Ellagic Acid Improves Antioxidant Capacity and Intestinal Barrier Function of Heat-Stressed Broilers via Regulating Gut Microbiota
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
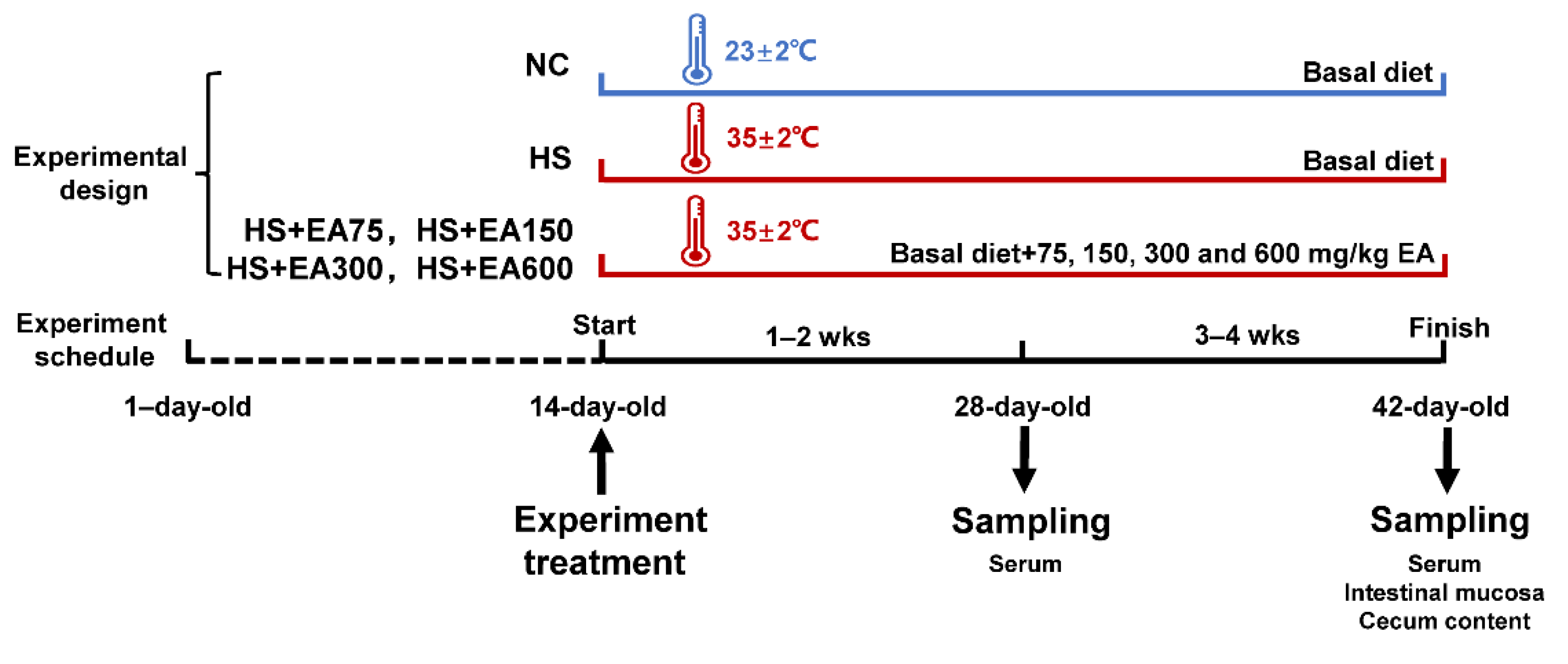
2.1. Animals and Experimental Design
2.2. Sample Collection
2.2.1. Serum Collection
2.2.2. Intestinal Mucosa Collection
2.2.3. Cecum Content Collection
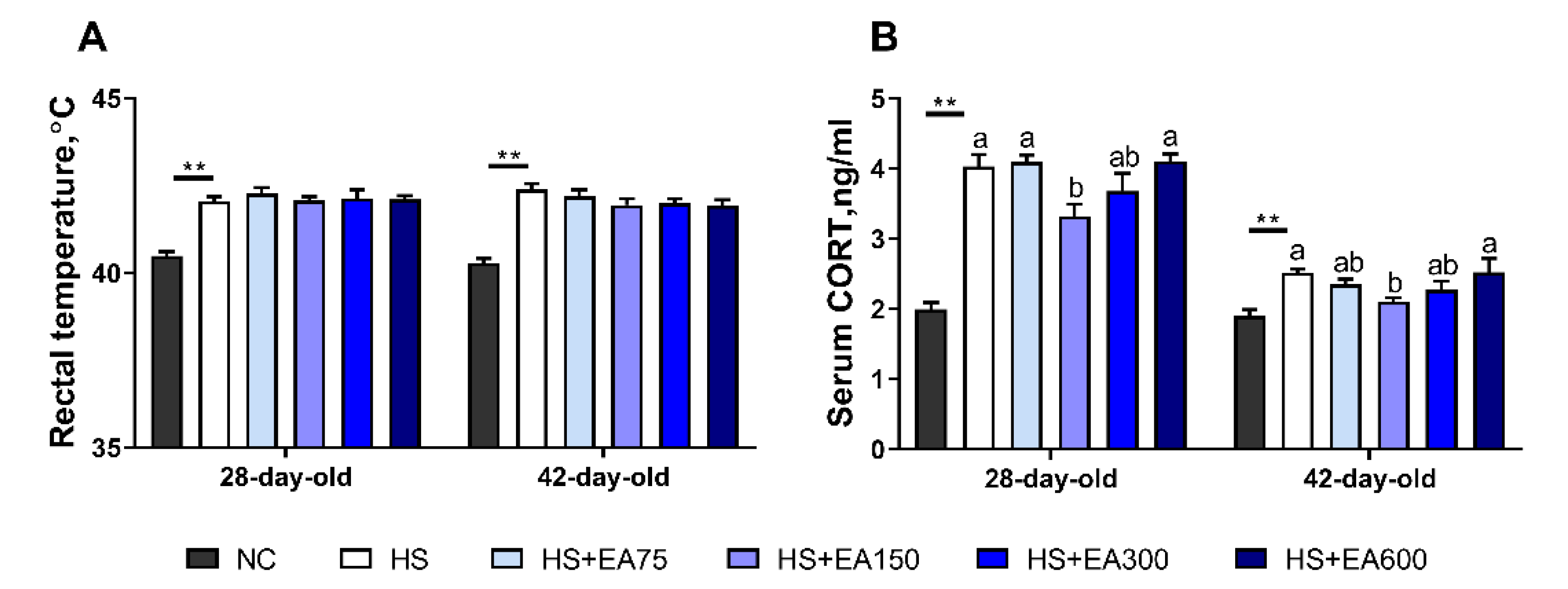
2.3. Measurement of Serum Corticosterone and Rectal Temperature
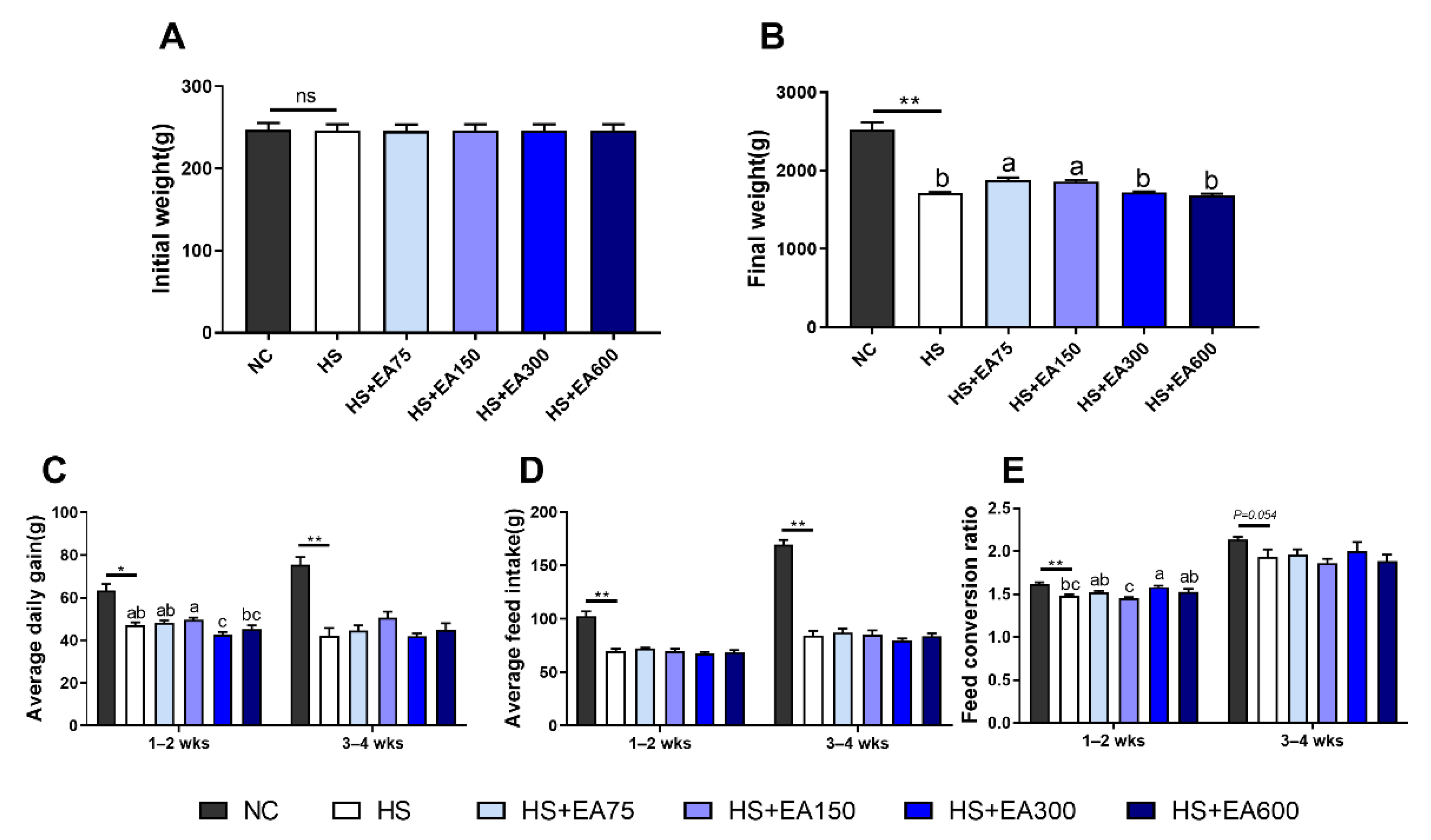
2.4. Growth Performance Determination
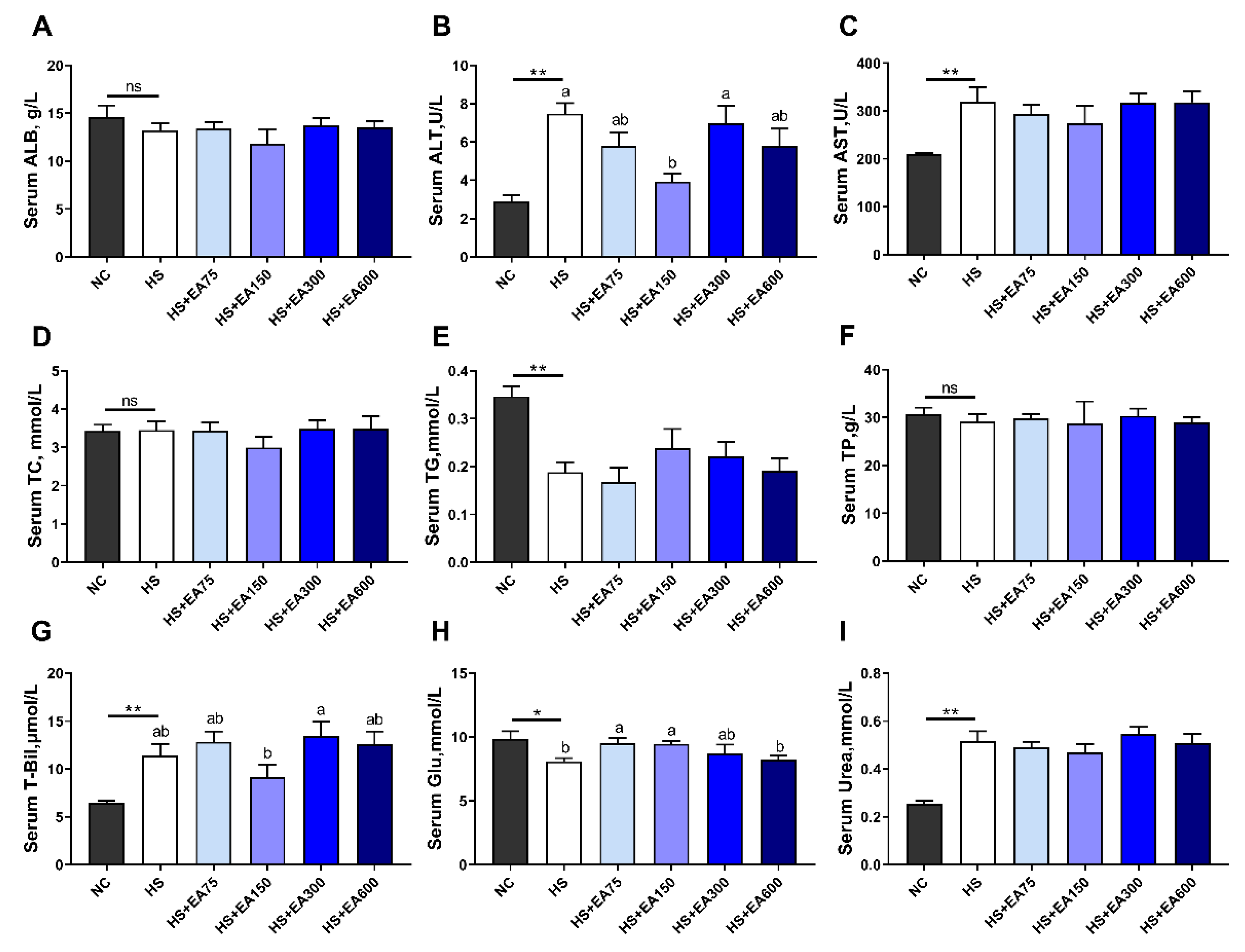
2.5. Measurement of Serum Biochemical Parameters
2.6. Measurement of Antioxidant Parameters in Serum and Intestinal Mucosa
2.7. Real-Time Quantitative Polymerase Chain Reaction (RT-PCR)
2.8. Analysis of Intestinal Microbial Community
2.9. Statistical Analysis
3. Results
3.1. Validation of Heat Stress Model of the Broilers
3.2. Effects of Dietary EA on the Growth Performance of Heat-Stressed Broilers
3.3. Effects of Dietary EA on Serum Biochemical Parameters of Heat-Stressed Broilers
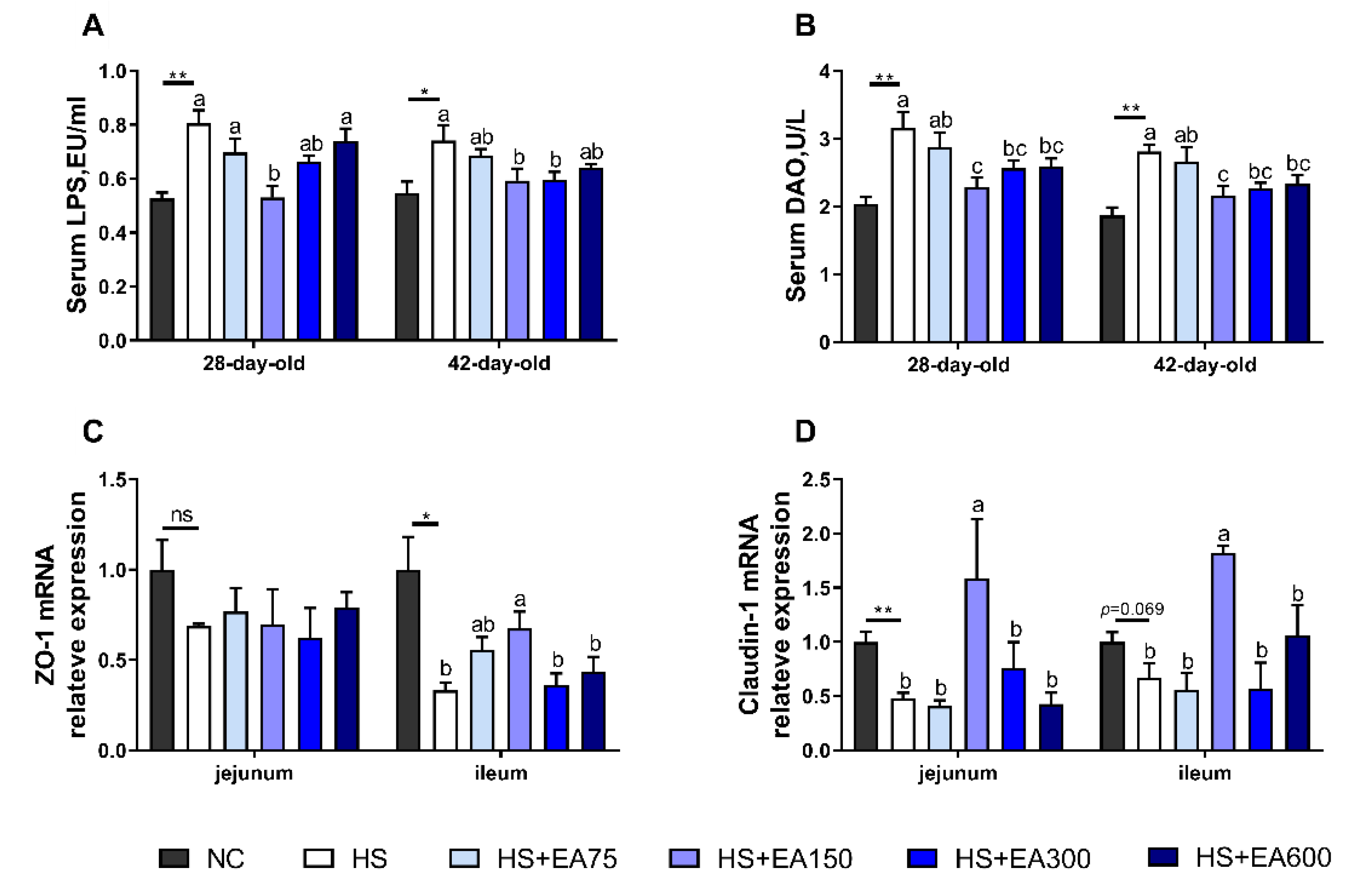
3.4. Effects of Dietary EA on Intestinal Permeability and mRNA Levels of Tight Junction Proteins of Heat-Stressed Broilers
3.5. Effects of Dietary EA on Antioxidant Enzyme Activity of Serum and Intestinal Mucosa in Heat-Stressed Broilers
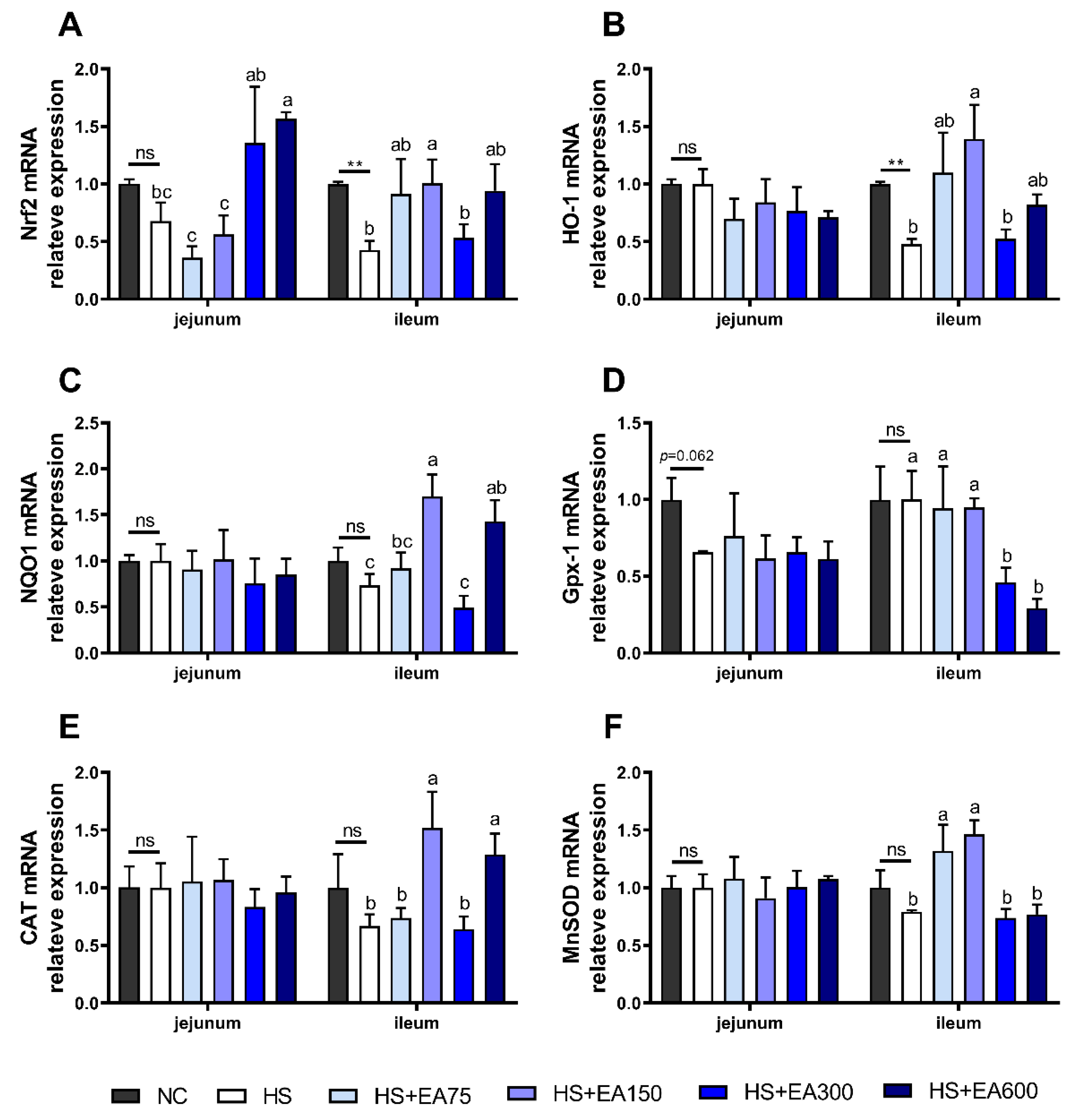
3.6. Effects of Dietary EA on the mRNA Levels Involved in the Antioxidant System in Heat-Stressed Broilers
3.7. Effects of Dietary EA on Cecum Microbiota in Heat-Stressed Broilers
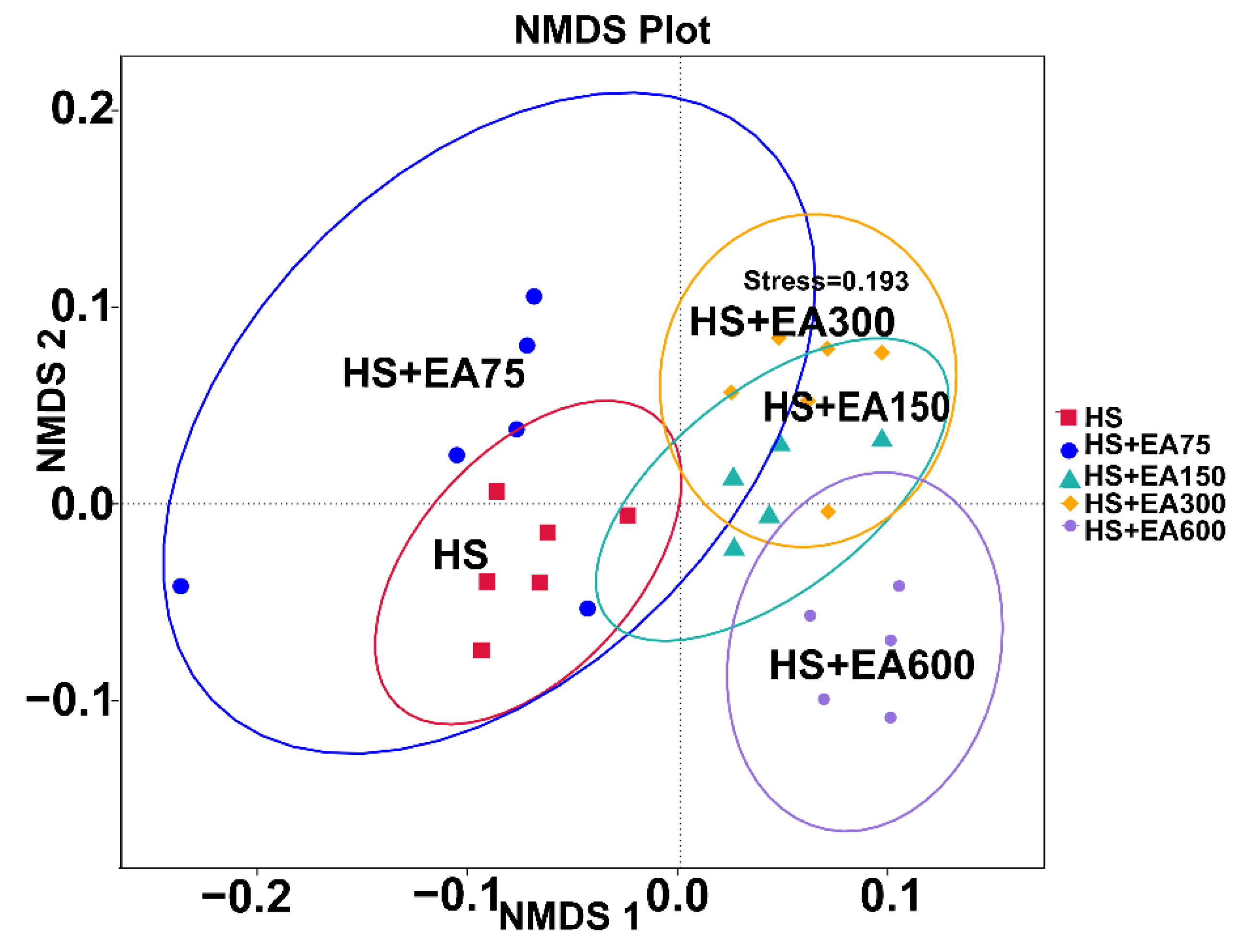
3.7.1. Two-Dimensional Nonparametric Multidimensional Scaling (NMDS) Analysis on Cecum Microbiota
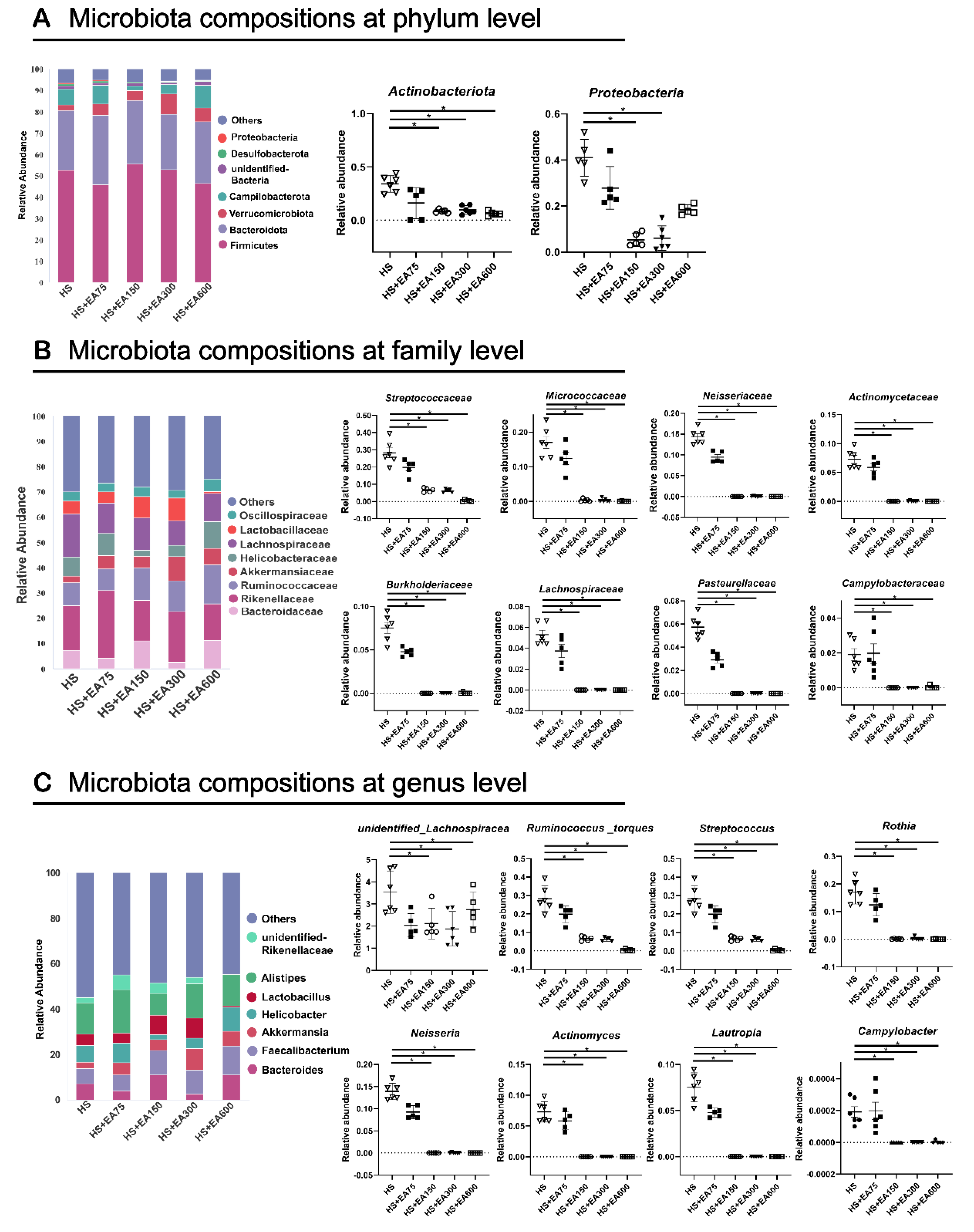
3.7.2. Cecum Microbiota Composition of Heat-Stressed Broilers
3.7.3. LEfSe Analysis of Cecum Microbiota in Heat-Stressed Broilers
3.7.4. PICRUSt Analysis of Intestinal Microbial Functions in the Heat-Stressed Broilers
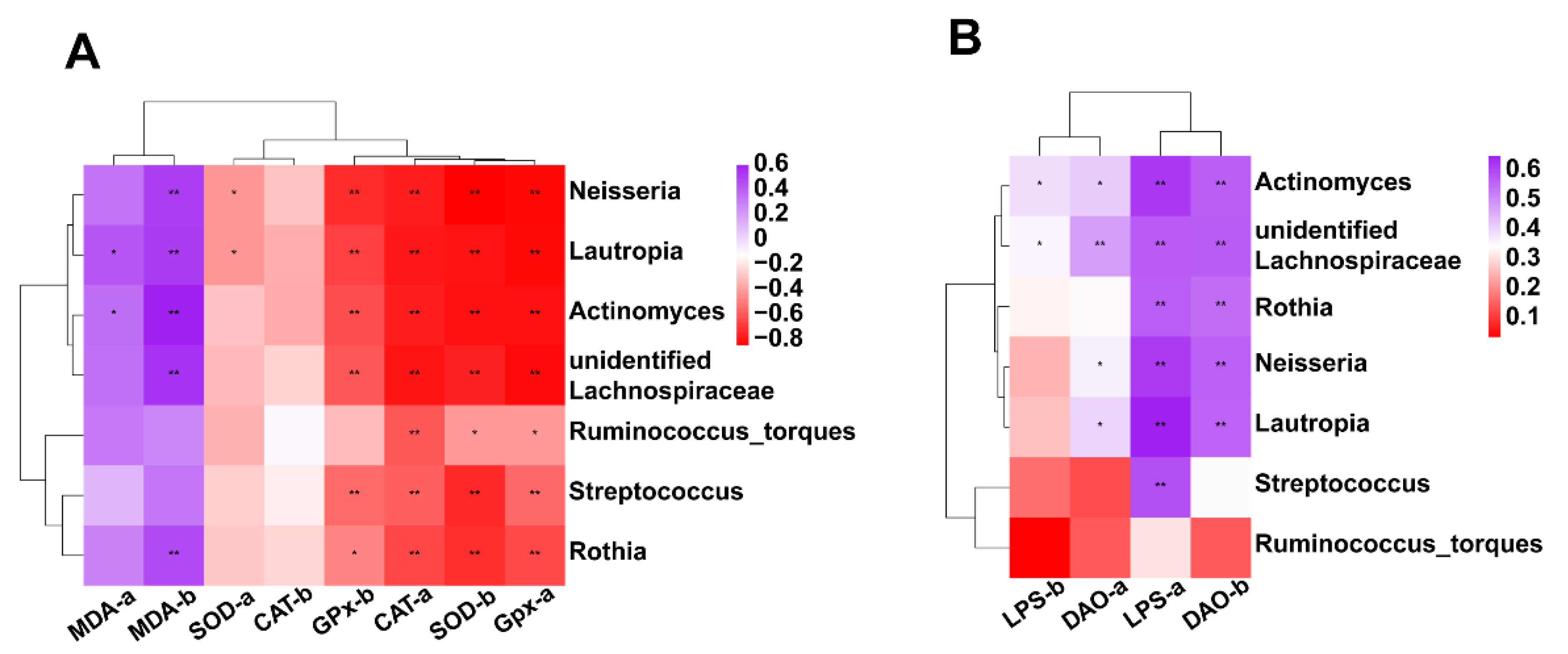
3.7.5. Correlation between Intestinal Microbiota (at Genus Level) and Antioxidant Activity or Intestinal Permeability
