) were normal, since no significant biometric anomalies were registered compared to the common shapes of both species considered.
4.3. Does the Phenanthrene Influence the Responses of M. galloprovincialis and R. decussatus to Stress on Stress?
Ideally, laboratory techniques and biomonitoring tools should be accurate, reliable, and cheap. In order to overcome the drawbacks imposed by reality, the stress on stress approach was implemented [30].
In the current study, the comparison of the tolerance to anoxia of R. decussatus compared to M. galloprovincialis showed that under the same environmental conditions, the former clam was more tolerant to anoxia compared to the latter mussel. This could be explained by different metabolic pathways and defence mechanisms of the two bivalves [38]. The shell closure of clams was tighter compared to that of mussels; the latter group needs the aperture for the protrusion of byssus, allowing the entrance of toxic substances at higher rates compared to clams [56].
The relative constancy of LT50 throughout the experimental may indicate a stability of the experimental conditions and that control bivalves better tolerated the open-air conditions compared to their congeners from contaminated treatments. However, the comparison of our results with those from literature (Table 3) showed that the tolerances were higher compared to those of bivalves collected from the same site of Menzel Abderrahmane (Bizerte Lagoon, Tunisia) by [60], but similar to the values observed by [56]. The contamination with phenanthrene reduced the LT50 in a time- and concentration-dependent way, altering the physiological status of both bivalves. The time and concentration dependency comprise typical hallmarks of expectable dose-response relationships: the toxicity typically increases with higher concentrations and with longer exposure durations, as previously demonstrated by [61]. Previous studies support the potential direct toxic effect of phenanthrene for bivalves [62,63,64]. PAHs are known for their carcinogenic, teratogenic, and mutagenic properties for aquatic organisms and humans [65]. Most PAHs have strong hydrophobic characteristics and tend to be absorbed by organic and inert suspended particles in water and finally accumulate in sediments, mainly applicable for the heaviest compounds [66,67,68,69,70]. Therefore, organisms with sessile lifestyles, such as M. galloprovincialis and with benthic preferences such as the European clam R. decussatus, could be doubly exposed to these contaminants, namely in the water column and at the water–sediment interface [21].

Table 3.
Comparison with data from literature on Median Lethal Time of 50% (LT50) of the populations of Mytilus galloprovincialis and Ruditapes decussatus after exposure to two concentrations of phenanthrene in water [50 µg/L (WC1) and 100 µg/L (WC2)] and sediment [50 µg/kg (SC1) and 100 µg/kg (SC2)] over 28 days.
The values of LT50 registered for R. decussatus support the assertion of higher toxicity of phenanthrene following the sediment contamination compared to water exposure, given that the LT50 declined faster in the former milieu. This result is in accordance with that of [71] in contaminated sediments with pesticides. The results of the current study showed that the sensitivity thresholds to phenanthrene for both bivalves decreased with exposure time.
4.4. Do Enzymatic Biomarkers Respond to Phenanthrene Contamination?
The concentrations of AChE and CAT were similar in time for control bivalves, suggesting that the experimental conditions had no significant effect on the activities of biomarkers for M. galloprovincialis and R. decussatus. Moreover, the specimens reared in the laboratory kept a healthy appearance and normal behaviour after 28 days. However, the inhibitory effect of phenanthrene was observed early in the experiment (i.e., after 7 days) and led to a maximum inhibition after 28 days. These results highlight the detrimental effects following exposure to this PAH, probably through bioconcentration in the tissues of bivalves and potential neurotoxic actions. These detrimental effects were observed for the mussel M. galloprovincialis in water and the clam R. decussatus in water and sediment contaminated with phenanthrene. The detrimental effects of phenanthrene on AChE activity became more visible for clams compared to mussels after 7 days of exposure. However, it can overall be concluded that the effects of contamination with phenanthrene were similar for both tested species. Comparable results were reported by [72,73], who showed the persistent inhibition of the AChE activity in the digestive glands of M. galloprovincialis after two days of exposure to benzopyrene. These authors reported that the reduction of the AChE activity was the result of an overall collapse of the health status of the animals following exposure to benzopyrene. Moreover, ref. [74] suggested that strong inhibitions of AChE activities may lead to the tetanization of muscles, and finally to the death of bivalves. Ref. [75] found similar impacts on gills and digestive glands following exposure of M. galloprovincialis and R. decussatus to benzo[a]pyrene. These findings, corroborated with similar effects following the exposure to organophosphorus and carbamates pesticides, which are considered as one of the most neurotoxic compounds, support the strong inhibition of AChE activity of bivalves by phenanthrene [75,76,77,78,79].
