Simple Summary
Due to environmental pollution, habitat loss, and overutilization, the wild population of Chinese giant salamanders (Andrias davidianus) is decreasing continuously. Therefore, artificial proliferation and release are effective ways to restore the wild population of this species. However, the sex of Chinese giant salamanders (CGSs) needs to be confirmed before reintroduction or rejuvenation into the wild. The sex identification of amphibians is difficult and is usually conducted through blood hormone testing, ultrasound, or even the observation of gonads and other invasive sampling methods, and the operation is cumbersome and expensive. As one of the main aspects of animal welfare, non-invasive or minimally invasive sampling is increasingly important in wildlife research. As one of the raw materials of non-invasive sampling, urine has been considered by more and more researchers engaged in wildlife physiology and biochemistry. Our objective was to identify the sex of CGSs by detecting the ratio of hormone testosterone (T) to estrone-3-glucuronide (E1G) in urine collected by minimally invasive sampling. Perhaps this method can be used for the determination of sex in other animals, especially amphibians.
Abstract
Minimally invasive sampling was used to determine the sex of Chinese giant salamanders (Andrias davidianus). Urine samples (n = 25) were collected from 6 adults in the breeding season and from 19 individuals (7 adults and 12 juveniles) in the non-breeding season. The hormone testosterone (T) and estrone-3-glucuronide (E1G) in urine were collected from Chinese giant salamanders (CGSs), and the hormone extracts were analyzed by enzyme immunoassays (EIA). The data demonstrated that the urine T concentration of the male CGSs was significantly higher than that of the females during the breeding season (p < 0.05) and even more pronounced during the non-breeding season (p < 0.01). The urine E1G concentration of the males was less pronounced than that of the females during the breeding season (p < 0.01) and significantly lower during the non-breeding season (p < 0.05). The urine T/E1G values of all the male salamanders were significantly higher than those of the females (p < 0.01) during both the breeding season and the non-breeding season. An interesting pattern was found in this study: the value of urine log10(T/E1G) of the male CGSs was higher than 1, whereas the value for the females was lower than 1, during both the breeding and non-breeding seasons, and in the adult and sub-adult age groups of CGSs. There were 25 salamanders in this study and the accuracy rate reached 100% by using a log10(T/E1G) value of 1. The results of the log10(T/E1G) value provide new insight into the future development of the sex identification of CGSs and also lay the foundation for accurate sex identification in the preparation for artificial release. This is the first study to show that the T/E1G ratio in urinary hormones is reliable for the sex identification of CGSs. Additionally, urinary hormone T/E1G measures are promising sex identification tools for amphibian or monomorphic species and for those whose secondary sex characteristics are visible only during the breeding season.
1. Introduction
The Cryptobranchid (giant salamanders) family consists of two extant genera and three currently recognized species, including the Japanese giant salamander (Andrias japonicus) and the North American giant salamander (Cryptobranchus alleganiensis) [1,2]. The Chinese giant salamander (A. davidianus) is endemic to China and is the largest amphibian of the three extant species of the family Cryptobranchidae (Figure 1). Moreover, the family Cryptobranchidae is distantly related to the family Salamandridae that emerged some 350 million years ago. As such, the Chinese giant salamander (CGS) is also considered a living fossil [3,4,5] since it represents a transitional form between aquatic and terrestrial organisms and is considered a valuable model for studying vertebrate evolution and biodiversity [3,6]. Due to the low number of wild resources caused by environmental change, water pollution, habitat loss, and overutilization in the past 50 years, the CGS has been designated as part of the national class ΙΙ protected species in China and listed in Appendices I of the Convention on International Trade in Endangered Species of Wild Fauna and Flora (CITES, 2014) since 1975. This species is also categorized as critically endangered by the IUCN Red List [7,8,9,10,11,12,13,14,15,16] because of its critically endangered status and apparent lack of recovery in the wild [14]. Moreover, the decline in the extant populations of the giant salamander in first- to third-order streams could have profound impacts on the trophic ecology within these aquatic systems [15].