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- OECD; Food and Agriculture Organization of the United Nations. OECD-FAO Agricultural Outlook 2015; OECD Publishing: Paris, France, 2015; Chapter 1; pp. 33–34. [Google Scholar]
- Ramiah, S.K.; Awad, E.A.; Mookiah, S.; Idrus, Z. Effects of zinc oxide nanoparticles on growth performance and concentrations of malondialdehyde, zinc in tissues, and corticosterone in broiler chickens under heat stress conditions. Poult. Sci. 2019, 98, 3828–3838. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Lai, X.; Li, Z.; Zhang, X.; Luo, Q. Effect of chronic heat stress on some physiological and immunological parameters in different breed of broilers. Poult. Sci. 2018, 97, 4073–4082. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.H.; Cheng, K.; Zheng, X.C.; Ahmad, H.; Zhang, L.L.; Wang, T. Effects of dietary supplementation with enzymatically treated Artemisia annua on growth performance, intestinal morphology, digestive enzyme activities, immunity, and antioxidant capacity of heat-stressed broilers. Poult. Sci. 2018, 97, 430–437. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Li, X.; Zhou, Y.; Feng, J.; Zhang, M. Effects of Heat Stress on Gut-Microbial Metabolites, Gastrointestinal Peptides, Glycolipid Metabolism, and Performance of Broilers. Animals 2021, 11, 1286. [Google Scholar] [CrossRef]
- Shi, D.; Bai, L.; Qu, Q.; Zhou, S.; Yang, M.; Guo, S.; Li, Q.; Liu, C. Impact of gut microbiota sturcture in heat-stressed broilers. Poult. Sci. 2019, 98, 2405–2413. [Google Scholar] [CrossRef]
- Cheng, Y.F.; Chen, Y.P.; Chen, R.; Su, Y.; Zhang, R.Q.; He, Q.F.; Wang, K.; Wen, C.; Zhou, Y.M. Dietary mannan oligosaccharide ameliorates cyclic heat stress-induced damages on intestinal oxidative status and barrier integrity of broilers. Poult. Sci. 2019, 98, 4767–4776. [Google Scholar] [CrossRef]
- Awad, E.A.; Zulkifli, I.; Ramiah, S.K.; Khalil, E.S.; Abdallh, M.E. Prebiotics supplementation: An effective approach to mitigate the detrimental effects of heat stress in broiler chickens. World′s Poult. Sci. J. 2021, 77, 135–151. [Google Scholar] [CrossRef]
- Burkholder, K.M.; Thompson, K.L.; Einstein, M.E.; Applegate, T.J.; Patterson, J.A. Influence of Stressors on Normal Intestinal Microbiota, Intestinal Morphology, and Susceptibility to Salmonella Enteritidis Colonization in Broilers. Poult. Sci. 2008, 87, 1734–1741. [Google Scholar] [CrossRef]
