Evaluation of Behavior and Affective State of Different-Parity Sows with Strong/Weak Pupil Light Reflex
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Management
2.2. PLR Test
2.3. Behavioral Observations
2.4. Sucrose/Quinine Response Test
2.5. Novel Object Test
2.6. Statistical Analyses
3. Results
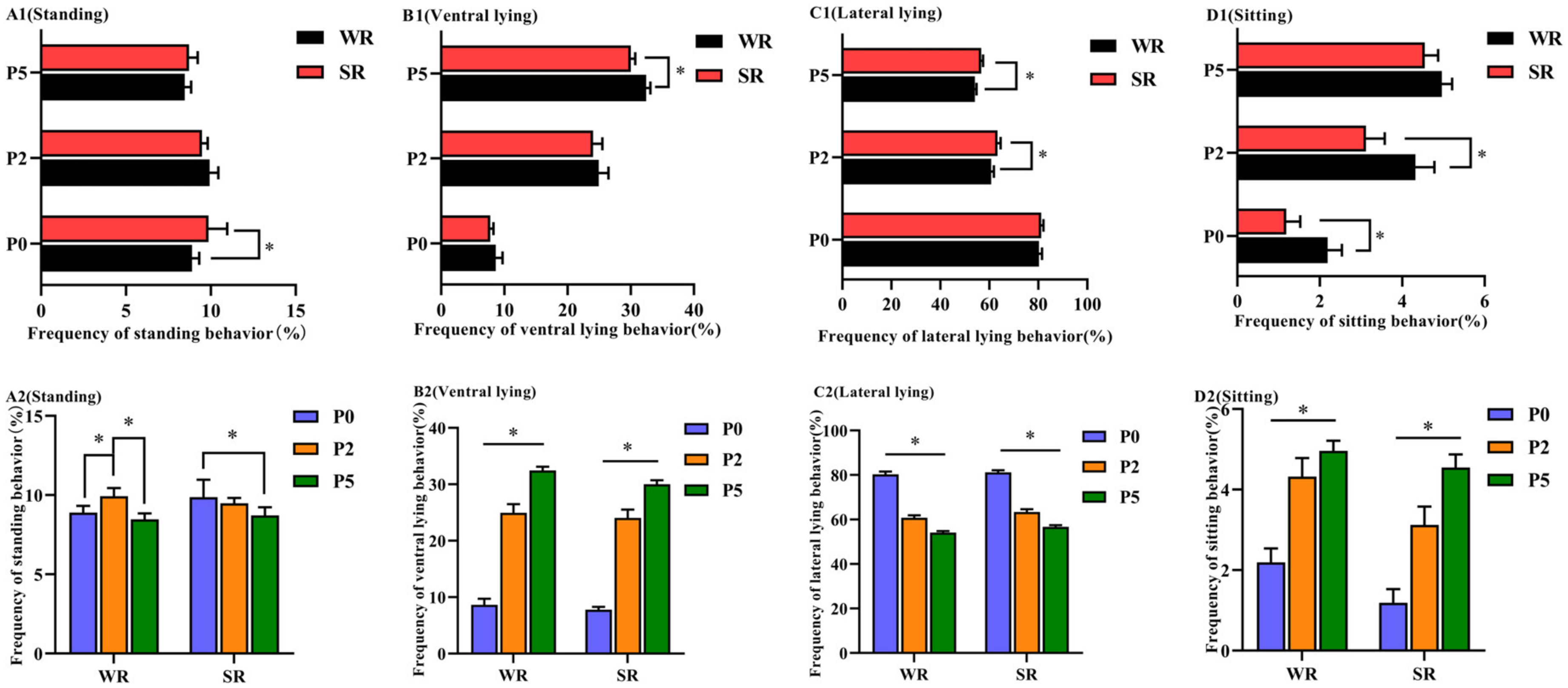
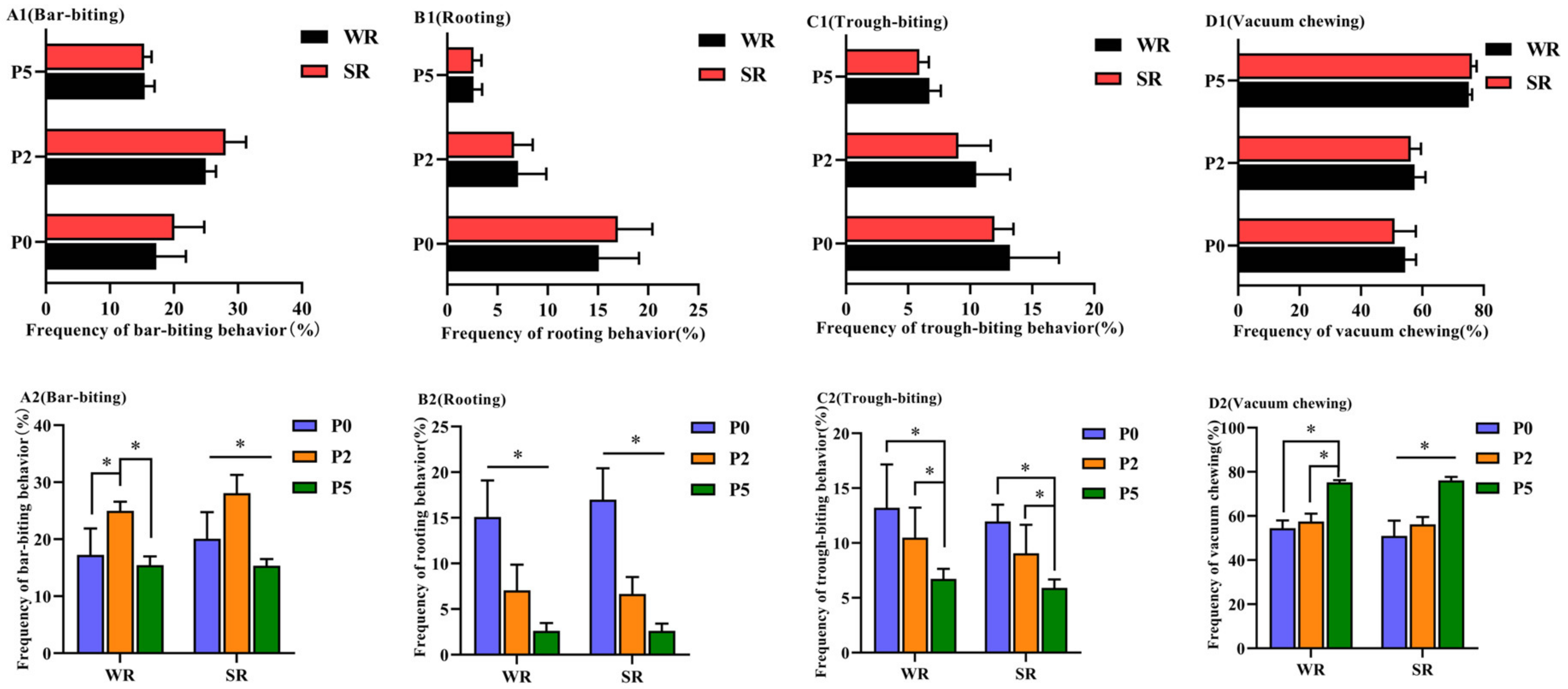
3.1. Behavioral Differences between Different-Parity and PLR Groups
3.1.1. Posture of Confined Sows with Different Parity and Strong/Weak PLR
3.1.2. Oral Behaviors of Confined Sows with Different Parity and Strong/Weak PLR
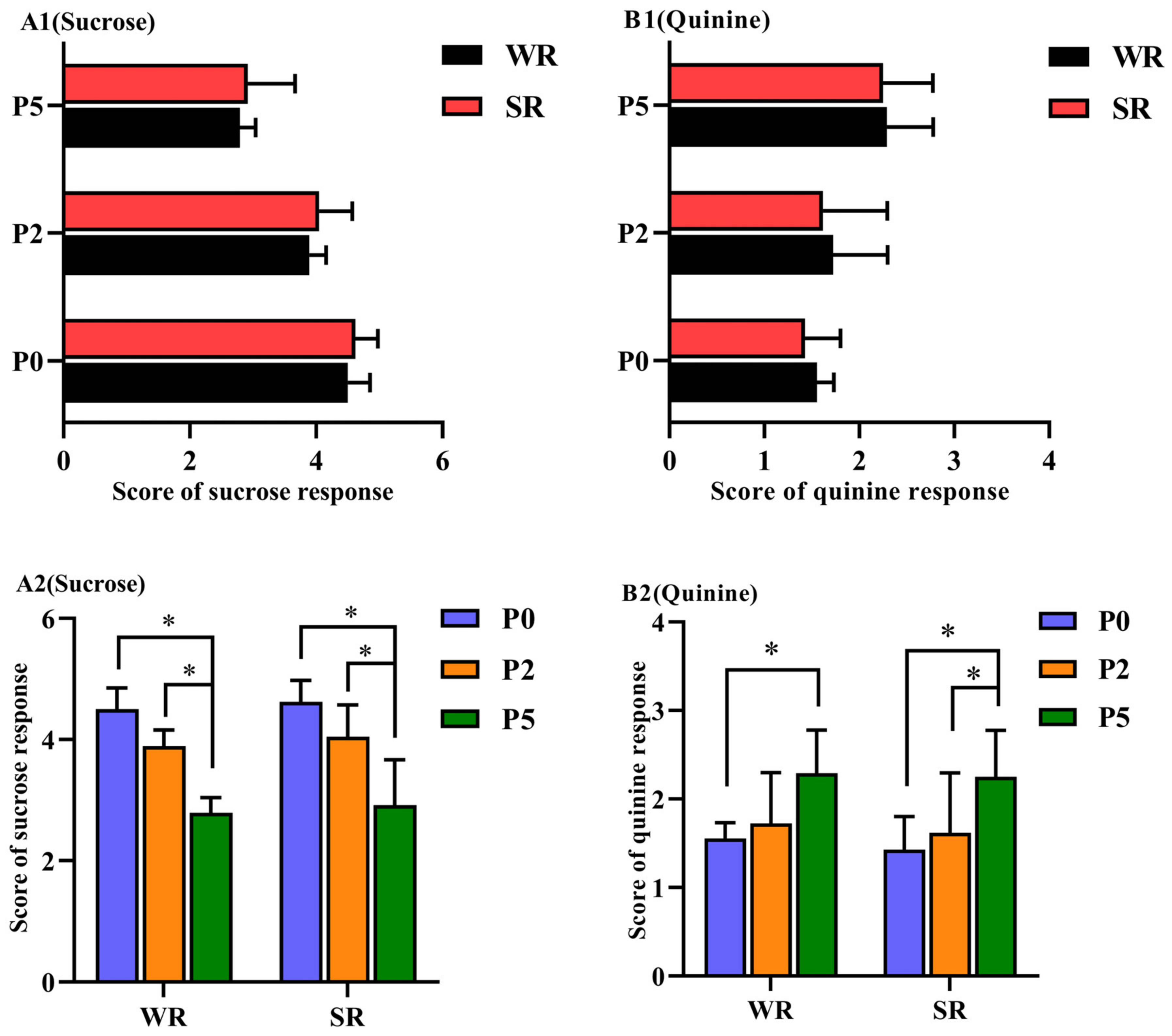
3.2. Sucrose/Quinine Response
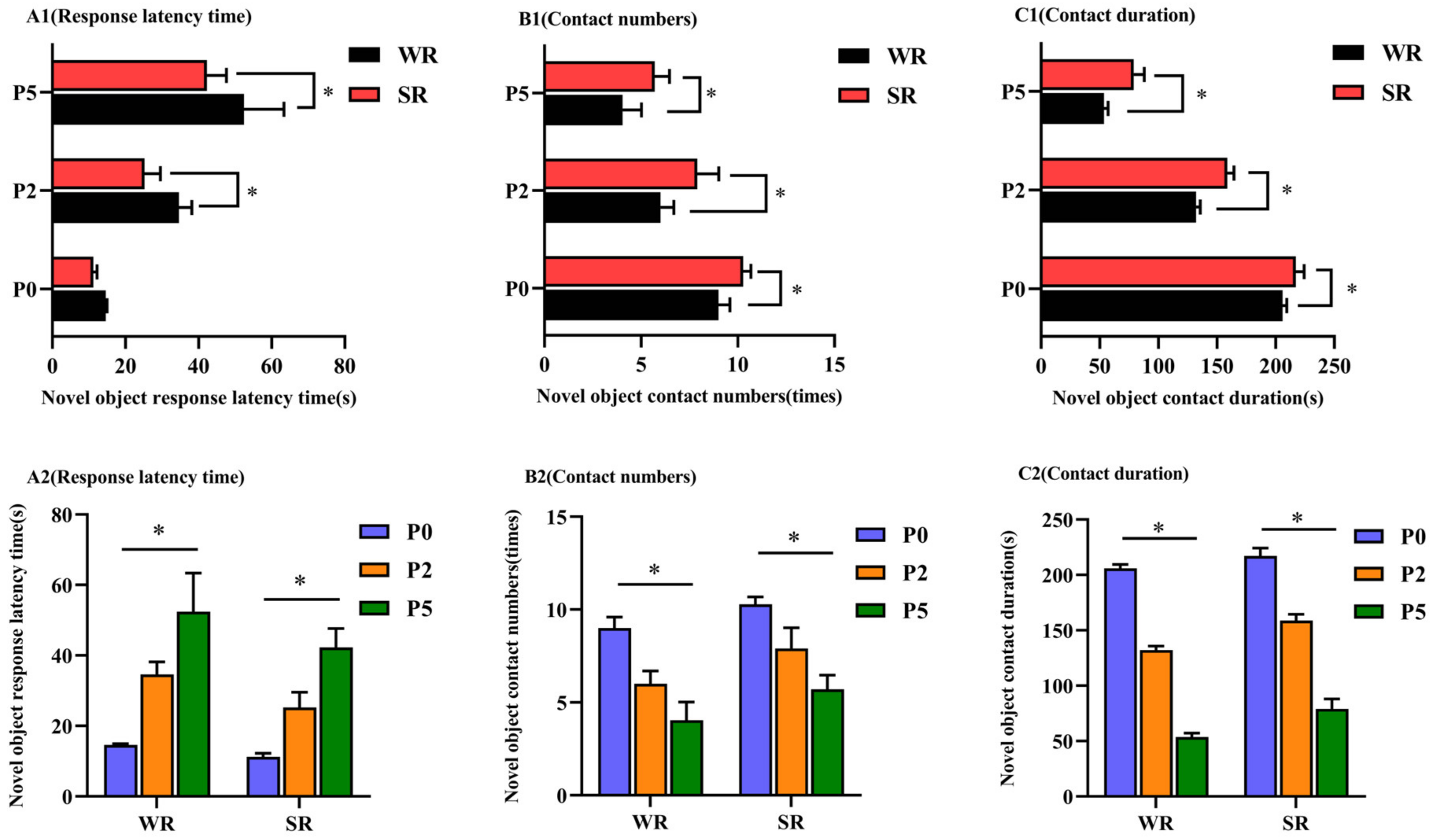
3.3. Novel Object Test
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Nicolaisen, T.; Risch, B.; Lühken, E.; van Meegen, C.; Fels, M.; Kemper, N. Comparison of three different farrowing systems: Skin lesions and behaviour of sows with special regard to nursing behaviour in a group housing system for lactating sows. Animal 2019, 13, 2612–2620. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yun, J.; Valros, A. Benefits of Prepartum Nest-building Behaviour on Parturition and Lactation in Sows—A Review. Asian-Australas. J. Anim. Sci. 2015, 28, 1519. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.; Song, P.; Yan, H.; Zhang, L.; Wang, L.; Zhao, F.; Gao, H.; Hou, X.; Shi, L.; Li, B.; et al. A Comparison of the Behavior, Physiology, and Offspring Resilience of Gestating Sows When Raised in a Group Housing System and Individual Stalls. Animals 2021, 11, 2076. [Google Scholar] [CrossRef]
- Salak-Johnson, J.L. Social status and housing factors affect reproductive performance of pregnant sows in groups. Mol. Reprod. Dev. 2017, 84, 905–913. [Google Scholar] [CrossRef] [Green Version]
- Cooper, J.J.; Mason, G.J. The identification of abnormal behaviour and behavioural problems in stabled horses and their relationship to horse welfare: A comparative review. Equine Vet. J. 1998, 30, 5–9. [Google Scholar] [CrossRef] [PubMed]