For the clam R. decussatus exposed to phenanthrene in water and sediment, the inhibition of AChE indicates a double effect. This may be achieved through the adsorption capacity of phenanthrene in sediment particles, which increases its bioavailability for benthic bivalves. Our results showed that the response of the antioxidant activity was similar in R. decussatus and M. galloprovincialis and that significant induction was observed for the former bivalve following contamination of water, as well as for the latter species in water and sediment. Following the exposure in the water column, the response was clearer for clams compared to mussels and started earlier for the highest concentration (i.e., 100 μg/L). A comparable trend was also registered for clams reared in contaminated sediments. Similar cases of early induction on the catalase activity were observed for Perna viridis exposed to PAHs [80] and Bathylomodiolus azoricus exposed to metals (i.e., mercury and copper, see [81]). Our results are convergent with those reported by Bebianno and Barreira (2009), who proved the strong accumulation of the most soluble PAHs in R. decussatus after just one day of exposure. The exposure to PAHs led to increased catalase activity in the mussels M. edulis [82], Perna viridis [20], Mya arenaria, and M. trossulus [83]. Moreover, ref. [84] showed that anthracene, benzopyrene, and other petrochemicals enhance the catalase activity in the fish Pomatoschistus microps. The enzyme catalase is normally present in many types of tissues and organs, intervening in cell defence mechanisms against oxidative stress by eliminating Reactive Oxygen Species (ROS) and accelerating the spontaneous reaction of dismutation of hydrogen peroxide (H2O2), which is toxic [85]. This enzyme prevents the peroxidation of biomolecules induced by H2O2. The catalase is sensitive to xenobiotics, such as PAHs, Polychlorinated biphenyls (PCBs), metals, and pesticides, known to induce oxidative stress in cell membranes and damages by protein oxidations, lipid peroxidations, and the formation of DNA adducts [80,81,86,87,88]. The low induction of catalase noticed at phenanthrene concentrations smaller than 50 µg/L could be explained by the fact that the oxidative stress, following the contamination, was first neutralized by antioxidant mechanisms rather than the enzymatic pathways. In the first step following exposure to toxic substances, the tissues trap ROS through numerous non-enzymatic compounds, such as vitamins (i.e., A, C and E), ubiquinone, carotenoids, flavonoids, and uric acid. Once the free ROS exceeds the capacity of these trappers, the antioxidant enzymes such as the catalase are mobilized, potentially explaining their concentration in bivalves until the 21st day of the experiment.
A delayed response was found for mussels and clams in treatments contaminated with the highest concentrations of phenanthrene after 28 days of experiment, given the significant collapse registered in the CAT activity. Ref. [85] reported an initial phase of increasing CAT activity in the digestive glands of the clam R. decussatus beginning after three days and reaching a maximum after 28 days of exposure, but followed by a decrease afterwards.
The contamination with phenanthrene may have also induced some behavioural changes for the reared on treated substrate R. decussatus. These clams were unable to close hermetically their valves, leaving the siphons extended outwards. These results are similar with previous works that reported changes in the behaviour of the bivalves Macoma balthica, Corbicula flumea and Crassostrea gigas, such as modifications in valves’ movement and reduced filtration rate following exposure to organic contaminants [89].
Regarding the in silico analyses, our results confirmed the possible toxicological outcomes of exposure to phenanthrene. It has been shown that phenanthrene is highly absorbed by the GI tract. It is also BBB permeable, but presented low skin permeability as assessed using Log Kp parameter. The possible inhibition of the major cytochrome P450 (CYP) isoforms was assessed. The phenanthrene inhibited both CYP1A2 and CYP3A4 but not CYPC19, CYPC9, and CYP2D6. These results confirmed recent results by Hedfi et al. (2022) [90] regarding phenanthrene and chrysene intoxication in nematodes. Tox21 stress response pathways (nrf2/ARE, HSE, MMP, TS-p53, and ATAD5) and Tox21 nuclear receptor signalling pathways (AhR, AR, AR-LBD, Aromatase, ER, ER-LBD, and PPAR-γ) are commonly assessed in toxicological approaches [90]. Excepting ER-LBD, all assessed pathways presented significant, high percentages, with associated mutagenicity and carcinogenicity, similar to those observed following exposure to polycylic aromatic hydrocarbons [90,91,92].
5. Conclusions
Following exposure to phenanthrene, the LT50 of bivalves decreased for the used concentrations and exposure time, a direct effect of the detrimental actions of this PAH on the physiological status of mussels and clams. The detrimental effects of phenanthrene were also reflected in a time- and concentration-dependent manner, with better tolerance observed for the clam R. decussatus compared to the mussel M. galloprovincialis. In the case of biochemical biomarkers, the effects were also time- and concentration- dependent. The AChE activity was inhibited in the bivalves after contamination with phenanthrene of water (i.e., the mussels and the clams) or just sediment (i.e., clams). In turn, the CAT activity was induced at a faster rate in mussels compared to clams until the 21st day of the experiment, afterwards followed by a collapse of the physiological status, reflected in the decrease of the enzyme activity in the 28th day of the experiment. The in silico findings (i.e., bioavailability, toxicokinetics, and toxicodynamics) supported and explained the measured morphological and physiological results, based on the biometric, stress on stress, and analyses of biomarkers.