- Tsiouris, V.; Georgopoulou, I.; Batzios, C.; Pappaioannou, N.; Ducatelle, R.; Fortomaris, P. Heat stress as a predisposing factor for necrotic enteritis in broiler chicks. Avian Pathol. 2018, 47, 616–624. [Google Scholar] [CrossRef]
- Wang, X.J.; Feng, J.H.; Zhang, M.H.; Li, X.M.; Ma, D.D.; Chang, S.S. Effects of high ambient temperature on the community structure and composition of ileal microbiome of broilers. Poult. Sci. 2018, 97, 2153–2158. [Google Scholar] [CrossRef]
- Ocejo, M.; Oporto, B.; Hurtado, A. 16S rRNA amplicon sequencing characterization of caecal microbiome composition of broilers and free-range slow-growing chickens throughout their productive lifespan. Sci. Rep. 2019, 9, 2506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alexander, D.; Carrière, J.; Mckay, K.A. Bacteriological studies of poultry litter fed to livestock. Can. Vet. J. 1968, 9, 127–131. [Google Scholar] [PubMed]
- Lovett, J.; Messer, J.W.; Read, R.B. The Microflora of Southern Ohio Poultry Litter. Poult. Sci. 1971, 50, 746–751. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Sanchez, S.; Hofacre, C.; Maurer, J.J.; Harmon, B.G.; Lee, M. Evaluation of Broiler Litter with Reference to the Microbial Composition as Assessed by Using 16S rRNA and Functional Gene Markers. Appl. Environ. Microbiol. 2003, 69, 901–908. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ngodigha, E.M.; Owen, O.J. Evaluation of the bacteriological characteristics of poultry litter as feedstuff for cattle. Sci. Res. Essays 2009, 4, 188–190. [Google Scholar]
- Bolan, N.S.; Szogi, A.A.; Chuasavathi, T.; Seshadri, B.; Panneerselvam, P. Uses and management of poultry litter. World′s Poult. Sci. J. 2010, 66, 673–698. [Google Scholar] [CrossRef] [Green Version]
- Singh, R.; Chandrashekharappa, S.; Bodduluri, S.R.; Baby, B.V.; Hegde, B.; Kotla, N.G.; Hiwale, A.A.; Saiyed, T.; Patel, P.; Vijay-Kumar, M. Enhancement of the gut barrier integrity by a microbial metabolite through the Nrf2 pathway. Nat. Commun. 2019, 10, 89. [Google Scholar] [CrossRef] [Green Version]
