Evaluation of Renal Blood Flow in Dogs during Short-Term Human-Dose Epoprostenol Administration Using Pulsed Doppler and Contrast-Enhanced Ultrasonography
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Anesthesia
2.3. Epoprostenol Administration
2.4. Measurements
2.5. Statistical Analyses
3. Results
3.1. Pulsed Doppler Ultrasonography
3.2. Contrast-Enhanced Ultrasonography
3.3. Systemic Circulation Parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Polzin, D.J. Chronic kidney disease in small animals. Vet. Clin. North. Am. Small Anim. Pract. 2011, 41, 15–30. [Google Scholar] [CrossRef]
- O’Neill, D.G.; Elliott, J.; Church, D.B.; McGreevy, P.D.; Thomson, P.C.; Brodbelt, D.C. Chronic kidney disease in dogs in UK veterinary practices: Prevalence, risk factors, and survival. J. Vet. Intern. Med. 2013, 27, 814–821. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Dunaevich, A.; Apfelbaum, N.; Kuzi, S.; Mazaki-Tovi, M.; Aroch, I.; Segev, G. Acute on chronic kidney disease in cats: Etiology, clinical and clinicopathologic findings, prognostic markers, and outcome. J. Vet. Intern. Med. 2020, 34, 1496–1506. [Google Scholar] [CrossRef] [PubMed]
- Sawashima, K.; Sawashima, Y.; Shitaka, H.; Mizuno, S.; Kudo, T.; Kurosawa, T. Correlation between Renal Function and Tubulo-Interstitial Lesions in Feline Progressive Kidney Disease. J. Jpn. Vet. Med. Assoc. 2001, 54, 687–691. [Google Scholar] [CrossRef]
- Wang, X.F.; Zhang, B.H.; Lu, X.Q.; Wang, P. Beraprost sodium, a stable analogue of PGI2, inhibits the renin-angiotensin system in the renal tissues of rats with chronic renal failure. Kidney Blood Press. Res. 2018, 43, 1231–1244. [Google Scholar] [CrossRef]
- First, M.R.; Ettenger, M.; Robson, M.; Pollak, V.E.; Ooi, B.S.; Goldberg, M. Acute deterioration in renal function in patients with preexisting renal insufficiency. Arch. Intern. Med. 1984, 144, 2233–2238. [Google Scholar] [CrossRef] [PubMed]
- Kubo, M.; Takeda, K.; Harada, A. Clinical analysis of cases with acute exacerbation of chronic renal failure. Nihon Jinzo Gakkai Shi. 1994, 36, 163–171. [Google Scholar]
- Morham, S.G.; Langenbach, R.; Loftin, C.D.; Tiano, H.F.; Vouloumanos, N.; Jennette, J.C.; Mahler, J.F.; Kluckman, K.D.; Ledford, A.; Lee, C.A.; et al. Prostaglandin synthase 2 gene disruption causes severe renal pathology in the mouse. Cell 1995, 83, 473–482. [Google Scholar] [CrossRef]