Author Contributions
Biomarker assessment, Writing—original draft, M.D.; Validation and Writing—original draft, K.M., Funding acquisition and review & editing, A.H.H., Funding acquisition and review & editing, L.M., Literature investigation, W.A., Literature investigation & formal analysis, S.N., Statistical analyses, A.F.A., Review & editing, O.P., In silico analyses and writing, R.B., Conceptualization, supervision, review & editing, F.B. All authors have read and agreed to the published version of the manuscript.
Funding
The authors extend their appreciation to the Deputyship for Research & Innovation, Ministry of Education in Saudi Arabia for funding this research work through the project no. (IFKSURG-2-672).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All the data in the article are available from the corresponding author upon reasonable request.
Acknowledgments
The authors extend their appreciation to the Deputyship for Research & Innovation, Ministry of Education in Saudi Arabia for funding this research work through the project no. (IFKSURG-2-672).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Andral, B.; Stanisiere, J.Y.; Sauzade, D.; Damier, E.; Thebault, H.; Galgani, F.; Boissery, P. Monitoring chemical contamination levels in the Mediterranean based on the use of mussel caging. Mar. Pollut. Bull. 2004, 49, 704–712. [Google Scholar] [CrossRef] [PubMed]
- Dellali, M.; Gnassia Barelli, M.; Romeo, M.; Aïssa, P. The use of acetylcholinésterase activity in Ruditapes decussatus and Mytilus galloprovincialis in the biomonitoring of Bizerta lagoon. Comp. Biochem. Phys. 2001, 130, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Baird, A.H.; Marshall, P.A. Mortality, growth and reproduction in scleractinian corals following bleaching on the Great Barrier Reef. Mar. Ecol. Prog. Ser. 2002, 237, 133–141. [Google Scholar] [CrossRef]
- US-EPA. Determination of Benzo(a)pyrene and Other Polynuclear Aromatic Hydrocarbons in Indoor Air; U.S. Environmental Protection Agency: Washington, DC, USA, 1990. [Google Scholar]
- Verschueren, K. Phenathrene. Handbook of Environmental Data on Organic Chemicals, 3rd ed.; Van Nostrand Reinhold Co.: New York, NY, USA, 1996; pp. 1756–1762. [Google Scholar]
- CITI. Biodegradation and Bioaccumulation Data of Existing Chemicals Based on the CSCL Japan; Chemicals Inspection and Testing Institute: Japan, 1992. [Google Scholar]
- Howard, P.H.; Boethling, R.S.; Jarvis, W.F. Phenantrene-Handbook of Environmental Degradation Rates; Meylan, W.M., Michalenko, E.M., Eds.; Lewis Publisher: Chealsea, MI, USA, 1991; p. 725. [Google Scholar]
- Maciorowski, A.G.; Sims, J.L.; Little, L.W.; Gerrard, V. Bioassays: Procedures and results. J. Water Pollut. Control. Fed. 1980, 53, 974–993. [Google Scholar]
- Jensen, L.K.; Honkanen, J.O.; Jæger, I.; Carroll, J. Bioaccumulation of phenanthrene and benzo[a]pyrene in Calanus finmarchicus. Ecotoxicol. Environ. Saf. 2012, 78, 225–231. [Google Scholar] [CrossRef]
- Allouche, M.; Nasri, A.; Harrath, A.H.; Mansour, L.; Alwasel, S.; Beyrem, H.; Plăvan, G.; Rohal-Lupher, M.; Boufahja, F. Meiobenthic nematode Oncholaimus campylocercoides as a model in laboratory studies: Selection, culture, and fluorescence microscopy after exposure to phenanthrene and chrysene. Environ. Sci. Pollut. Res. 2021, 28, 29484–29497. [Google Scholar] [CrossRef]
- Swartz, R.C.; Ferraro, S.P.; Lamberson, J.O.; Cole, F.A.; Ozretich, R.J.; Boese, B.L.; Schults, D.W. Photoactivation and toxicity of mixtures of polycyclic aromatic hydrocarbon compounds in marine sediment. Environ. Toxicol. Chem. 1997, 16, 2151–2157. [Google Scholar] [CrossRef]
- INERIS. Données Technico-économiques Sur Les Substances Chimiques en France. In Rapport D’Étude 3; INERIS: Verneuil-en-Halatte, France, 2006; p. 37. [Google Scholar]
- Chen, H.; Zhang, Z.; Tian, F.; Zhang, L.; Li, Y.; Cai, W.; Jia, X. The effect of pH on the acute toxicity of phenanthrene in a marine microalgae Chlorella salina. Sci. Rep. 2018, 8, 17577. [Google Scholar] [CrossRef]