- Aslan, A.; Hussein, Y.T.; Gok, O.; Beyaz, S.; Erman, O.; Baspinar, S. Ellagic acid ameliorates lung damage in rats via modulating antioxidant activities, inhibitory effects on inflammatory mediators and apoptosis-inducing activities. Environ. Sci. Pollut. Res. Int. 2020, 27, 7526–7537. [Google Scholar] [CrossRef]
- Kilic, I.; Yeşiloğlu, Y.; Bayrak, Y. Spectroscopic studies on the antioxidant activity of ellagic acid. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 130, 447–452. [Google Scholar] [CrossRef]
- Dhingra, D.; Jangra, A. Antiepileptic activity of ellagic acid, a naturally occurring polyphenolic compound, in mice. J. Funct. Foods 2014, 10, 364–369. [Google Scholar] [CrossRef]
- Bensaad, L.A.; Kim, K.H.; Quah, C.C.; Kim, W.R.; Shahimi, M. Anti-inflammatory potential of ellagic acid, gallic acid and punicalagin A&B isolated from Punica granatum. BMC Complement. Altern. Med. 2017, 17, 47. [Google Scholar]
- Evtyugin, D.D.; Magina, S.; Evtuguin, D.V. Recent Advances in the Production and Applications of Ellagic Acid and Its Derivatives. A Review. Molecules 2020, 25, 2745. [Google Scholar] [CrossRef] [PubMed]
- Qi, M.; Wang, N.; Xiao, Y.; Deng, Y.; Zha, A.; Tan, B.; Wang, J.; Yin, Y.; Liao, P. Ellagic acid ameliorates paraquat-induced liver injury associated with improved gut microbial profile. Environ. Pollut. 2022, 293, 118572. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.X.; Huang, R.; Wang, N.; Deng, Y.K.; Tan, B.E.; Yin, Y.L.; Qi, M.; Wang, J. Ellagic Acid Alleviates Oxidative Stress by Mediating Nrf2 Signaling Pathways and Protects against Paraquat-Induced Intestinal Injury in Piglets. Antioxidants 2022, 11, 252. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Chen, F.; Zhou, B. Antioxidative, anti-inflammatory and anti-apoptotic effects of ellagic acid in liver and brain of rats treated by D.-galactose. Sci. Rep. 2018, 8, 1465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Wang, S.; Wu, Y.; Liu, X.; Wang, J.; Han, D. Ellagic Acid Alleviates Diquat-Induced Jejunum Oxidative Stress in C57BL/6 Mice through Activating Nrf2 Mediated Signaling Pathway. Nutrients 2022, 14, 1103. [Google Scholar] [CrossRef]