- Mason, G.J. Stereotypies—A critical review. Anim. Behav. 1991, 41, 1015–1037. [Google Scholar] [CrossRef] [Green Version]
- Lawrence, A.B.; Claudia, T.E.M. A review of behavioral factors involved in the development and continued performance of stereotypic behaviors in pigs. J. Anim. Sci. 1993, 10, 2815–2825. [Google Scholar] [CrossRef]
- Li, J. Study on Stereotyped Behavior of Sows and Its Relationship to Opioid Receptor Genes. Master’s Thesis, Northest Agricultural University, Harbin, China, 2003. [Google Scholar]
- Novak, J.; Bailoo, J.D.; Melotti, L.; Würbel, H. Effect of Cage-Induced Stereotypies on Measures of Affective State and Recurrent Perseveration in CD-1 and C57BL/6 Mice. PLoS ONE 2016, 11, e0153203. [Google Scholar] [CrossRef] [Green Version]
- Pomerantz, O.; Terkel, J.; Suomi, S.J.; Paukner, A. Stereotypic head twirls, but not pacing, are related to a ‘pessimistic’-like judgment bias among captive tufted capuchins (Cebus apella). Anim. Cogn. 2012, 15, 689–698. [Google Scholar] [CrossRef] [Green Version]
- Zhang, M.Y.; Li, X.; Zhang, X.H.; Liu, H.G.; Li, J.H.; Bao, J. Effects of confinement duration and parity on behavioural responses and the degree of psychological fear in pregnant sows. Appl. Anim. Behav. Sci. 2017, 193, 21–28. [Google Scholar] [CrossRef]
- Liu, X. Study on Effect of Long-Term Confinement on Behavior and miRNA and Depression Related Protein of Sow. Master’s Thesis, Noreheast Agricultural University, Harbin, China, 2018. [Google Scholar]
- Laeng, B.; Sirois, S.; Gredebäck, G. Pupillometry: A Window to the Preconscious? Perspect. Psychol. Sci. 2012, 7, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Bao, J.; Li, X.; Lv, F.L.; Chen, D.H.; Li, J.H. Prolonged latency of pupillary light reflex in confined sows: Possible stress-related symptom? J. Vet. Behav. Clin. Appl. Res. 2013, 8, 475–478. [Google Scholar] [CrossRef]
- Drouet, A.; Carvalho, A.D.; Mage, F.; Guilloton, L.; Felten, D. Pupille tonique d’Adie et migraine: Une association fortuite? J. Français D’ophtalmol. 2008, 31, 185.e1–185.e3. [Google Scholar] [CrossRef]