- Evans, A.D.; Nipper, M. Toxicity of phenanthrene and lindane mixtures to marine invertebrates. Environ. Toxicol. 2007, 22, 495–501. [Google Scholar] [CrossRef]
- Emery, V.L., Jr.; Dillon. T.M. Chronic Toxicity of Phenanthrene to the Marine Polychaete Worm, Nereis (Neanthes) Arenaceodenta. Bull. Environ. Contam. Toxicol. 1996, 56, 265–270. [Google Scholar]
- Mu, J.; Wang, J.; Jin, F.; Wang, X.; Hong, H. Comparative embryotoxicity of phenanthrene and alkyl-phenanthrene to marine medaka (Oryzias melastigma). Mar. Pollut. Bull. 2014, 85, 505–515. [Google Scholar] [CrossRef] [PubMed]
- Storer, J.S.; DeLeon, I.; Millikan, L.E.; Laseter, J.L.; Griffing, C. Human Absorption of Crude Coal Tar Products. Arch. Dermatol. 1984, 120, 874–877. [Google Scholar] [CrossRef] [PubMed]
- Bayne, B.; Hawkins, A.; Navarro, E.; Iglesias, I. Effects of seston concentration on feeding, digestion and growth in the mussel Mytilus edulis. Mar. Ecol. Prog. Ser. 1989, 55, 47–54. [Google Scholar] [CrossRef]
- Lagadic, L.; Caquet, T.; Amiard, J.C. Biomarqueurs en Ecotoxicologie: Aspects Fondamentaux; Masson: Paris, France, 1997; pp. 1–9. [Google Scholar]
- Cheung, C.; Zheng, G.; Li, A.; Richardson, B.; Lam, P. Relationships between tissue concentrations of polycyclic aromatic hydrocarbons and antioxidative responses of marine mussels, Perna viridis. Aquat. Toxicol. 2001, 52, 189–203. [Google Scholar] [CrossRef]
- Sellami, B. Réponse Protéique et Multimarqueurs de la Palourde à la Contamination Par Certains Polluants Organiques (Pesticides, HAP et Cocktail). Ph.D. Thesis, University of Carthage, Bizerte, Tunisia, 2014; 130p. [Google Scholar]
- Livingstone, D.R. Contaminant-stimulated Reactive Oxygen Species production and oxidative damage in aquatic arganisms. Mar. Poll. Bull. 2000, 42, 656–666. [Google Scholar] [CrossRef]
- Cheung, C.; Zheng, G.; Lam, P.; Richardson, B. Relationships between tissue concentrations of chlorinated hydrocarbons (polychlorinated biphenyls and chlorinated pesticides) and antioxidative responses of marine mussels, Perna viridis. Mar. Pollut. Bull. 2002, 45, 181–191. [Google Scholar] [CrossRef]
- Widdows, J.; Donkin, P.; Staff, F.; Matthiessen, P.; Law, R.; Allen, Y.; Thain, J.; Allchin, C.; Jones, B. Measurement of stress effects (scope for growth) and contaminant levels in mussels (Mytilus edulis) collected from the Irish Sea. Mar. Environ. Res. 2002, 53, 327–356. [Google Scholar] [CrossRef]
- Thoré, E.S.; Philippe, C.; Brendonck, L.; Pinceel, T. Towards improved fish tests in ecotoxicology—Efficient chronic and multi-generational testing with the killifish Nothobranchius furzeri. Chemosphere 2021, 273, 129697. [Google Scholar] [CrossRef]
- Perić, L.; Nerlović, V.; Žurga, P.; Žilić, L.; Ramšak, A. Variations of biomarkers response in mussels Mytilus galloprovincialis to low, moderate and high concentrations of organic chemicals and metals. Chemosphere 2017, 174, 554–562. [Google Scholar] [CrossRef]
- Verbruggen, E.M.J. Environmental Risk Limits for Polycyclic Aromatic Hydrocarbons (PAHs) for Direct Aquatic, Benthic, and Terrestrial Toxicity; RIVM Report 607711007/2012; Rijksinstituut voor Volksgezondheid en Milieu RIVM: Utrecht, The Netherlands, 1990; 337p. [Google Scholar]
- Canadian Council of Ministers of the Environment. Protocol for the derivation of Canadian sediment quality guidelines for the protection of aquatic life. CCME EPC-98E. Prepared by Environment Canada, Guideline Division. In Technical Secretariat of the CCME Task Group on Water Quality Guidelines; Canadian Council of Ministers of the Environment: Ottawa, ON, Canada, 1999; 35p. [Google Scholar]
- Macdonald, D.D.; Carr, R.S.; Calder, F.D.; Long, E.R.; Ingersoll, C.G. Development and evaluation of sediment quality guidelines for Florida coastal waters. Ecotoxicology 1996, 5, 253–278. [Google Scholar] [CrossRef]