- Najafi, A.; Taheri, R.A.; Mehdipour, M.; Martínez-Pastor, F.; Rouhollahi, A.A.; Nourani, M.R. Improvement of post-thawed sperm quality in broiler breeder roosters by ellagic acid-loaded liposomes. Poult Sci. 2019, 98, 440–446. [Google Scholar] [CrossRef]
- Ziesche, A.; Bergelt, J.; Deubel, H.; Hamker, F.H. Pre-and post-saccadic stimulus timing in saccadic suppression of displacement–A computational model. Vision Res. 2017, 138, 1–11. [Google Scholar] [CrossRef]
- Khan, R.U.; Naz, S.; Ullah, H.; Ullah, Q.; Laudadio, V.; Qudratullah; Bozzo, G.; Tufarelli, V. Physiological dynamics in broiler chickens under heat stress and possible mitigation strategies. Anim. Biotechnol. 2021, 2, 1–10. [Google Scholar] [CrossRef]
- Varasteh, S.; Braber, S.; Akbari, P.; Garssen, J.; Fink-Gremmels, J. Differences in Susceptibility to Heat Stress along the Chicken Intestine and the Protective Effects of Galacto-Oligosaccharides. PLoS ONE 2015, 10, e0138975. [Google Scholar] [CrossRef] [Green Version]
- Kikusato, M.; Xue, G.; Pastor, A.; Niewold, T.A.; Toyomizu, M. Effects of plant-derived isoquinoline alkaloids on growth performance and intestinal function of broiler chickens under heat stress. Poult. Sci. 2021, 100, 957–963. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Y.; Huang, H.; Liu, S.; Liu, F.; Tu, Q.; Yin, Y.; He, S. Resveratrol Improves Growth Performance, Intestinal Morphology, and Microbiota Composition and Metabolism in Mice. Front. Microbiol. 2021, 12, 726878. [Google Scholar] [CrossRef] [PubMed]
- Koch, F.; Thom, U.; Albrecht, E.; Weikard, R.; Nolte, W.; Kuhla, B.; Kuehn, C. Heat stress directly impairs gut integrity and recruits distinct immune cell populations into the bovine intestine. Proc. Natl. Acad. Sci. USA 2019, 116, 10333–10338. [Google Scholar] [CrossRef] [Green Version]
- Esmaeillou, M.; Moharamnejad, M.; Hsankhani, R.; Tehrani, A.A.; Maadi, H. Toxicity of ZnO nanoparticles in healthy adult mice. Environ. Toxicol. Pharmacol. 2013, 35, 67–71. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; Zhao, A.; Wang, Q.; Yang, X.; Ren, D.; Haiping, D.; Qi, W. Supplementation of Inulin with Various Degree of Polymerization Ameliorates Liver Injury and Gut Microbiota Dysbiosis in High Fat-Fed Obese Mice. J. Agric. Food Chem. 2020, 68, 779–787. [Google Scholar] [CrossRef]
