Effects of Hormone, NEFA and SCFA on the Migration of Neutrophils and the Formation of Neutrophil Extracellular Traps in Dairy Cows
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. PMN Isolation
2.3. Cell Culture
2.4. PMN Transepithelial Migration Detection with Transwell
2.5. Visual Inspection of NETs
2.6. Statistical Analysis
3. Results
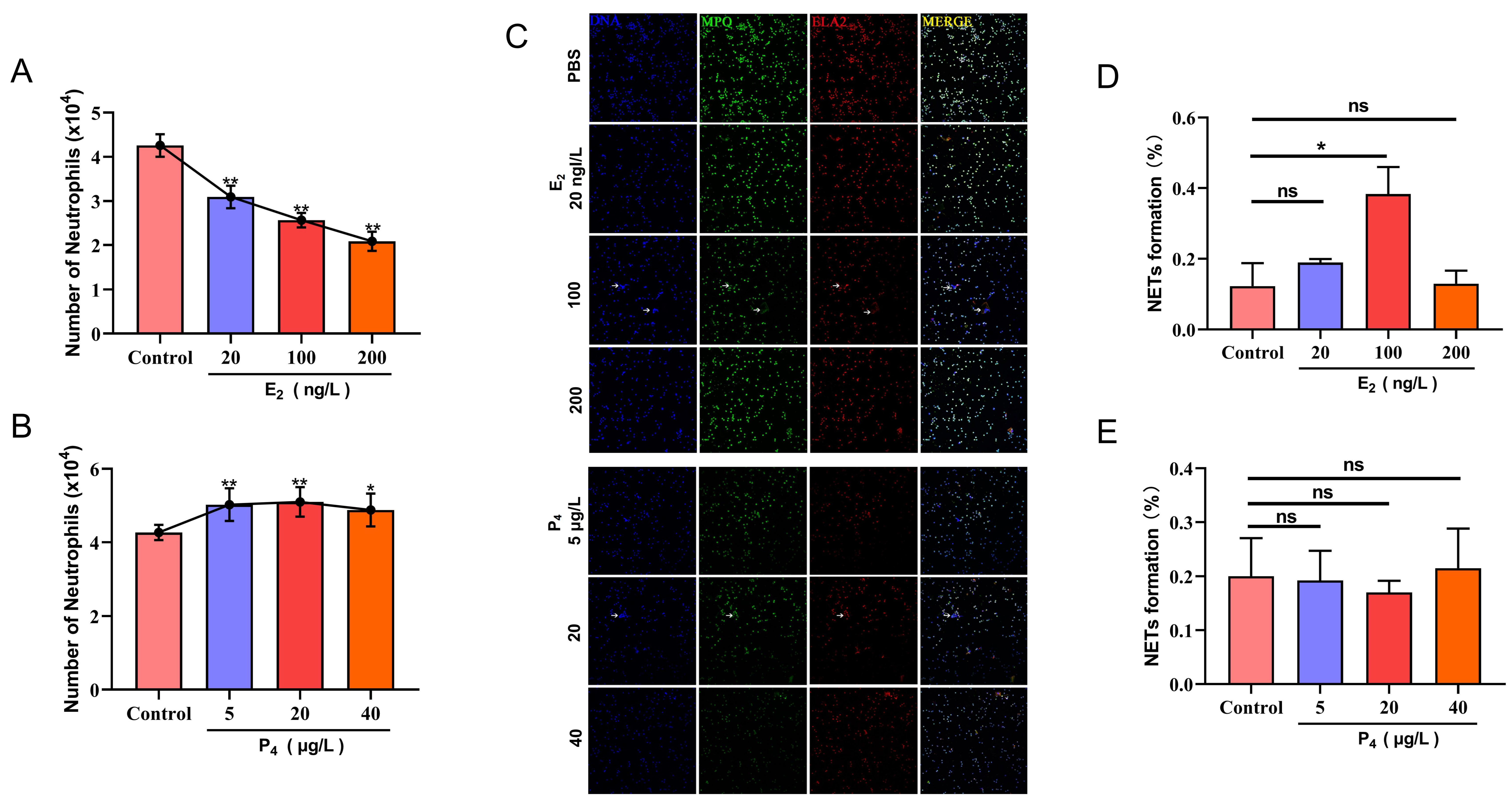
3.1. The Effects of E2 and P4 on PMN Migration and NET Formation
3.2. The Effects of OA, PA, and SA on PMN Migration and NET Formation
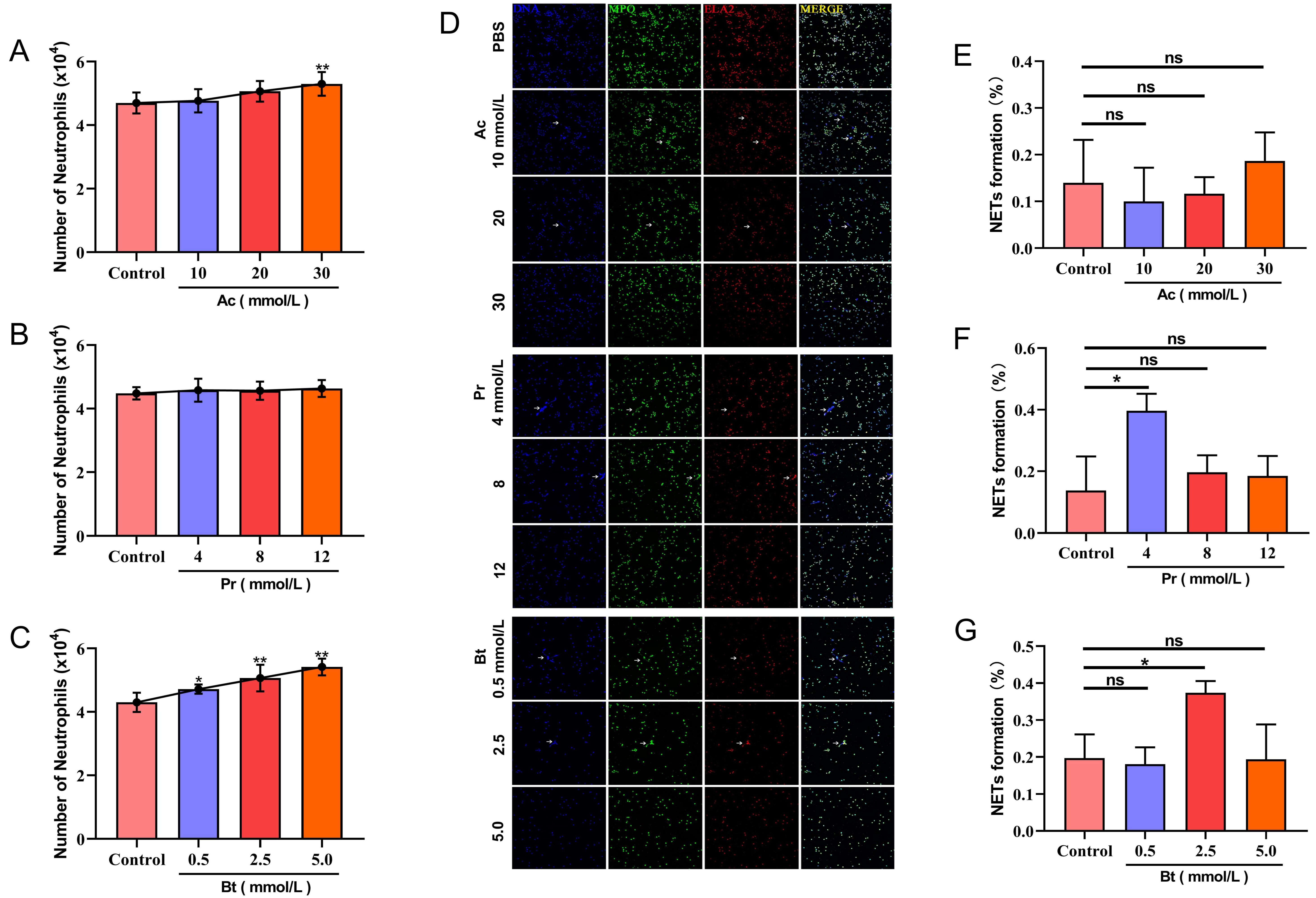
3.3. The Effects of Ac, Pr, and Bt on PMN Migration and NET Formation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Amulic, B.; Cazalet, C.; Hayes, G.L.; Metzler, K.D.; Zychlinsky, A. Neutrophil function: From mechanisms to disease. Annu. Rev. Immunol. 2012, 30, 459–489. [Google Scholar] [CrossRef] [PubMed]
- Paape, M.J.; Bannerman, D.D.; Zhao, X.; Lee, J.W. The bovine neutrophil: Structure and function in blood and milk. Vet. Res. 2003, 34, 597–627. [Google Scholar] [PubMed] [Green Version]
- Elazar, S.; Gonen, E.; Livneh-Kol, A.; Rosenshine, I.; Shpigel, N.Y. Neutrophil recruitment in endotoxin-induced murine mastitis is strictly dependent on mammary alveolar macrophages. Vet. Res. 2010, 41, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Papayannopoulos, V. Neutrophil extracellular traps in immunity and disease. Nat. Rev. Immunol. 2018, 18, 134–147. [Google Scholar] [CrossRef]
- Sordillo, L.M. New concepts in the causes and control of mastitis. J. Mammary Gland Biol. Neoplasia 2011, 16, 271–273. [Google Scholar] [CrossRef] [Green Version]
- Mulligan, F.J.; Doherty, M.L. Production diseases of the transition cow. Vet. J. 2008, 176, 3–9. [Google Scholar] [CrossRef]
- Cai, T.Q.; Weston, P.G.; Lund, L.A.; Brodie, B.; Wagner, W.C. Association between neutrophil functions and periparturient disorders in cows. Am. J. Vet. Res. 1994, 55, 934–943. [Google Scholar]
- Drackley, J.K. ADSA Foundation Scholar Award. Biology of dairy cows during the transition period: The final frontier? J. Dairy Sci. 1999, 82, 2259–2273. [Google Scholar] [CrossRef]