- Yokoyama, C.; Yabuki, T.; Shimonishi, M.; Wada, M.; Hatae, T.; Ohkawara, S.; Takeda, J.; Kinoshita, T.; Okabe, M.; Tanabe, T. Prostacyclin-deficient mice develop ischemic renal disorders, including nephrosclerosis and renal infarction. Circulation 2002, 106, 2397–2403. [Google Scholar] [CrossRef]
- Hill, T.W.; Moncada, S. The renal haemodynamic and excretory actions of prostacyclin (PGI2) in anaesthetized dogs. Br. J. Pharmacol. 1978, 62, 413–414. [Google Scholar]
- Tobimatsu, M.; Ueda, Y.; Toyoda, K.; Saito, S.; Konomi, K. Effect of a stable prostacyclin analogue on canine renal allograft rejection. Ann. Surg. 1987, 205, 199–202. [Google Scholar] [CrossRef] [PubMed]
- Tobimatsu, M.; Ueda, Y.; Saito, S.; Tsumagari, T.; Konomi, K. Effects of a stable prostacyclin analog on experimental ischemic acute renal failure. Ann. Surg. 1988, 208, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Saito, Y.; Nakamura, K.; Akagi, S.; Sarashina, T.; Ejiri, K.; Miura, A.; Ogawa, A.; Matsubara, H.; Ito, H. Epoprostenol sodium for treatment of pulmonary arterial hypertension. Vasc. Health. Risk. Manag. 2015, 11, 265–270. [Google Scholar] [PubMed]
- Zwissler, B.; Welte, M.; Habler, O.; Kleen, M.; Messmer, K. Effects of inhaled prostacyclin as compared with inhaled nitric oxide in a canine model of pulmonary microembolism and oleic acid edema. J. Cardiothorac. Vasc. Anesth. 1995, 9, 634–640. [Google Scholar] [CrossRef]
- Fulghum, T.G.; DiMarco, J.P.; Supple, E.W.; Nash, I.; Gendlerman, J.; Eton, D.F.; Newell, J.B.; Zusman, R.M.; Powell, W.J., Jr. Effect of prostacyclin on vascular capacity in the dog. J. Clin. Investig. 1985, 76, 999–1006. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.H.; Jung, C.D.; Kim, S.H.; Kim, S.H. Renal venous doppler ultrasonography in normal subjects and patients with diabetic nephropathy: Value of venous impedance index measurements. J. Clin. Ultrasound. 2011, 39, 512–518. [Google Scholar] [CrossRef] [PubMed]
- Oktar, S.O.; Yücel, C.; Ozdemir, H.; Karaosmanoglu, D. Doppler sonography of renal obstruction: Value of venous impedance index measurements. J. Ultrasound Med. 2004, 23, 929–936. [Google Scholar] [CrossRef]
- Bragato, N.; Borges, N.C.; Fioravanti, M.C.S. B-mode and Doppler ultrasound of chronic kidney disease in dogs and cats. Vet. Res. Commun. 2017, 41, 307–315. [Google Scholar] [CrossRef]