- Jastrebski, S.F.; Lamont, S.J.; Schmidt, C. Chicken hepatic response to chronic heat stress using integrated transcriptome and metabolome analysis. PLoS ONE 2017, 12, e0181900. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lara, L.; Rostagno, M. Impact of Heat Stress on Poultry Production. Animals 2013, 3, 356–369. [Google Scholar] [CrossRef]
- Sammad, A.; Wang, Y.J.; Umer, S.; Lirong, H.; Khan, I.; Khan, A.; Ahmad, B.; Wang, Y. Nutritional Physiology and Biochemistry of Dairy Cattle under the Influence of Heat Stress: Consequences and Opportunities. Animals 2020, 10, 793. [Google Scholar] [CrossRef]
- Alhenaky, A.; Abdelqader, A.; Abuajamieh, M.; Al-Fataftah, A.-R. The effect of heat stress on intestinal integrity and Salmonella invasion in broiler birds. J. Therm. Biol. 2017, 70, 9–14. [Google Scholar] [CrossRef]
- Abdelqader, A.M.; Abuajamieh, M.; Hammad, H.M.; Al-Fataftah, A.R.A. Effects of dietary butyrate supplementation on intestinal integrity of heat-stressed cockerels. J. Anim. Physiol. Anim. Nutr. 2017, 101, 1115–1121. [Google Scholar] [CrossRef]
- Zhang, C.; Zhao, X.H.; Yang, L.; Chen, X.Y.; Jiang, R.S.; Jin, S.H.; Geng, Z.Y. Resveratrol alleviates heat stress-induced impairment of intestinal morphology, microflora, and barrier integrity in broilers. Poult. Sci. 2017, 96, 4325–4332. [Google Scholar] [CrossRef] [PubMed]
- Tekeli, O.; Ateşşahin, A.; Sakin, F.; Aslan, A.; Çeribaşı, S.; Yipel, M. Protective effects of conventional and colon-targeted lycopene and linalool on ulcerative colitis induced by acetic acid in rats. Inflammopharmacology 2018, 27, 313–322. [Google Scholar] [CrossRef] [PubMed]
- Iglesias, D.E.; Cremonini, E.; Fraga, C.G.; Oteiza, P.I. Ellagic acid protects Caco-2 cell monolayers against inflammation-induced permeabilization. Free Radic. Biol. Med. 2020, 152, 776–786. [Google Scholar] [CrossRef] [PubMed]
- Tarique, H.; Bie, T.; Yulong, Y.; Francois, B.; Tossou, M.C.B.; Najma, R. Oxidative Stress and Inflammation: What Polyphenols Can Do for Us? Oxid. Med. Cell Longev. 2016, 2016, 7432797. [Google Scholar]
- Fang, L.; Li, M.; Zhao, L.; Han, S.; Li, Y.; Xiong, B.; Jiang, L. Dietary grape seed procyanidins suppressed weaning stress by improving antioxidant enzyme activity and mRNA expression in weanling piglets. J. Anim. Physiol. Anim. Nutr. 2020, 104, 1178–1185. [Google Scholar] [CrossRef]