Figure 1.
Photograph of Chinese giant salamander.
The farming of the CGS has been a great success in China since 2005, and approximately, 2 million CGSs are artificially reproduced per year [10]. In order to restore the historical range of the species, the rejuvenation and reintroduction of the population, funded by the Chinese government, is used as a conservation tool for the CGS via creating a captive breeding population, which has strict requirements for the sex ratio [17]. A reasonable sex ratio (1:1) is the guarantee of an artificial, successful, and stable population balance of CGSs. Unfortunately, sex identification is difficult for CGSs, especially for juveniles. Accurate sex identification has always been the “bottleneck” problem for the selection and breeding of parent CGSs in the development of the CGS breeding industry [10,12]. Moreover, sex identification is also needed in the commercial breeding farms for juvenile giant salamanders.
In the breeding season, experienced breeders can identify the sex of CGSs by their behavior, abdominal skin folds, body size, and cloacal orifice. However, there are some disadvantages to the above methods because the giant salamander is late in sexual maturity, and these characteristics are illegible in the non-breeding season. Moreover, the sex of salamanders can be determined by directly scanning the salamander’s gonads with ultrasonography methodologies or by drawing blood from the salamander’s tail vein, in which the levels of serum hormones such as estradiol, progesterone, and testosterone (T) can be detected. However, ultrasonography is more expensive and only suitable for adult CGSs during breeding season, and drawing blood is invasive to the CGS [18,19].
As one of the main components of animal welfare, non-invasive hormone monitoring has become an important part of wildlife and conservation research, especially in mammals and birds [20,21,22,23,24]. These techniques, which measure hormone metabolites in voided urine or feces, allow reproductive monitoring, stress evaluation, and sex identification in animals, either from a distance or with minimal handling. For the most part, this work has been carried out in mammals, with a few studies on birds and reptiles. In recent years, non-invasive hormone monitoring has been applied to amphibians in several sexually dimorphic anuran species [25,26,27,28].
At present, most studies on biological estrogens focus on estradiol, but there are no significant sex differences in estradiol concentration in some species. Therefore, researchers also began to select E1G, the primary metabolite of estradiol, as an indicator for detection. Although no relevant experiments have been conducted on amphibians, E1G has been used as a detection indicator for mammals. For example, E1G has been used as a major detection indicator in the study on the reproductive hormone pattern of female gibbons (Hoolock Leuconedys) during the whole maturity stage [29]. Moreover, a significant relationship was found between the color and mean concentration of the fecal E1G of white-cheeked gibbons (Nomascus leucogenys) [30]. Changes in E1G in feces have also been used as a method to detect estrus in sea lions (Eumetopias Jubatus) [31].
Previous studies found that there were no significant differences in the expression of estradiol in the blood of male and female CGSs [32]; hence, urine T and E1G, which are both primary metabolites of estradiol, were selected as research indicators of CGSs in this study. The aim of our study was to find a new minimally invasive method for the rapid and accurate sex identification of CGSs, which will benefit the captive breeding and the design of future rejuvenation and reintroduction programs for this critically endangered species.
2. Materials and Methods
2.1. Study Animals
On 27 May 2017, the urine of 6 sexually mature CGSs (6 years old, 3 males and 3 females) from a giant salamander farm in the Chenggu county of Shaanxi Province was collected and stored at −80 °C for future use. On 11 November 2021, 19 CGSs were purchased from another giant salamander farm in Xi’an city, at the northern foot of the Qinling Mountains. Among them, 7 salamanders were sexually mature (7.5 years old, 3 males and 4 females) and 12 were sub-adults (4.5 years old). The salamanders were deep anesthetized in a water bath containing tricaine methane sulfonate (MS-222) at a concentration of 600 mg·L−1. The method of anesthesia was based on the results of our previous study [33], and the anesthesia lasted about 35 min for the 7.5-year-old CGSs and 30 min for the 4.5-year-old CGSs. Finally, the deep anesthetized CGSs were euthanized for dissection to confirm their sex after the urine was collected. The urine was stored in an ultra-low-temperature freezer (−80 °C) until analysis for T and E1G hormones.