- Ingvartsen, K.L.; Moyes, K. Nutrition, immune function and health of dairy cattle. Animal 2013, 7 (Suppl. S1), 112–122. [Google Scholar] [CrossRef] [Green Version]
- Mallard, B.A.; Dekkers, J.C.; Ireland, M.J.; Leslie, K.E.; Sharif, S.; Vankampen, C.L.; Wagter, L.; Wilkie, B.N. Alteration in immune responsiveness during the peripartum period and its ramification on dairy cow and calf health. J. Dairy Sci. 1998, 81, 585–595. [Google Scholar] [CrossRef]
- Kehrli, M.E., Jr.; Shuster, D.E. Factors affecting milk somatic cells and their role in health of the bovine mammary gland. J. Dairy Sci. 1994, 77, 619–627. [Google Scholar] [CrossRef]
- van Knegsel, A.T.; de Vries Reilingh, G.; Meulenberg, S.; van den Brand, H.; Dijkstra, J.; Kemp, B.; Parmentier, H.K. Natural antibodies related to energy balance in early lactation dairy cows. J. Dairy Sci. 2007, 90, 5490–5498. [Google Scholar] [CrossRef] [PubMed]
- Henricks, D.M.; Dickey, J.F.; Hill, J.R.; Johnston, W.E. Plasma estrogen and progesterone levels after mating, and during late pregnancy and postpartum in cows. Endocrinology 1972, 90, 1336–1342. [Google Scholar] [CrossRef] [PubMed]
- Lamote, I.; Meyer, E.; De Ketelaere, A.; Duchateau, L.; Burvenich, C. Expression of the estrogen receptor in blood neutrophils of dairy cows during the periparturient period. Theriogenology 2006, 65, 1082–1098. [Google Scholar] [CrossRef]
- Hammon, D.S.; Evjen, I.M.; Dhiman, T.R.; Goff, J.P.; Walters, J.L. Neutrophil function and energy status in Holstein cows with uterine health disorders. Vet. Immunol. Immunopathol. 2006, 113, 21–29. [Google Scholar] [CrossRef]
- Ster, C.; Loiselle, M.C.; Lacasse, P. Effect of postcalving serum nonesterified fatty acids concentration on the functionality of bovine immune cells. J. Dairy Sci. 2012, 95, 708–717. [Google Scholar] [CrossRef]
- Sutton, J.D. Digestion and Absorption of Energy Substrates in the Lactating Cow. J. Dairy Sci. 1985, 68, 3376–3393. [Google Scholar] [CrossRef]