- Trachtman, J.N. Post-traumatic stress disorder and vision. Optometry 2010, 81, 240–252. [Google Scholar] [CrossRef] [PubMed]
- Bär, K.J.; Greiner, W.; Jochum, T.; Friedrich, M.; Wagner, G.; Sauer, H. The influence of major depression and its treatment on heart rate variability and pupillary light reflex parameters. J. Affect. Disord. 2004, 82, 245–252. [Google Scholar] [CrossRef]
- Li, X.; Sun, H.; Zhang, L.; Liu, H.; Li, J.; Wang, C.; Zhang, M.; Bao, J. Technical Note: Effects of age and confinement on pupillary light reflex in sows1. J. Anim. Sci. 2019, 97, 2009–2014. [Google Scholar] [CrossRef]
- Wang, L.; Yu, L.; Yin, G. Physiology and miRNA expression in confined sows with different pupillary light reflex. Ital. J. Anim. Sci. 2021, 20, 526–538. [Google Scholar] [CrossRef]
- Désiré, L.; Boissy, A.; Veissier, I. Emotions in farm animals: A new approach to animal welfare in applied ethology. Behav. Processes 2002, 60, 165–180. [Google Scholar] [CrossRef]
- Burgdorf, J.; Panksepp, J. The neurobiology of positive emotions. Neurosci. Biobehav. Rev. 2006, 30, 173–187. [Google Scholar] [CrossRef]
- Paul, E.S.; Harding, E.J.; Mendl, M. Measuring emotional processes in animals: The utility of a cognitive approach. Neurosci. Biobehav. Rev. 2005, 29, 469–491. [Google Scholar] [CrossRef]
- Hemsworth, P.H.; Mellor, D.J.; Cronin, G.M.; Tilbrook, A.J. Scientific assessment of animal welfare. N. Z. Vet. J. 2015, 63, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Kirkden, R.D.; Pajor, E.A. Using preference, motivation and aversion tests to ask scientific questions about animals’ feelings. Appl. Anim. Behav. Sci. 2006, 97, 29–47. [Google Scholar] [CrossRef]
- Kojima, M.; Shioiri, T.; Hosoki, T.; Kitamura, H.; Bando, T.; Someya, T. Pupillary light reflex in panic disorder. A trial using audiovisual stimulation. Eur. Arch. Psychiatry Clin. Neurosci. 2004, 254, 242–244. [Google Scholar] [CrossRef] [PubMed]
- Joseph, M.H.; Datla, K.; Young, A.M. The interpretation of the measurement of nucleus accumbens dopamine by in vivo dialysis: The kick, the craving or the cognition? Neurosci. Biobehav. Rev. 2003, 27, 527–541. [Google Scholar] [CrossRef]