- Choi, H.; Won, S.; Chung, W.; Lee, K.; Chang, D.; Lee, H.; Eom, K.; Lee, Y.; Yoon, J. Effect of intravenous mannitol upon the resistive index in complete unilateral renal obstruction in dogs. J. Vet. Intern. Med. 2003, 17, 158–162. [Google Scholar] [CrossRef]
- Novellas, R.; Ruiz de Gopegui, R.; Espada, Y. Assessment of renal vascular resistance and blood pressure in dogs and cats with renal disease. Vet. Rec. 2010, 166, 618–623. [Google Scholar] [CrossRef]
- Tipisca, V.; Murino, C.; Cortese, L.; Mennonna, G.; Auletta, L.; Vulpe, V.; Meomartino, L. Resistive index for kidney evaluation in normal and diseased cats. J. Feline Med. Surg. 2016, 18, 471–475. [Google Scholar] [CrossRef] [PubMed]
- Szczepankiewicz, B.; Pasławska, U.; Siwińska, N.; Plens, K.; Pasławski, R. Evaluation of the diagnostic value of the renal resistive index as a marker of the subclinical development of cardiorenal syndrome in MMVD dogs. J. Renin. Angiotensin. Aldosterone. Syst. 2021, 22, 1470320321995082. [Google Scholar] [CrossRef] [PubMed]
- Koma, L.M.; Kirberger, R.M.; Scholtz, L. Doppler ultrasonographic changes in the canine kidney during normovolaemic anaemia. Res. Vet. Sci. 2006, 80, 96–102. [Google Scholar] [CrossRef]
- Liu, D.J.X.; Hesta, M.; Stock, E.; Bogaerts, E.; Broeckx, B.J.G.; Saunders, J.H.; Vanderperren, K. Renal perfusion parameters measured by contrast-enhanced ultrasound in healthy dogs demonstrate a wide range of variability in the long-term. Vet. Radiol. Ultrasound. 2019, 60, 201–209. [Google Scholar] [CrossRef]
- Mannucci, T.; Lippi, I.; Rota, A.; Citi, S. Contrast enhancement ultrasound of renal perfusion in dogs with acute kidney injury. J. Small Anim. Pract. 2019, 60, 471–476. [Google Scholar] [CrossRef]
- Stock, E.; Paepe, D.; Daminet, S.; Duchateau, L.; Saunders, J.H.; Vanderperren, K. Influence of ageing on quantitative contrast-enhanced ultrasound of the kidneys in healthy cats. Vet. Rec. 2018, 182, 515. [Google Scholar] [CrossRef] [PubMed]
- Stock, E.; Vanderperren, K.; Bosmans, T.; Dobbeleir, A.; Duchateau, L.; Hesta, M.; Lybaert, L.; Peremans, K.; Vandermeulen, E.; Saunders, J. Evaluation of Feline Renal Perfusion with Contrast-EnhancedUltrasonography and Scintigraphy. PLoS ONE 2016, 11, e0164488. [Google Scholar] [CrossRef]