- Hu, H.; Dai, S.; Li, J.; Wen, A.; Bai, X. Glutamine improves heat stress–induced oxidative damage in the broiler thigh muscle by activating the nuclear factor erythroid 2–related 2/Kelch-like ECH-associated protein 1 signaling pathway. Poult. Sci. 2020, 99, 1454–1461. [Google Scholar] [CrossRef]
- Loboda, A.; Damulewicz, M.; Pyza, E.; Jozkowicz, A.; Dulak, J. Role of Nrf2/HO-1 system in development, oxidative stress response and diseases: An evolutionarily conserved mechanism. Cell. Mol. Life. Sci. 2016, 73, 3221–3247. [Google Scholar] [CrossRef] [Green Version]
- Lu, Z.; He, X.; Ma, B.; Zhang, L.; Li, J.; Jiang, Y.; Zhou, G.; Gao, F. Dietary taurine supplementation improves breast meat quality in chronic heat-stressed broilers via activating the Nrf2 pathway and protecting mitochondria from oxidative attack. J. Sci. Food Agric. 2019, 99, 1066–1072. [Google Scholar] [CrossRef]
- Akbarian, A.; Michiels, J.; Degroote, J.; Majdeddin, M.; Golian, A.; De Smet, S. Association between heat stress and oxidative stress in poultry; mitochondrial dysfunction and dietary interventions with phytochemicals. J. Anim. Sci. Biotechnol. 2016, 28, 37. [Google Scholar] [CrossRef] [Green Version]
- Tomás-Barberán, F.A.; Selma, M.V.; Espín, J.C. Interactions of gut microbiota with dietary polyphenols and consequences to human health. Curr. Opin. Clin. Nutr. Metab. Care 2016, 19, 471–476. [Google Scholar] [CrossRef]
- Zhu, G.; Jiang, Y.; Yao, Y.; Wu, N.; Luo, J.; Hu, M.; Tu, Y.; Xu, M. Ovotransferrin ameliorates the dysbiosis of immunomodulatory function and intestinal microbiota induced by cyclophosphamide. Food Funct. 2019, 10, 1109–1122. [Google Scholar] [CrossRef] [PubMed]
- Malinen, E.; Krogius-Kurikka, L.; Lyra, A.; Nikkilä, J.; Jääskeläinen, A.; Rinttilä, T.; Vilpponen-Salmela, T.; von Wright, A.J.; Palva, A. Association of symptoms with gastrointestinal microbiota in irritable bowel syndrome. World J. Gastroenterol. 2010, 16, 4532. [Google Scholar] [CrossRef] [PubMed]