- Willner, P. Validity, reliability and utility of the chronic mild stress model of depression: A 10-year review and evaluation. Psychopharmacology 1997, 134, 319–329. [Google Scholar] [CrossRef]
- Willner, P. Animal models of depression: An overview. Pharmacol. Ther. 1990, 45, 425–455. [Google Scholar] [CrossRef]
- Paul, I.A.; English, J.A.; Halaris, A. Sucrose and quinine intake by maternally-deprived and control rhesus monkeys. Behav. Brain Res. 2000, 112, 127–134. [Google Scholar] [CrossRef]
- Hellekant, G.; Danilova, V. Taste in domestic pig, Sus scrofa. J. Anim. Physiol. Anim. Nutr. 2010, 82, 8–24. [Google Scholar] [CrossRef]
- Damiano, C.R.; Aloi, J.; Burrus, C.; Garbutt, J.C.; Kampov-Polevoy, A.B.; Dichter, G.S. Intact Hedonic Responses to Sweet Tastes in Autism Spectrum Disorder. Res. Autism Spectr. Disord. 2014, 8, 230–236. [Google Scholar] [CrossRef] [Green Version]
- Willner, P.; Towell, A.; Sampson, D.; Sophokleous, S.; Muscat, R. Reduction of sucrose preference by chronic unpredictable mild stress, and its restoration by a tricyclic antidepressant. Psychopharmacology 1987, 93, 358–364. [Google Scholar] [CrossRef]
- Box, H.O. Responsiveness to environmental change: Interrelationships among parameters. In Primate Responses to Environmental Change; Springer: Dordrecht, The Netherlands, 1991. [Google Scholar]
- Sabbatini, G.; Stammati, M.; Tavares, M.C.; Visalberghi, E. Response toward novel stimuli in a group of tufted capuchins (Cebus libidinosus) in Brasília National Park, Brazil. Am. J. Primatol. 2007, 69, 457–470. [Google Scholar] [CrossRef] [PubMed]
- Boissy, A. Fear and fearfulness in animals. Q. Rev. Biol. 1995, 70, 165–191. [Google Scholar] [CrossRef] [PubMed]
- Dulawa, S.C.; Grandy, D.K.; Low, M.J.; Paulus, M.P.; Geyer, M.A. Dopamine D4 receptor-knock-out mice exhibit reduced exploration of novel stimuli. J. Neurosci. 1999, 19, 9550–9556. [Google Scholar] [CrossRef] [PubMed]