2.2. Husbandry, Management, and Sexing of the Animals
The experimental salamanders were placed in white foam boxes with a size of 60 × 40 × 20 cm and a water depth of 8 cm when purchased from aquafarms, then temporarily bred in the laboratory for two weeks and fed with fresh wild fish at 9 a.m. every day before urine collection. The sex of the CGSs could be determined by the morphological characteristics of the gonads. The female gonads showed a wide band of milky white, and the middle and rear sections were obviously curved, showing an “S” line. The eggs on the surface of the ovary could be clearly observed visually.
2.3. Experimental Reagents and Consumables
A Testosterone Enzyme Immunoassay Kit (Arbor Assays, catalog number k032-H1), an Estrone-3-Glucuronide Enzyme Immunoassay Kit (Arbor Assays, catalog number k036-H1), and a 15 mL centrifuge tube (for collecting urine samples), etc., were used in this experiment.
2.4. Experimental Apparatus
An enzyme standard instrument (Bio-Rad, Hercules, CA, USA) was used as the experimental apparatus in this study.
2.5. Experimental Methods
The concentrations of T and E1G in the urine of 6 sexually mature CGSs during breeding season and 19 salamanders during non-breeding season (7 sexually mature adults and 12 sub-adults) were detected by ELISA. The specific detection and analysis methods are described in the following section.
2.5.1. Urine Collection
After binding a CGS with a cloth bag, the CGS was placed on its back on the experimental table. The water around the cloacal orifice was gently wiped off with a clean dry towel, and urine was collected by gently squeezing the salamander’s abdomen. A 50 mL centrifuge tube was laced in the front of the orifice of the salamander to collect urine and was then quickly stored in a refrigerator at −80 °C for future detection.
2.5.2. Steps for Determining the Concentration of T and E1G in Urine
We made a standard curve according to the instructions of the Testosterone Enzyme Immunoassay Kit and the Estrone-3-Glucuronide Enzyme Immunoassay Kit, respectively. The T and E1G concentrations were detected using the two kits, following the manufacturer’s protocol.
2.6. Statistical Analysis
The standard curve was drawn by the Hill fitting curve method in ELISACalc. An independent sample T test was performed for the concentration of T and E1G in urine, obtaining the log10(T/E1G) of male and female CGSs using SPSS Statistics 24.0 software. GraphPad Prism 8.0.2 was used to plot the results, and the significant difference (p < 0.05) and extremely significant difference (p < 0.01) were represented by * and **, respectively. The T tests plus the paired test were used for the data statistics.
3. Results
3.1. Determining the Concentration of T and E1G in the Urine of CGSs during the Breeding Season
EIA was used to determine the T and E1G concentration’s standard curve. The urine of six CGSs (three males, three females) was collected for three-fold dilution. Then, the T and E1G concentration was detected. The T and E1G concentration in the urine of the six salamanders is shown in Table 1. Hill curve fitting was adopted, and the standard curve equations of the two hormones were yT = 243.00903/(103.8438 + x^0.66824, R2 = 0.9961), and yE1G = 386.21935/(240.21305 + x^1.18952, R2 = 0.9854). The T concentration in the urine of the males (4289.085 ± 2135.082) was significantly higher than that of the female salamanders during the breeding season (67.971 ± 12.406), (p < 0.05, Figure 2). The concentration of E1G in the males (12.328 ± 4.563) was extremely significantly lower than in the females (197.636 ± 64.441) during the breeding season, (p < 0.01, Figure 2).

Table 1.
T and E1G concentration in the urine of adult CGSs during the breeding season.