- Vinolo, M.A.; Hatanaka, E.; Lambertucci, R.H.; Newsholme, P.; Curi, R. Effects of short chain fatty acids on effector mechanisms of neutrophils. Cell Biochem. Funct. 2009, 27, 48–55. [Google Scholar] [CrossRef]
- Carretta, M.D.; Hidalgo, A.I.; Burgos, J.; Opazo, L.; Castro, L.; Hidalgo, M.A.; Figueroa, C.D.; Taubert, A.; Hermosilla, C.; Burgos, R.A. Butyric acid stimulates bovine neutrophil functions and potentiates the effect of platelet activating factor. Vet. Immunol. Immunopathol. 2016, 176, 18–27. [Google Scholar] [CrossRef]
- Weisz-Carrington, P.; Roux, M.E.; Lamm, M.E. Plasma cells and epithelial immunoglobulins in the mouse mammary gland during pregnancy and lactation. J. Immunol. 1977, 119, 1306–1307. [Google Scholar]
- Meegan, J.E.; Yang, X.; Coleman, D.C.; Jannaway, M.; Yuan, S.Y. Neutrophil-mediated vascular barrier injury: Role of neutrophil extracellular traps. Microcirculation 2017, 24, e12352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meng, H.; Yalavarthi, S.; Kanthi, Y.; Mazza, L.F.; Elfline, M.A.; Luke, C.E.; Pinsky, D.J.; Henke, P.K.; Knight, J.S. In Vivo Role of Neutrophil Extracellular Traps in Antiphospholipid Antibody-Mediated Venous Thrombosis. Arthritis Rheumatol. 2017, 69, 655–667. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, Z.; Wang, J.; Wang, Y.; Wang, C.; Liu, X.; Han, Z.; Fu, Y.; Yang, Z. Effects of Neutrophil Extracellular Traps on Bovine Mammary Epithelial Cells in vitro. Front. Immunol. 2019, 10, 1003. [Google Scholar] [CrossRef] [Green Version]
- Morris, D.G.; Waters, S.M.; McCarthy, S.D.; Patton, J.; Earley, B.; Fitzpatrick, R.; Murphy, J.J.; Diskin, M.G.; Kenny, D.A.; Brass, A.; et al. Pleiotropic effects of negative energy balance in the postpartum dairy cow on splenic gene expression: Repercussions for innate and adaptive immunity. Physiol. Genom. 2009, 39, 28–37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lacetera, N.; Scalia, D.; Bernabucci, U.; Ronchi, B.; Pirazzi, D.; Nardone, A. Lymphocyte functions in overconditioned cows around parturition. J. Dairy Sci. 2005, 88, 2010–2016. [Google Scholar] [CrossRef] [Green Version]