- Eertman, R.H.M.; Zwaan, A. Survival of the Fittest: Resistance of Mussels to Aerial Exposure. In Biomonitoring of Coastal Waters and Estuaries; Kramer, K.I.M., Ed.; CRC Press: Boca Raton, FL, USA, 1994; pp. 269–282. [Google Scholar]
- Maryoung, L.A.; Lavado, R.; Schlenk, D. Impacts of hypersaline acclimation on the acute toxicity of the organophosphate chlorpyrifos to salmonids. Aquat. Toxicol. 2014, 152, 284–290. [Google Scholar] [CrossRef] [PubMed]
- Verma, V.; Yu, Q.J.; Connell, D.W. Evaluation of effects of long term exposure on lethal toxicity with mammals. Environ. Pollut. 2014, 185, 234–239. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantization of microgram quantities of protein utilizing the principle of protein-dry binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Ellman, G.L.; Courtney, K.D.; Andres, V., Jr.; Featherstone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Galgani, F.; Bocquené, G.; Cadiou, Y. Evidence of variation in cholinesterase activity in fish along a pollution gradient in the North Sea. Mar. Ecol. Prog. Ser. 1992, 91, 77–82. [Google Scholar] [CrossRef]
- Aebi, H. Methods of Enzymatic Analysis, 2nd ed.; Chemia Weinheium: New York, NY, USA, 1974. [Google Scholar]
- Beutler, E. Red Cell Metabolism: A Manual of Biochemical Methods; Grune & Stratton: New York, NY, USA, 1975. [Google Scholar]
- Claiborne, A. Catalase activity. Handbook of Methods for Oxygen Radical Research; Greenwald, R.A., Ed.; CRC Press: Boca Raton, FL, USA, 1985; pp. 283–284. [Google Scholar]
- Trabelsi, S.; Driss, M.R. Polycyclic aromatic hydrocarbons in superficial coastal sediments from Bizerte lagoon, Tunisia. Mar. Pollut. Bull. 2005, 50, 344–359. [Google Scholar] [CrossRef] [PubMed]
- Zrafi-Nouira, I.; Khedir-Ghenim, Z.; Bahri, R.; Cheraeif, I.; Rouabhia, M.; Saidane-Mosbahi, D. Hydrocarbons in Seawater and Water Extract of Jarzouna-Bizerte Coastal of Tunisia (Mediterranean Sea): Petroleum Origin Investigation Around Refinery Rejection Place. Water Air Soil Pollut. 2009, 202, 19–31. [Google Scholar] [CrossRef]
- Zrafi-Nouira, I.; Safi, N.M.D.; Bahri, R.; Mzoughi, N.; Aissi, A.; Ben Abdennebi, H.; Saidane-Mosbahi, D. Distribution and Sources of Polycyclic Aromatic Hydrocarbons Around a Petroleum Refinery Rejection Area in Jarzouna-Bizerte (Coastal Tunisia). Soil Sediment Contam. Int. J. 2010, 19, 292–306. [Google Scholar] [CrossRef]
- Badraoui, R.; Adnan, M.; Bardakci, F.; Alreshidi, M. Chloroquine and Hydroxychloroquine Interact Differently with ACE2 Domains Reported to Bind with the Coronavirus Spike Protein: Mediation by ACE2 Polymorphism. Molecules 2021, 26, 673. [Google Scholar] [CrossRef]
- Hchicha, K.; Korb, M.; Badraoui, R.; Naïli, H. A novel sulfate-bridged binuclear copper (II) complex: Structure, optical, ADMET and in vivo approach in a murine model of bone metastasis. New J. Chem. 2021, 45, 13775–13784. [Google Scholar] [CrossRef]
- Mhadhbi, N.; Issaoui, N.; Hamadou, W.S.; Alam, J.M.; Elhadi, A.S.; Adnan, M.; Naϊli, H.; Badraoui, R. Physico-Chemical Properties, Pharmacokinetics, Molecular Docking and In-Vitro Pharmacological Study of a Cobalt (II) Complex Based on 2-Aminopyridine. Chem. Select. 2022, 7, e202103592. [Google Scholar] [CrossRef]
- Badraoui, R.; Saeed, M.; Bouali, N.; Hamadou, W.S.; Elkahoui, S.; Alam, M.J.; Siddiqui, A.J.; Adnan, M.; Saoudi, M.; Rebai, T. Expression Profiling of Selected Immune Genes and Trabecular Microarchitecture in Breast Cancer Skeletal Metastases Model: Effect of α–Tocopherol Acetate Supplementation. Calcif. Tissue Int. 2022, 110, 475–488. [Google Scholar] [CrossRef] [PubMed]
- Jedli, O.; Ben-Nasr, H.; Zammel, N.; Rebai, T.; Saoudi, M.; Elkahoui, S.; Jamal, A.; Siddiqui, A.J.; Sulieman, A.E.; Alreshidi, M.M.; et al. Attenation of ovalbumin-induced inflammation and lung oxidative injury in asthamtic rats by Zingiber officinale extract: Combined in silico and in vivo study on antioxidant potential, STAT6 and TNF-pathways. 3 Biotech 2022, 12, 191. [Google Scholar] [CrossRef] [PubMed]