- Dong, Y.; Wang, W.; Cao, J.; Fan, P.; Lin, X. Quantitative evaluation of contrast-enhanced ultrasonography in the diagnosis of chronic ischemic renal disease in a dog model. PLoS ONE 2013, 8, e70337. [Google Scholar] [CrossRef]
- Itami, T.; Hanazono, K.; Oyama, N.; Sano, T.; Makita, K.; Yamashita, K. Cardiovascular effects of intravenous colforsin in normal and acute respiratory acidosis canine models: A dose-response study. PLoS ONE 2019, 14, e0213414. [Google Scholar] [CrossRef]
- Endo, Y.; Tamura, J.; Ishizuka, T.; Itami, T.; Hanazono, K.; Miyoshi, K.; Sano, T.; Yamashita, K.; Muir, W.W. Stroke volume variation (SVV) and pulse pressure variation (PPV) as indicators of fluid responsiveness in sevoflurane anesthetized mechanically ventilated euvolemic dogs. J. Vet. Med. Sci. 2017, 79, 1437–1445. [Google Scholar] [CrossRef]
- Takenaka, M.; Iio, A.; Sato, R.; Sakamoto, T.; Kurumatani, H.; KT-140 Clinical Study Group. A double-blind, placebo-controlled, multicenter, prospective, randomized study of beraprost sodium treatment for cats with chronic kidney disease. J. Vet. Intern. Med. 2018, 32, 236–248. [Google Scholar] [CrossRef] [PubMed]
- Tsuruoka, K.; Yasuda, T.; Koitabashi, K.; Yazawa, M.; Shimazaki, M.; Sakurada, T.; Shirai, S.; Shibagaki, Y.; Kimura, K.; Tsujimoto, F. Evaluation of renal microcirculation by contrast-enhanced ultrasound with Sonazoid as a contrast agent. Int. Heart. J. 2010, 51, 176–182. [Google Scholar] [CrossRef] [PubMed]
- Akaishi, T.; Abe, M.; Miki, T.; Funamizu, Y.; Ito, S.; Abe, T.; Ishii, T. Ratio of diastolic to systolic blood pressure represents renal resistive index. J. Hum. Hypertens. 2020, 34, 512–519. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.Y.; Jeong, W.C.; Lee, Y.W.; Choi, H.J. Contrast enhanced ultrasonography of kidney in conscious and anesthetized beagle dogs. J. Vet. Med. Sci. 2015, 78, 239–244. [Google Scholar] [CrossRef]
- Li, S.; Wang, Y.; Chen, L.; Wang, Z.; Liu, G.; Zuo, B.; Liu, C.; Sun, D. Beraprost sodium mitigates renal interstitial fibrosis through repairing renal microvessels. J. Mol. Med. 2019, 97, 777–791. [Google Scholar] [CrossRef]
- Gerber, J.G.; Nies, A.S.; Friesinger, G.C.; Gerkens, J.F.; Branch, R.A.; Oates, J.A. The effect of PGI2 on canine renal function and hemodynamics. Prostaglandins 1978, 16, 519–528. [Google Scholar] [CrossRef]
- Lifschitz, M.D. Prostaglandins and Renal Blood Flow: In Vivo Studies. Kidney Int. 1981, 19, 781–785. [Google Scholar] [CrossRef]