- Alarcon, P.; Manosalva, C.; Quiroga, J.; Belmar, I.; Alvarez, K.; Diaz, G.; Taubert, A.; Hermosilla, C.; Carretta, M.D.; Burgos, R.A.; et al. Oleic and Linoleic Acids Induce the Release of Neutrophil Extracellular Traps via Pannexin 1-Dependent ATP Release and P2X1 Receptor Activation. Front. Vet. Sci. 2020, 7, 260. [Google Scholar] [CrossRef]
- Ledderose, C.; Liu, K.; Kondo, Y.; Slubowski, C.J.; Dertnig, T.; Denicolo, S.; Arbab, M.; Hubner, J.; Konrad, K.; Fakhari, M.; et al. Purinergic P2X4 receptors and mitochondrial ATP production regulate T cell migration. J. Clin. Investig. 2018, 128, 3583–3594. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Qin, W.; Xu, X.; Xiong, Y.; Zhang, Y.; Zhang, H.; Sun, B. Endotoxin-induced autocrine ATP signaling inhibits neutrophil chemotaxis through enhancing myosin light chain phosphorylation. Proc. Natl. Acad. Sci. USA 2017, 114, 4483–4488. [Google Scholar] [CrossRef] [Green Version]
- Villagra-Blanco, R.; Silva, L.M.R.; Munoz-Caro, T.; Yang, Z.; Li, J.; Gartner, U.; Taubert, A.; Zhang, X.; Hermosilla, C. Bovine Polymorphonuclear Neutrophils Cast Neutrophil Extracellular Traps against the Abortive Parasite Neospora caninum. Front. Immunol. 2017, 8, 606. [Google Scholar] [CrossRef]
- Tan, J.; Mckenzie, C.; Potamitis, M.; Thorburn, A.N.; Mackay, C.R.; Macia, L. The role of short-chain fatty acids in health and disease. Adv. Immunol. 2014, 121, 91–119. [Google Scholar]
- Le Poul, E.; Loison, C.; Struyf, S.; Springael, J.Y.; Lannoy, V.; Decobecq, M.E.; Brezillon, S.; Dupriez, V.; Vassart, G.; Detheux, M.; et al. Functional Characterization of Human Receptors for Short Chain Fatty Acids and Their Role in Polymorphonuclear Cell Activation—ScienceDirect. J. Biol. Chem. 2003, 278, 25481–25489. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vinolo Marco, A.R.; Rodrigues Hosana, G.; Hatanaka, E.; Hebeda Cristina, B.; Farsky Sandra, H.P.; Curi, R. Short-chain fatty acids stimulate the migration of neutrophils to inflammatory sites. Clin. Sci. 2009, 117, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Vinolo, M.; John, F.G.; Suhasini, K.; George, D.; Karen, A.; Mohammad, B.Y.; Len, S.; Hawkins, P.T.; Rui, C.; Ko, B. SCFAs Induce Mouse Neutrophil Chemotaxis through the GPR43 Receptor. PLoS ONE 2011, 6, e21205. [Google Scholar] [CrossRef] [PubMed]