- Zammel, N.; Jedli, O.; Rebai, T.; Hamadou, W.S.; Elkahoui, S.; Jamal, A.; Alam, J.M.; Adnan, M.; Siddiqui, A.J.; Alreshidi, M.M.; et al. Kidney injury and oxidative damage alleviation by Zingiber officinale: Phramacokinetics and protective approach in a combined murine model of osteoporosis. 3 Biotech 2022, 12, 112. [Google Scholar] [CrossRef]
- Roosenburg, W.M.; Dennis, T. Egg Component Comparisons within and among Clutches of the Diamondback Terrapin, Malaclemys terrapin. Copeia 2005, 2, 417–423. [Google Scholar] [CrossRef]
- Hawthorne, S.B.; Grabanski, C.B. Vaporization of Polycyclic Aromatic Hydrocarbons (PAHs) from Sediments at Ambient Conditions. Environ. Sci. Technol. 2000, 34, 4348–4353. [Google Scholar] [CrossRef]
- Afli, A.; Boufahja, F.; Sadraoui, S.; Ben Mustapha, K.; Aïssa, P.; Mrabet, R. Functional organization of the benthic macrofauna in the Bizerte lagoon (SW Mediterranean Sea), semi-enclosed area subject to strong environmental/anthropogenic variations. Cah. Biol. Mar. 2009, 50, 105–117. [Google Scholar]
- Boufahja, F.; Hedfi, A.; Amorri, J.; Aïssa, P.; Beyrem, H.; Mahmoudi, E. Examination of the bioindicator potential of Oncholaimus campylocercoides (Nematoda, Oncholaimidae) from Bizerte bay (Tunisia). Ecol. Indic 2011, 11, 1139–1148. [Google Scholar] [CrossRef]
- Boufahja, F.; Hedfi, A.; Essid, N.; Aïssa, P.; Mahmoudi, E.; Beyrem, H. An observational study on changes in biometry and generation time of Odontophora villoti (Nematoda, Axonolaimidae) related to petroleum pollution in Bizerte bay, Tunisia. Environ. Sci. Pollut. Res. 2012, 19, 646–655. [Google Scholar] [CrossRef]
- Seed, R. Factors Influencing Shell Shape in the Mussel Mytilus edulis. J. Mar. Biol. Assoc. United Kingd. 1968, 48, 561–584. [Google Scholar] [CrossRef]
- Seed, R. Absolute and allometric Growth in the mussel Mytilus edulis (Mollusca, bivalvia). Proc. Malacol. Soc. Lond. 1973, 40, 343–357. [Google Scholar]
- Brown, S.B. Stereospecific haem cleavage. A model for the formation of bile-pigment isomers in vivo and in vitro. Biochem. J. 1976, 159, 23–27. [Google Scholar] [CrossRef] [PubMed]
- Dellali, M. Utilisation D’indicateurs Microbiologiques et Biochimiques Chez Ruditapes Decussatus et Mytilus galloprovincialis Dans la Biosurveillance de la Lagune de Bizerte: Validation de Certains Biomarqueurs. Ph.D. Thesis, University of Carthage, Carthage, Tunisia, 2001. [Google Scholar]
- Trigui-El Menif, N. La Palourde Ruditapes decussatus (Linnée, 1758) des côtes Tunisiennes: Biométrie, Reproduction et Impact de L’environnement sur la Bioaccumulation en Métaux traces. Ph.D. Thesis, University of Tunis II, Tunis, Tunisia, 1995; 261p. [Google Scholar]
- Parache, A.; Massé, H. Croissance de Mytilus galloprovincialis (LMK) sur filières en mer ouverte en Méditerranée Nord-Occidentale. Haliotis 1986, 15, 163–171. [Google Scholar]
- Seed, R.; Suchanek, T.H. Population and Community Ecology of Mytilus. In The Mussel Mytilus; Gosling, E.G., Ed.; Elsevier: Amsterdam, The Netherlands, 1992; pp. 87–169. [Google Scholar]
- Khessiba, A. Premières Données sur la Moule de la Lagune de Bizerte: Potentialités Mytilicoles d’une Ferme Aquacole et Etude des Biomarqueurs; DEA, University of Carthage: Bizerte, Tunisia, 1999; 89p. [Google Scholar]
- Philippe, C.; Gregoir, A.F.; Thoré, E.S.J.; De Boeck, G.; Brendonck, L.; Pinceel, T. Protocol for Acute and Chronic Ecotoxicity Testing of the Turquoise Killifish Nothobranchius furzeri. J. Vis. Exp. 2018, 134, e57308. [Google Scholar] [CrossRef] [PubMed]