- Nishio, S.; Kurumatani, H. Pharmacological and clinical properties of beraprost sodium, orally active prostacyclin analogue. Nihon Yakurigaku Zasshi. 2001, 117, 123–130. [Google Scholar] [CrossRef][Green Version]
- Novellas, R.; Ruiz de Gopegui, R.; Espada, Y. Effects of Sedation with Midazolam and Butorphanol on Resistive and Pulsatility Indices in Healthy Dogs. Vet. Radiol. Ultrasound. 2007, 48, 276–280. [Google Scholar] [CrossRef]
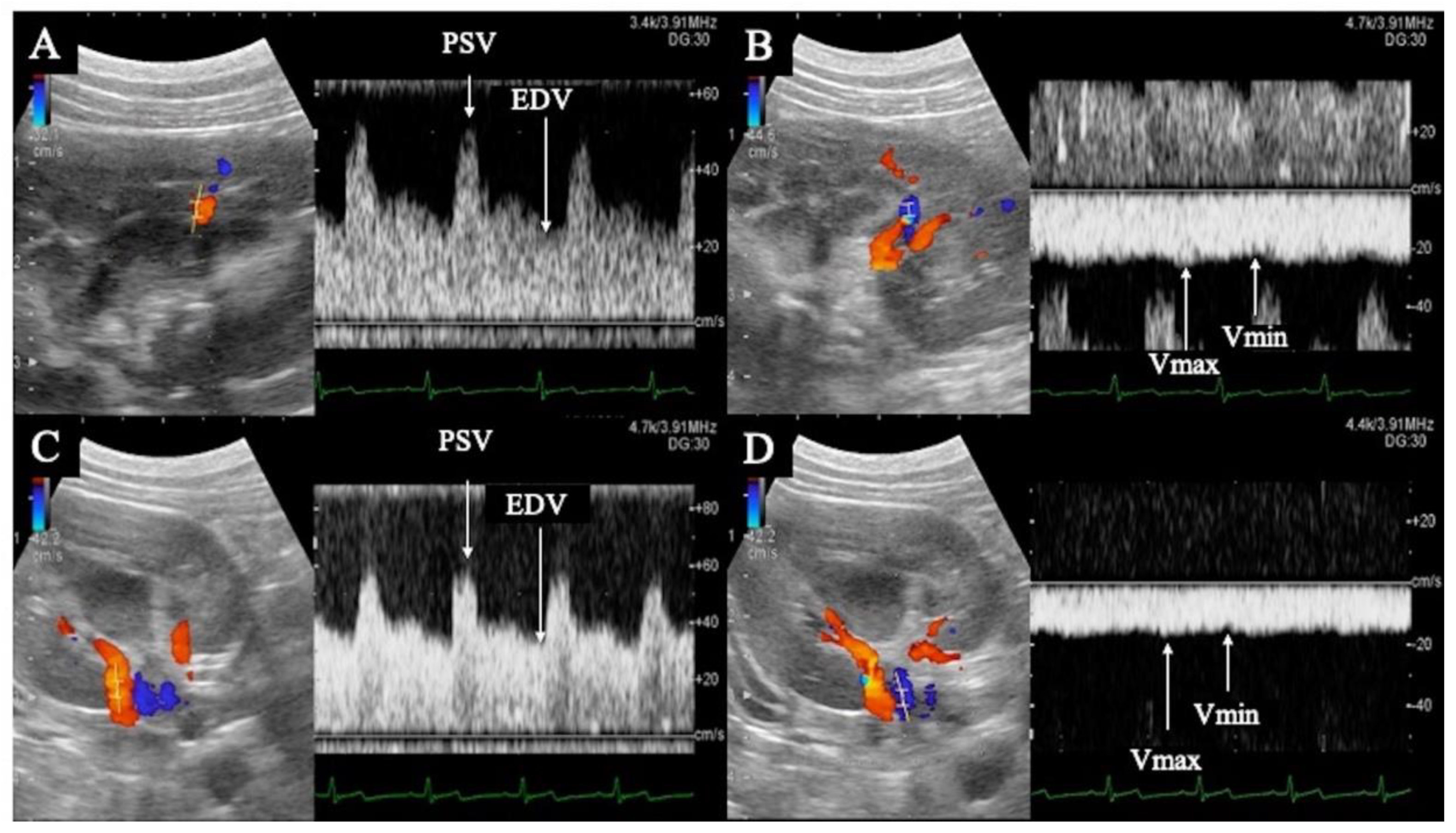


| Parts | Variables | Norms | Pre-Administration | 2 ng/kg/min | 5 ng/kg/min | 10 ng/kg/min |
|---|---|---|---|---|---|---|
| Interlobular artery | PSV (cm/sec) | - | 33.48 (18.83–52.07) | 37.93 (13.57–53.33) | 39.37 (20.77–52.33) | 39.25 (27.87–49.10) |
| EDV (cm/sec) | - | 14.87 (7.40–23.83) | 15.95 (5.47–28.70) | 17.77 (9.13–20.40) | 16.23 (8.57–24.33) | |
| PI | 1.15 ± 0.15 [20] | 0.99 (0.72–1.41) | 0.90 (0.651.16) | 0.93 (0.72–1.29) | 0.96 (0.71–1.39) | |
| RI | 0.62 ± 0.04 [20] 0.66 ± 0.05 [22] | 0.60 (0.50–0.70) | 0.55 (0.46–0.75) | 0.57 (0.50–0.68) | 0.58 (0.49–0.70) | |
| Interlobular vein | Vmax (cm/sec) | - | 12.48 (8.97–21.17) | 12.92 (6.73–50.07) | 13.75 (8.77–58.30) | 16.78 (10.80–6.13) |
| Vmin (cm/sec) | - | 9.17 (6.83–16.27) | 9.87 (4.87–40.00) | 10.43 (6.47–48.43) | 16.61 (7.90–38.03) | |
| VII | - | 0.25 (0.21–0.31) | 0.23 (0.20–0.38) | 0.25 (0.17–0.29) | 0.25 (0.18–0.39) | |
| Renal artery | PSV (cm/sec) | 75.2 ± 22.0 [23] | 54.77 (35.80–74.30) | 58.02 (46.80–91.07) | 63.80 (48.20–70.10) | 68.67 (43.40–111.40) |
| EDV (cm/sec) | 25.7 ± 8.2 [23] | 25.23 (15.13–36.27) | 27.90 (23.90–42.97) | 29.83 (19.37–35.80) | 30.42 (16.83–53.07) | |
| PI | 1.34 ± 0.32 [23] | 0.77 (0.65–1.26) | 0.75 (0.60–1.25) | 0.91 (0.57–1.38) | 0.94 (0.57–1.28) | |
| RI | 0.66 ± 0.07 [23] | 0.50 (0.46–0.67) | 0.50 (0.44–0.66) | 0.56 (0.41–0.70) | 0.56 (0.42–0.67) | |
| Renal vein | Vmax (cm/sec) | - | 14.28 (13.00–25.57) | 16.95 (15.07–25.00) | 19.27 (12.43–27.10) | 20.32 (12.27–30.77) |
| Vmin (cm/sec) | - | 11.35 (9.83–21.10) | 13.27 (9.70–19.70) | 14.95 (6.73–22.27) | 15.68 (6.77–24.47) | |
| VII | - | 0.22 (0.17–0.28) | 0.24 (0.19–0.36) | 0.22 (0.18–0.46) | 0.21 (0.20–0.45) |
| Parts | Variables | Pre-Administration | 2 ng/kg/min | 5 ng/kg/min | 10 ng/kg/min |
|---|---|---|---|---|---|
| Renal cortex | TTP (sec) | 13.10 (12.00–14.60) | 13.80 (11.20–16.20) | 13.20 (10.00–14.80) | 12.60 (10.00–15.20) |