- Thodberg, K.; Jensen, K.H.; Herskin, M.S. A general reaction pattern across situations in prepubertal gilts. Appl. Anim. Behav. Sci. 1999, 63, 103–119. [Google Scholar] [CrossRef]
- Binda, P.; Gamlin, P.D. Renewed Attention on the Pupil Light Reflex. Trends Neurosci. 2017, 40, 455–457. [Google Scholar] [CrossRef]
- Bradley, M.M.; Sapigao, R.G.; Lang, P.J. Sympathetic ANS modulation of pupil diameter in emotional scene perception: Effects of hedonic content, brightness, and contrast. Psychophysiology 2017, 54, 1419–1435. [Google Scholar] [CrossRef]
- Liu, H.; Yi, R.; Wang, C.; Zhao, P.; Zhang, M.; Xu, S.; Bao, J. Behavior and physiology of two different sow breeds in a farrowing environment during late 35-day lactation. PLoS ONE 2018, 13, e0197152. [Google Scholar] [CrossRef] [Green Version]
- Kandola, A.; Lewis, G.; Osborn, D.P.J.; Stubbs, B.; Hayes, J.F. Depressive symptoms and objectively measured physical activity and sedentary behaviour throughout adolescence: A prospective cohort study. Lancet Psychiatry 2020, 7, 262–271. [Google Scholar] [CrossRef] [Green Version]
- Correll, C.U.; Schooler, N.R. Negative Symptoms in Schizophrenia: A Review and Clinical Guide for Recognition, Assessment, and Treatment. Neuropsychiatr. Dis. Treat. 2020, 16, 519–534. [Google Scholar] [CrossRef] [Green Version]
- Hulbert, L.E.; McGlone, J.J. Evaluation of drop versus trickle-feeding systems for crated or group-penned gestating sows. J. Anim. Sci. 2006, 84, 1004–1014. [Google Scholar] [CrossRef]
- Von Borell, E.; Hurnik, J.F. The effect of haloperidol on the performance of stereotyped behavior in sows. Life Sci. 1991, 49, 309–314. [Google Scholar] [CrossRef]
- Barwick, V.S.; Jones, D.H.; Richter, J.T.; Hicks, P.B.; Young, K.A. Subthalamic nucleus microinjections of 5-HT2 receptor antagonists suppress stereotypy in rats. Neuroreport 2000, 11, 267–270. [Google Scholar] [CrossRef] [PubMed]
- Rincón-Cortés, M.; Grace, A.A. Antidepressant effects of ketamine on depression-related phenotypes and dopamine dysfunction in rodent models of stress. Behav. Brain Res. 2020, 379, 112367. [Google Scholar] [CrossRef]