- Bates, P.A.; Kurtz, M.K.; Gottlieb, M.; Dwyer, D.M. Leishmania donovani: Generation of monospecific antibody reagents to soluble acid phosphatase. Exp. Parasitol. 1987, 64, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Doong, R.-A.; Lin, Y.-T. Characterization and distribution of polycyclic aromatic hydrocarbon contaminations in surface sediment and water from Gao-ping River, Taiwan. Water Res. 2004, 38, 1733–1744. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, O.; Santos, S.; Junqueira, A.; Jansen, A.M.; Cupolillo, E.; Campbell, D.; Zingales, B.; Coura, J.R. Populational heterogeneity of Brazilian Trypanosoma cruzi isolates revealed by the mini-exon and ribosomal spacers. Mem. Inst. Oswaldo Cruz 1999, 94, 195–197. [Google Scholar] [CrossRef] [PubMed][Green Version]
- White, L.E.; Andrews, T.J.; Hulette, C.; Richards, A.; Groelle, M.; Paydarfar, J.; Purves, D. Structure of the human sensorimotor system. I: Morphology and cytoarchitecture of the central sulcus. Cereb. Cortex 1997, 7, 18–30. [Google Scholar] [CrossRef]
- Gschwend, P.M.; Hites, R.A. Fluxes of polycyclic aromatic hydrocarbons to marine and lacustrine sediments in the northeastern United States. Geochim. Cosmochim. Acta 1981, 45, 2359–2367. [Google Scholar] [CrossRef]
- Mackay, D.; Shiu, W.Y.; Ma, K.C. Illustrated Handbook of Physical-Chemical Properties and Environmental Fate for Organic Chemicals; Lewis Publishers: Chelsea, UK, 1992; 697p. [Google Scholar]
- Hughes, J.B.; Beckles, D.M.; Chandra, S.D.; Ward, C.H. Utilization of bioremediation processes for the treatment of PAH-contaminated sediments. J. Ind. Microbiol. Biotechnol. 1997, 18, 152–160. [Google Scholar] [CrossRef]
- Ke, L.; Wong, T.W.; Wong, Y.; Tam, N.F. Fate of polycyclic aromatic hydrocarbon (PAH) contamination in a mangrove swamp in Hong Kong following an oil spill. Mar. Pollut. Bull. 2002, 45, 339–347. [Google Scholar] [CrossRef] [PubMed]
- Stark, A.; Brennecke, J.; Russell, R.B.; Cohen, S.M. Identification of Drosophila MicroRNA Targets. PLoS Biol. 2003, 1, e60. [Google Scholar] [CrossRef] [PubMed]
- Singh, H.; Bhattacharya, A.K. Susceptibility of Spodoptera litura to diflubenzuron. Ann. Pl. Protec. Sci. 2003, 11, 243–245. [Google Scholar]
- Akcha, F.; Izuel, C.; Venier, P.; Budzinski, H.; Burgeot, T.; Narbonne, J.F. Enzymatic biomarker measurement and study of DNA adduct formation in benzo [a] pyrene-contaminated mussels, Mytilus galloprovincialis. Aquat. Toxicol. 2000, 49, 269–287. [Google Scholar] [CrossRef]
- Banni, M.; Negri, A.; Dagnino, A.; Jebali, J.; Ameur, S.; Boussetta, H. Acute effects of benzo[a]pyrene on digestive gland enzymatic biomarkers and DNA damage on mussel Mytilus galloprovincialis. Ecotoxicol. Environ. Saf. 2010, 73, 842–848. [Google Scholar] [CrossRef]
- Bocquene, G. L’Acétylcholinestérase, Marqueur de Neurotoxicité. Application à la Surveillance des Effets Biologiques des Polluants Chez les Organismes Marins. Ph.D. Thesis, University of Montpellier, Montpellier, France, 1996; 254p. [Google Scholar]
- Dellali, M.; Hedfi, A.; Ben Ali, M.; Noureldeen, A.; Darwish, H.; Beyrem, H.; Gyedu-Ababio, T.; Dervishi, A.; Karachle, P.K.; Boufahja, F. Multi-biomarker approach in Mytilus galloprovincialis and Ruditapes decussatus as a predictor of pelago-benthic responses after exposure to Benzo[a]Pyrene. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2021, 249, 109141. [Google Scholar] [CrossRef]
- Moulton, C.A.; Fleming, W.J.; Purnell, C.E. Effects of two cholinesterase-inhibiting pesticides on freshwater mussels. Environ. Toxicol. Chem. 1996, 15, 131–137. [Google Scholar] [CrossRef]
- Dellali, M.; Romeo, M.; Gnassia-Barelli, M.; Aïssa, P. A Multivariate Data Analysis of the Clam Ruditapes decussatus as Sentinel Organism of the Bizerta Lagoon (Tunisia). Water Air Soil Pollut. 2004, 156, 131–144. [Google Scholar] [CrossRef]
- Kevin, B.; Temeyer Andrew, Y.; Li Kimberly, H.; Lohmeyer Andrew, C.; Chen Pia, U.; Olafson, D.W.; Sanson Lane Foil, D. Acetylcholinesterase mutation in diazinon resistant Haematobia irritans (L.) (Diptera), Muscidae. Vet. Parasitol. 2008, 154, 300–310. [Google Scholar]