| RT (sec) | 4.30 (3.40–6.00) | 4.55 (3.00–6.00) | 4.90 (3.00–6.20) | 4.80 (4.20–5.60) | |
| WiR | 38.55 (26.00–64.80) | 43.25 (31.40–76.80) | 49.65 (29.20–60.60) | 46.10 (30.70–81.40) | |
| WoR | 0.60 (0.50–0.70) | 0.60 (0.40–0.70) | 0.55 (0.40–0.80) | 0.60 (0.50–0.80) | |
| PE | 139.55 (108.50–186.70) | 155.55 (135.30–188.70) | 156.25 (115.40–205.30) | 155.65 (133.10–202.20) | |
| BI | 10.50 (0.60–44.40) | 15.75 (3.10–50.20) | 11.95 (4.40–57.10) | 12.95 (6.00–49.30) | |
| AUC | 8451.30 (7468.80–11,105.90) | 11,089. 10 (8263.00–12,154.50) | 10,796.00 (5841.10–11,930.50) | 9953.20 (7560.50–12,580.60) | |
| WiAUC | 621.25 (386.20–845.60) | 466.40 (383.30–717.40) | 528.30 (445.60–724.70) | 489.45 (388.60–726.50) | |
| WoAUC | 7743.10 (7082.60–10,477.50) | 10538.70 (7703.00–11,762.70) | 10335.40 (5338.00–11,241.90) | 9556.90 (6989.50–11,854.10) | |
| Renal medulla | TTP (sec) | 18.70 (16.60–22.80) | 19.50 (15.20–25.20) | 20.80 (15.20–25.20) | 18.90 (15.20–25.00) |
| RT (sec) | 8.30 (6.80–9.20) | 8.90 (5.60–12.40) | 9.50 (5.00–13.40) | 8.50 (7.40–11.80) | |
| WiR | 21.85 (8.60–25.40) | 14.25 (8.70–40.20) | 16.50 (10.50–47.30) | 17.75 (14.80– 31.60) | |
| WoR | 0.75 (0.60–1.10) | 0.80 (0.60–1.50) | 1.00 (0.40–1.20) | 0.85 (0.60–1.00) | |
| PE | 138.20 (90.60–174.00) | 132.05 (83.50–197.40) | 137.80 (109.50–193.70) | 138.75 (79.0–193.00) | |
| BI | 16.10 (11.60–20.90) | 14.60 (10.60–26.10) | 18.65 (3.40–30.80) | 18.35 (14.10–28.00) | |
| AUC | 5560.60 (1861.80–7720.80) | 3646.80 (1848.90–8293.00) | 5235.80 (1890.90–8284.00) | 4324.10 (946.90–9601.00) | |
| WiAUC | 593.90 (255.80–917.20) | 571.00 (347.80–1054.20) | 635.20 (385.30–841.20) | 491.60 (227.20–816.80) | |
| WoAUC | 4891.9 (1606.00–7071.60) | 2859.7 (1501.10–7238.90) | 4489.40 (1505.60–7665.20) | 3813.5 (719.70–8810.50) |
| Variables | Norms | Pre-Administration | 2 ng/kg/min | 5 ng/kg/min | 10 ng/kg/min |
|---|---|---|---|---|---|
| SABP (mmHg) | 96.1 ± 3.5 [29] | 118.53 (107.5–129.2) | 123.85 (118.66–134.0) | 121.54 (114.60–137.4) | 124.25 (112.0–136.5) |
| MABP (mmHg) | 68.5 ± 2.6 [29] | 80.40 (75.20–85.00) | 84.80 (79.66–93.00) | 84.17 (82.25–93.20) | 81.33 (78.75–95.00) |
| DABP (mmHg) | 54.2 ± 2.0 [29] | 67.98 (57.80–72.00) | 71.83 (62.66–79.75) | 68.85 (63.80–78.00) | 66.30 (61.40–80.33) |