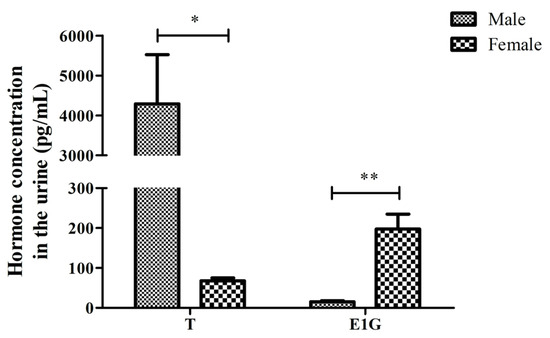
Figure 2.
The T and E1G concentration in the urine of male and female adult CGSs during the breeding season (* p < 0.05, ** p < 0.01). The values are the means (±SD) of three salamanders per group.
In this study, the differences in urine T/E1G between the adult sexes during the breeding season were found (Figure 3), and the specific ratio is shown in Table 1. The ratio of the urine T/E1G of the male salamanders ranged from 174.675 to 367.470, and that of the females ranged from 0.238 to 0.606. The value of log10(T/E1G) of the adult male salamanders (2.429 ± 0.168) was extremely significantly higher than that of the adult females (−0.452 ± 0.210), (p < 0.01, Figure 3).
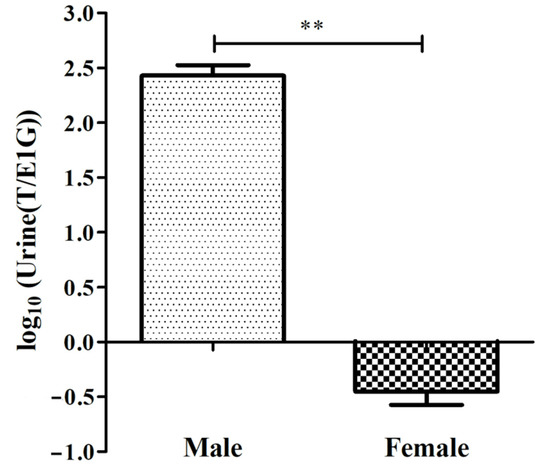
Figure 3.
log10(Urine(T/E1G)) of male and female adult CGSs during the breeding season (** p < 0.01). The values are the means (±SD) of three salamanders per group.
3.2. Verification of the Determination of CGSs’ Sex Using the T/E1G Ratio during the Non-Breeding Season
A total of 19 CGSs during the non-breeding season were prepared, including 7 adults and 12 sub-adults; the urine samples of each salamander were collected, and the salamanders were dissected to observe the gonads to determine the sex. The autopsy results showed that there were 3 males and 4 females among the 7 adult salamanders, and 7 males and 5 females among the 12 sub-adult salamanders.
The hormone test results of the urine T from the 19 salamanders are shown in Table 2. The T concentration of the male salamanders (1175.511 ± 936.903) was extremely significantly higher than that of the females (149.370 ± 203.154) (p < 0.01, Figure 4). There were no significant differences between the adult and juvenile salamanders of the same sex. The results showed that the urine T concentration in the non-reproductive season was not related to adult status but was significantly related to sex difference.

Table 2.
The concentration of urine T and E1G of 19 CGSs during the non-breeding season.
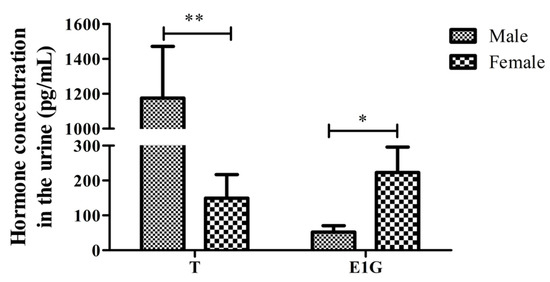
Figure 4.
The T and E1G concentration in the urine of male and female CGSs during the non-breeding season (* p < 0.05, ** p < 0.01).
The results of the urine hormone tests from the 19 CGSs during the non-breeding season are shown in Table 2. The urine E1G concentration varies greatly between individuals, with the male E1G concentrations (52.057 ± 58.885) lower than the female E1G concentrations (222.946 ± 218.721), and the difference was significant (p < 0.05). Similarly, there was no significant difference in E1G concentration between the adult and juvenile salamanders of the same sex (p > 0.05).