- Kaczmarczyk, M.; Löber, U.; Adamek, K.; Węgrzyn, D.; Skonieczna-Żydecka, K.; Malinowski, D.; Łoniewski, I.; Markó, L.; Ulas, T.; Forslund, S.K. The gut microbiota is associated with the small intestinal paracellular permeability and the development of the immune system in healthy children during the first two years of life. J. Transl. Med. 2021, 19, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Bai, H.; Deng, F.; Zhong, R.; Liu, L.; Chen, L.; Zhang, H. Chemically Protected Sodium Butyrate Improves Growth Performance and Early Development and Function of Small Intestine in Broilers as One Effective Substitute for Antibiotics. Antibiotics 2022, 11, 132. [Google Scholar] [CrossRef]
- Kong, Y.; Olejar, K.J.; On, S.; Chelikani, V. The Potential of Lactobacillus spp. for Modulating Oxidative Stress in the Gastrointestinal Tract. Antioxidants 2020, 9, 610. [Google Scholar] [CrossRef] [PubMed]
- Schwarzer, M.; Makki, K.; Storelli, G.; Machuca-Gayet, I.; Srutkova, D.; Hermanova, P.; Martino, M.E.; Balmand, S.; Hudcovic, T.; Heddi, A. Lactobacillus plantarum strain maintains growth of infant mice during chronic undernutrition. Science 2016, 351, 854–857. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, T.; Liu, B.; Wang, Y.; Huang, X.; Yan, Z.; Jiang, Q.; Chen, Q. Ellagic Acid Improves Antioxidant Capacity and Intestinal Barrier Function of Heat-Stressed Broilers via Regulating Gut Microbiota. Animals 2022, 12, 1180. https://doi.org/10.3390/ani12091180
Yang T, Liu B, Wang Y, Huang X, Yan Z, Jiang Q, Chen Q. Ellagic Acid Improves Antioxidant Capacity and Intestinal Barrier Function of Heat-Stressed Broilers via Regulating Gut Microbiota. Animals. 2022; 12(9):1180. https://doi.org/10.3390/ani12091180
Chicago/Turabian StyleYang, Tai, Bifan Liu, Yujie Wang, Xiangying Huang, Zhaoming Yan, Qian Jiang, and Qinghua Chen. 2022. "Ellagic Acid Improves Antioxidant Capacity and Intestinal Barrier Function of Heat-Stressed Broilers via Regulating Gut Microbiota" Animals 12, no. 9: 1180. https://doi.org/10.3390/ani12091180
APA StyleYang, T., Liu, B., Wang, Y., Huang, X., Yan, Z., Jiang, Q., & Chen, Q. (2022). Ellagic Acid Improves Antioxidant Capacity and Intestinal Barrier Function of Heat-Stressed Broilers via Regulating Gut Microbiota. Animals, 12(9), 1180. https://doi.org/10.3390/ani12091180