| mPAP (mmHg) | 10.7 ± 0.2 [29] | 11.63 (9.00–13.60) | 12.83 (8.33–13.00) | 12.97 (8.40–14.16) | 12.50 (9.00–13.50) |
| mRAP (mmHg) | 3.8 ± 0.3 [29] | 3.50 (2.66–4.75) | 4.00 (1.00–4.00) | 3.00 (1.00–5.20) | 2.50 (1.00–4.80) |
| PCWP (mmHg) | 4.5 ± 0.2 [29] | 5.25 (4.00–7.00) | 5.75 (4.50–7.00) | 5.25 (3.50–8.00) | 5.00 (3.00–7.50) |
| HR (/min) | 86.0 ± 3.9 [29] | 98.50 (71.20–118.33) | 105.50 (74.33–116.6) | 113.07 (77.20–129.5) | 119.88 (75.60–130.0) |
| CO (L/min) | 1.8 ± 0.3 [30] | 2.08 (1.30–2.66) | 2.25 (1.20–2.48) | 2.24 (1.49–2.44) | 2.18 (1.48–2.59) |
| Parts | Variables | SABP | MABP | DABP | HR | CO | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| r | p | r | p | R | p | r | p | r | p | ||
| Interlobular artery | PSV | 0.38 | 0.07 | −0.01 | 0.98 | −0.03 | 0.9 | −0.16 | 0.44 | −0.18 | 0.4 |
| EDV | 0.33 | 0.12 | 0.46 | 0.03 | 0.52 | <0.01 | 0.31 | 0.15 | 0.2 | 0.35 | |
| PI | −0.15 | 0.49 | −0.67 | <0.01 | −0.82 | <0.01 | −0.67 | <0.01 | −0.41 | 0.046 | |
| RI | −0.14 | 0.5 | −0.7 | <0.01 | −0.81 | <0.01 | −0.63 | <0.01 | −0.43 | 0.04 | |
| Interlobular vein | Vmax | 0.05 | 0.82 | 0.25 | 0.23 | 0.41 | 0.049 | 0.58 | <0.01 | 0.45 | 0.03 |
| Vmin | 0.05 | 0.83 | 0.28 | 0.19 | 0.41 | 0.04 | 0.58 | <0.01 | 0.51 | 0.01 | |
| VII | −0.08 | 0.73 | −0.29 | 0.16 | −0.34 | 0.11 | −0.43 | 0.04 | −0.38 | 0.07 | |
| Renal artery | PSV | 0.6 | <0.01 | 0.12 | 0.57 | −0.03 | 0.91 | −0.13 | 0.55 | 0.18 | 0.41 |
| EDV | 0.25 | 0.25 | 0.55 | <0.01 | 0.6 | <0.01 | 0.41 | 0.048 | 0.63 | <0.01 | |
| PI | 0.16 | 0.45 | −0.51 | 0.01 | −0.73 | <0.01 | −0.51 | 0.01 | −0.45 | 0.03 | |
| RI | 0.18 | 0.39 | −0.5 | 0.01 | −0.73 | <0.01 | −0.49 | 0.02 | −0.47 | 0.02 | |
| Renal vein | Vmax | 0.05 | 0.8 | 0.63 | <0.01 | 0.55 | <0.01 | 0.57 | <0.01 | 0.59 | <0.01 |
| Vmin | 0.02 | 0.92 | 0.66 | <0.01 | 0.59 | <0.01 | 0.60 | <0.01 | 0.64 | <0.01 | |
| VII | −0.09 | 0.67 | −0.44 | 0.03 | −0.48 | 0.02 | −0.31 | 0.14 | −0.43 | 0.04 | |
| Renal cortex | TTP | 0.25 | 0.24 | 0.02 | 0.92 | −0.27 | 0.21 | −0.61 | <0.01 | −0.06 | 0.77 |
| RT | 0.36 | 0.08 | −0.05 | 0.8 | −0.31 | 0.14 | −0.35 | 0.09 | 0.05 | 0.83 | |
| WiR | −0.22 | 0.31 | 0.25 | 0.24 | 0.33 | 0.12 | 0.58 | <0.01 | 0.23 | 0.27 | |
| WoR | 0.35 | 0.1 | −0.25 | 0.23 | −0.21 | 0.33 | −0.2 | 0.35 | −0.52 | 0.01 | |
| PE | −0.24 | 0.26 | 0.12 | 0.59 | 0.12 | 0.57 | 0.58 | <0.01 | 0.15 | 0.5 | |