- Fox, M.E.; Lobo, M.K. The molecular and cellular mechanisms of depression: A focus on reward circuitry. Mol. Psychiatry 2019, 24, 1798–1815. [Google Scholar] [CrossRef] [PubMed]
- Sagioglou, C.; Greitemeyer, T. Individual differences in bitter taste preferences are associated with antisocial personality traits. Appetite 2016, 96, 299–308. [Google Scholar] [CrossRef]
- Zhu, L.L.; Shen, J.D.; Wang, B.Y.; Lu, S.F.; Ming, B.; Xu, E.P.; Li, Y.C.; Fang, X.Y. Depressive-like behaviors are induced by chronic liver injury in male and female mice. Neurosci. Lett. 2020, 718, 134750. [Google Scholar] [CrossRef]
- Jiang, S.; Zhang, Q.A.; Guo, Q.; Di, Z. The glutamatergic system and astrocytic impairment in rat hippocampus: A comparative study of underlying etiology and pathophysiology of depression. J. Integr. Neurosci. 2019, 18, 387–392. [Google Scholar] [CrossRef]
- Das, L.; Patel, B.; Patri, M. Adolescence benzo[a]pyrene treatment induces learning and memory impairment and anxiolytic like behavioral response altering neuronal morphology of hippocampus in adult male Wistar rats—ScienceDirect. Toxicol. Rep. 2019, 6, 1104–1113. [Google Scholar] [CrossRef]
- Olney, J.J.; Marshall, S.A.; Thiele, T.E. Assessment of depression-like behavior and anhedonia after repeated cycles of binge-like ethanol drinking in male C57BL/6J mice. Pharmacol. Biochem. Behav. 2018, 168, 1–7. [Google Scholar] [CrossRef]
- Scinska, A.; Swiecicki, L.; Korkosz, I.; Mierzejewski, P.; Kolaczkowski, M. Immobility in the tail suspension test predicts quinine but not saccharin intake in mice. Neurosci. Lett. 2009, 461, 285–288. [Google Scholar] [CrossRef]
- Forkman, B.; Boissy, A.; Meunier-Salaün, M.C.; Canali, E.; Jones, R.B. A critical review of fear tests used on cattle, pigs, sheep, poultry and horses. Physiol. Behav. 2007, 92, 340–374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Proyer, R.T.; Gander, F.; Wellenzohn, S.; Ruch, W. Positive psychology interventions in people aged 50-79 years: Long-term effects of placebo-controlled online interventions on well-being and depression. Aging Ment Health 2014, 18, 997–1005. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fehnel, S.E.; Forsyth, B.H.; DiBenedetti, D.B.; Danchenko, N.; François, C.; Brevig, T. Patient-centered assessment of cognitive symptoms of depression. CNS Spectr. 2016, 21, 43–52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dickson, J.M.; Moberly, N.J.; O’Dea, C.; Field, M. Goal Fluency, Pessimism and Disengagement in Depression. PLoS ONE 2016, 11, e0166259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosenström, T.; Jokela, M. Reconsidering the definition of Major Depression based on Collaborative Psychiatric Epidemiology Surveys. J. Affect. Disord. 2017, 207, 38–46. [Google Scholar] [CrossRef] [Green Version]
| Number of Sows | WR | SR |
|---|---|---|
| P0 | n = 6 | n = 7 |
| P2 | n = 6 | n = 7 |
| P5 | n = 8 | n = 8 |
| Categories of Postures | Definition |
|---|---|
| standing | The stretched limbs maintain an upright posture of the body. |
| ventral lying | The chest and belly touch the ground, and the forelimbs extend forward or under the body. |
| lateral lying | The entire flank of the sow contacts with the ground, and all four limbs can be seen. |
| sitting | The buttocks of the hindlegs touch the ground, hind limbs are angled, and the forelimbs are upright to support the forebody weight. |
| Categories of Oral Behaviors | Definition |
|---|---|
| bar-biting | Licking, nibbling, sniffing, and lifting the arched railings. |
| rooting | Close to or touching the ground with sniffing, licking, gnawing, or arching. |
| trough-biting | Licking, nibbling, sniffing, and gnawing the feeding trough. |
| vacuum-chewing | Chewing motion when there appears to be no food in the mouth. |
| Score | Definition |
|---|---|
| 1 | Sows avoid the source of the sucrose injection, their snout is close to the ground or moving back. |
| 2 | Sucrose is injected into the mouth of sows, but sows do not drink sucrose, and there are no marked or only minor changes of the snout. |
| 3 | Sow’s posture suggests it is drinking sucrose, and the snout shows obvious tension and closure of the jaw, but the frequency of tongue extension is low. |