- Laguerre, C.; Sanchez-Hernandez, J.C.; Köhler, H.R.; Triebskorn, R.; Capowiez, Y.; Rault, M.; Mazzia, C. B-type esterases in the snail Xeropicta derbentina. Environ. Pollut. 2009, 157, 199–207. [Google Scholar] [CrossRef]
- Richardson, C.; Alessi, D. The regulation of salt transport and blood pressure by the WNK-SPAK/OSR1 signalling pathway. J. Cell Sci. 2008, 121, 3293–3304. [Google Scholar] [CrossRef] [PubMed]
- Serafim, A.; Lopes, B.; Company, R.; Ferreira, A.M.; Bebianno, M.J. Comparative petroleum hydrocarbons levels and biochemical responses in mussels from hydrothermal vents (Bathymodiolus azoricus) and coastal environments (Mytilus galloprovincialis). Mar. Pollut. Bull. 2008, 57, 529–537. [Google Scholar] [CrossRef] [PubMed]
- Eertman, R.H.M.; Groenink, C.L.F.M.G.; Sandee, B.; Hummel, H.; Smaal, A.C. Response of the blue mussel Mytilus edulis L. following exposure to PAHs or contaminated sediment. Mar. Environ. Res. 1994, 39, 169–173. [Google Scholar] [CrossRef]
- Downs, C.A.; Shigenaka, G.; Fauth, J.E.; Robinson, C.E.; Huang, A. Cellular Physiological Assessment of Bivalves after Chronic Exposure to Spilled Exxon valdez Crude Oil Using a Novel Molecular Diagnostic Biotechnology. Environ. Sci. Technol. 2002, 36, 2987–2993. [Google Scholar] [CrossRef]
- Vieira, L.; Sousa, A.; Frasco, M.; Lima, I.; Morgado, F.; Guilhermino, L. Acute effects of Benzo[a]pyrene, anthracene and a fuel oil on biomarkers of the common goby Pomatoschistus microps (Teleostei, Gobiidae). Sci. Total Environ. 2008, 395, 87–100. [Google Scholar] [CrossRef]
- Bebianno, M.J.; Barreira, L.A. Polycyclic aromatic hydrocarbons concentrations and biomarker responses in the clam Ruditapes decussatus transplanted in the Ria Formosa lagoon. Ecotoxicol. Environ. Saf. 2009, 72, 1849–1860. [Google Scholar] [CrossRef]
- Khessiba, P.H.A.; Hoarau, P.; Gnassia-Barelli, M.; Aissa, P.; Roméo, M. Biochemical Response of the Mussel Mytilus galloprovincialis from Bizerta (Tunisia) to Chemical Pollutant Exposure. Arch. Environ. Contam. Toxicol. 2001, 40, 222–229. [Google Scholar] [CrossRef]
- Altenburger, R.; Segner, H.; van der Oost, R. PAHs—Prospects for the Assessment of Exposure and Effects in Aquatic Systems; PAHs: An Ecotoxicological Perspective; Wiley: Hoboken, NJ, USA, 2003; p. 297. [Google Scholar]
- Olga-Lopez Antonio, F.; Hernandez Lourdes, R.; Fernando, G.; Gloria, P.; Jose, L.S.; Tesifon, P.; Enrique, V. Changes in antioxidant enzymes in humans with long-term exposure to pesticides. Toxicol. Lett. 2007, 171, 146–153. [Google Scholar]
- Anguiano, V.; Llera-Herrera, R.; Rojas, E.; Vazquez Boucard, C. Subchronic organismal toxicity, cytotoxicity, genotoxicity, and feeding response of Pacific oyster (Crassostrea gigas) to lindane (gamma-HCH) exposure under experimental conditions. Environ. Toxicol. Chem. 2007, 26, 2192–2197. [Google Scholar] [CrossRef]
- Badraoui, R.; Allouche, M.; El Ouaer, D.; Siddiqui, A.J.; Ishak, S.; Hedfi, A.; Beyrem, H.; Pacioglu, O.; Rudayni, H.A.; Boufahja, F. Ecotoxicity of chrysene and phenanthrene on meiobenthic nematodes with a case study of Terschellingia longicaudata: Taxonomics, toxicokinetics, and molecular interactions modelling. Environ. Pollut. 2023, 316, 120459. [Google Scholar] [CrossRef]
- Allouche, M.; Ishak, S.; Ben Ali, M.; Hedfi, A.; Almalki, M.; Karachle, P.K.; Harrath, A.H.; Abu-Zied, R.H.; Badraoui, R.; Boufahja, F. Molecular interactions of polyvinyl chloride microplastics and beta-blockers (Diltiazem and Bisoprolol) and their effects on marine meiofauna: Combined in vivo and modeling study. J. Hazard. Mater. 2022, 431, 128609. [Google Scholar] [CrossRef] [PubMed]
- Hedfi, A.; Ben Ali, M.; Korkobi, M.; Allouche, M.; Harrath, A.H.; Beyrem, H.; Pacioglu, O.; Badraoui, R.; Boufahja, F. The exposure to polyvinyl chloride microplastics and chrysene induces multiple changes in the structure and functionality of marine meiobenthic communities. J. Hazard. Mater. 2022, 436, 129161. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).