| BI | 0.04 | 0.84 | 0.22 | 0.3 | 0.27 | 0.21 | 0.46 | 0.02 | −0.05 | 0.82 | |
| AUC− | −0.28 | 0.19 | 0.27 | 0.2 | 0.15 | 0.48 | 0.31 | 0.14 | 0.39 | 0.06 | |
| WiAUC | −0.32 | 0.12 | −0.43 | 0.04 | −0.54 | <0.01 | −0.44 | 0.03 | −0.26 | 0.22 | |
| WoAUC | −0.24 | 0.26 | 0.33 | 0.11 | 0.21 | 0.31 | 0.36 | 0.09 | 0.44 | 0.03 | |
| Renal medulla | TTP | 0.29 | 0.18 | −0.08 | 0.72 | −0.36 | 0.08 | −0.69 | <0.01 | −0.15 | 0.48 |
| RT− | 0.09 | 0.69 | 0.16 | 0.47 | −0.002 | 0.99 | −0.29 | 0.17 | 0.15 | 0.47 | |
| WiR | −0.28 | 0.18 | −0.04 | 0.85 | −0.11 | 0.62 | 0.4 | 0.05 | 0.16 | 0.46 | |
| WoR | −0.08 | 0.72 | 0.19 | 0.38 | 0.22 | 0.29 | 0.38 | 0.07 | −0.27 | 0.21 | |
| PE | −0.39 | 0.06 | 0.02 | 0.92 | 0.05 | 0.83 | 0.49 | 0.02 | 0.11 | 0.6 | |
| BI | 0.21 | 0.32 | 0.07 | 0.75 | 0.15 | 0.48 | 0.3 | 0.15 | 0.05 | 0.8 | |
| AUC | −0.25 | −0.25 | 0.06 | 0.79 | −0.02 | 0.94 | 0.36 | 0.08 | 0.43 | 0.04 | |
| WiAUC | −0.23 | 0.28 | 0.33 | 0.12 | 0.14 | 0.5 | 0.09 | 0.66 | 0.28 | 0.19 | |
| WoAUC | −0.28 | 0.18 | 0.001 | 0.99 | −0.05 | 0.81 | 0.37 | 0.08 | 0.39 | 0.06 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hanazono, K.; Itami, T.; Hayasaka, I.; Miyoshi, K.; Hori, A.; Kato, K.; Endoh, D. Evaluation of Renal Blood Flow in Dogs during Short-Term Human-Dose Epoprostenol Administration Using Pulsed Doppler and Contrast-Enhanced Ultrasonography. Animals 2022, 12, 1175. https://doi.org/10.3390/ani12091175
Hanazono K, Itami T, Hayasaka I, Miyoshi K, Hori A, Kato K, Endoh D. Evaluation of Renal Blood Flow in Dogs during Short-Term Human-Dose Epoprostenol Administration Using Pulsed Doppler and Contrast-Enhanced Ultrasonography. Animals. 2022; 12(9):1175. https://doi.org/10.3390/ani12091175
Chicago/Turabian StyleHanazono, Kiwamu, Takaharu Itami, Ikuto Hayasaka, Kenjiro Miyoshi, Ai Hori, Keiko Kato, and Daiji Endoh. 2022. "Evaluation of Renal Blood Flow in Dogs during Short-Term Human-Dose Epoprostenol Administration Using Pulsed Doppler and Contrast-Enhanced Ultrasonography" Animals 12, no. 9: 1175. https://doi.org/10.3390/ani12091175
APA StyleHanazono, K., Itami, T., Hayasaka, I., Miyoshi, K., Hori, A., Kato, K., & Endoh, D. (2022). Evaluation of Renal Blood Flow in Dogs during Short-Term Human-Dose Epoprostenol Administration Using Pulsed Doppler and Contrast-Enhanced Ultrasonography. Animals, 12(9), 1175. https://doi.org/10.3390/ani12091175