| 4 | Sows show enjoyment by opening their mouths and sticking out their tongues, with a faster frequency of tongue extension and a larger range in tension and closure of the jaw. |
| 5 | Sows quickly stretch their tongues and approach the jet source, their tension and closure of the jaw are quickly with fluid sputtering, and the frequency of blinking is higher. |
| Score | Definition |
|---|---|
| 1 | The jaw of sows is wide open and they rub their snout, accompanied by shaking the head and trampling on the forelimbs. |
| 2 | The opening and closing of the jaw of sows causes fluid to flow out of the mouth, alternately showing enjoyment or disgust behaviors. |
| 3 | Sows stick out their tongues and lick, the frequency of tension and closure of the jaw is higher. |
| Categories | Definition |
|---|---|
| Contact numbers | Number of times the sow sniffed, bit, or manipulated the novel object. |
| Response latency time | The time between the novel object being placed in the crate and first contact by the sow. |
| Contact duration | The time from the first contact with the novel object in the crate to the time when the sow leaves the novel object. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Yu, L.; Yin, G. Evaluation of Behavior and Affective State of Different-Parity Sows with Strong/Weak Pupil Light Reflex. Animals 2022, 12, 1184. https://doi.org/10.3390/ani12091184
Zhang J, Yu L, Yin G. Evaluation of Behavior and Affective State of Different-Parity Sows with Strong/Weak Pupil Light Reflex. Animals. 2022; 12(9):1184. https://doi.org/10.3390/ani12091184
Chicago/Turabian StyleZhang, Jinyue, Langchao Yu, and Guoan Yin. 2022. "Evaluation of Behavior and Affective State of Different-Parity Sows with Strong/Weak Pupil Light Reflex" Animals 12, no. 9: 1184. https://doi.org/10.3390/ani12091184
APA StyleZhang, J., Yu, L., & Yin, G. (2022). Evaluation of Behavior and Affective State of Different-Parity Sows with Strong/Weak Pupil Light Reflex. Animals, 12(9), 1184. https://doi.org/10.3390/ani12091184