- Vinolo, M.A.; Rodrigues, H.G.; Nachbar, R.T.; Curi, R. Regulation of inflammation by short chain fatty acids. Nutrients 2011, 3, 858–876. [Google Scholar] [CrossRef] [Green Version]
- Ohbuchi, A.; Kono, M.; Takenokuchi, M.; Imoto, S.; Saigo, K. Acetate moderately attenuates the generation of neutrophil extracellular traps. Blood Res 2018, 53, 177–180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iniguez-Gutierrez, L.; Godinez-Mendez, L.A.; Fafutis-Morris, M.; Padilla-Arellano, J.R.; Corona-Rivera, A.; Bueno-Topete, M.R.; Rojas-Rejon, O.A.; Delgado-Rizo, V. Physiological concentrations of short-chain fatty acids induce the formation of neutrophil extracellular traps in vitro. Int. J. Immunopathol. Pharmacol. 2020, 34, 2058738420958949. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lv, G.; Wang, H.; Zhou, X.; Lian, S.; Wang, J.; Wu, R. Effects of Hormone, NEFA and SCFA on the Migration of Neutrophils and the Formation of Neutrophil Extracellular Traps in Dairy Cows. Animals 2022, 12, 1190. https://doi.org/10.3390/ani12091190
Lv G, Wang H, Zhou X, Lian S, Wang J, Wu R. Effects of Hormone, NEFA and SCFA on the Migration of Neutrophils and the Formation of Neutrophil Extracellular Traps in Dairy Cows. Animals. 2022; 12(9):1190. https://doi.org/10.3390/ani12091190
Chicago/Turabian StyleLv, Guanxin, Hai Wang, Xiechen Zhou, Shuai Lian, Jianfa Wang, and Rui Wu. 2022. "Effects of Hormone, NEFA and SCFA on the Migration of Neutrophils and the Formation of Neutrophil Extracellular Traps in Dairy Cows" Animals 12, no. 9: 1190. https://doi.org/10.3390/ani12091190
APA StyleLv, G., Wang, H., Zhou, X., Lian, S., Wang, J., & Wu, R. (2022). Effects of Hormone, NEFA and SCFA on the Migration of Neutrophils and the Formation of Neutrophil Extracellular Traps in Dairy Cows. Animals, 12(9), 1190. https://doi.org/10.3390/ani12091190