In our study, there was no regularity in the urine T or E1G in the male and female CGSs during the non-breeding season, except for the adult salamanders during the breeding season. Moreover, the value of urine log10(T/E1G) during the non-breeding season showed significant sexual differences (p < 0.05) (Figure 5 and Table 3). The value of the urine log10(T/E1G) of male CGSs was higher than 1, whereas that of the females was lower than 1. The accuracy of determining the sex of the adult CGSs during the breeding season was 100% when log10(T/E1G) was set to the limit of 1. The verification test of the log10(T/E1G) value “1” showed that all the 19 adult salamanders (both male and female salamanders) during the non-breeding season were completely consistent with the value “1”. That is, the urine log10(T/E1G) values of the male CGSs were all greater than 1 (between 1.050 and 2.490), and the values of the females were all less than 1 (between −2.155 and 0.720). The rate of accuracy for determining the sex of CGSs could reach 100% by using this value.

Figure 5.
log10(Urine(T/E1G)) of male and female CGSs during the non-breeding season (** p < 0.01).

Table 3.
The T/E1G ratio and log10(T/E1G) of 19 CGSs during the non-breeding season.
4. Discussion
Regarding sex differentiation, Klein and Bogart proposed a sex-determination hypothesis based on the ratio of androgen to estrogen in the gonads in the process of sexual differentiation. The hypothesis suggests that gonadal sex is determined by the local gonadal ratio of androgens to estrogen steroids. These androgens and estrogens compete to initiate and maintain different steroid-induced gene transcription pathways. In vertebrates, this steroid ratio is usually controlled by the activity of aromatase, which converts the androgen T into estrogen. The early ratio of androgen to estrogen can determine sex differentiation. When the ratio of androgen to estrogen is low, the ovary develops, whereas the testis develops when the ratio is high; this idea is known as the “equilibrium hypothesis” [34]. Our findings showed that T/E1G was lower in female CGSs and higher in male salamanders, which is in agreement with this “equilibrium hypothesis”. In this study, the urine T and E1G expression levels of the young CGSs (e.g., 2 years old and 3 years old) were lacking, and we will pay attention to this area in future studies.
Sex hormones are one of the main factors affecting sex determination in amphibian species [35]. The three common estrogens, estradiol, estrone, and estriol, can combine with the estrogen receptor to produce a range of biological effects. Studies have shown that estradiol from ovarian granulosa cells is rich in content and has strong activity, which can act on organs such as the uterus and the pituitary gland [35,36]. The primary metabolite is E1G, which was also commonly used to detect female ovarian function in basic research and clinical research [37]. At present, there are few studies on sex hormones in CGSs, and only the annual changes in the urine sex hormones and their relationship with gonadal development and reproduction have been reported [38].
However, to identify the sex of the animals, the ratio of estrogens to androgens in fecal samples was the most common measurement utilized [39,40]. No researchers had ever linked T/E1G ratio to the sex of animals; thus, it was difficult to discuss or compare the results of this study with those of other studies. The ability to measure sex hormone concentrations within individuals has important research and conservation implications. Conventional studies have used invasive blood collection to achieve this in the past [41,42,43,44]. Although collecting fecal samples is much less invasive and easier than collecting blood, there are also many disadvantages to this method [25]. In particular, for the aquatic animals such as the CGS, the feces are washed away or diluted when they defecate, and the urine is discharged directly into the water and cannot be collected directly. Indeed, we have not found the opportunity to sample the urine in other non-invasive approaches at present. The urine sampling method adopted in this study is easier to operate with minimal invasion than drawing blood from the tail vein. Compared with ultrasonography, our method is not limited only to adult CGSs during the breeding season but is also suitable for sub-adults, both in the breeding and non-breeding season. We hope to develop a urine detection kit for CGSs in the future, which will make the sex detection of CGSs more convenient, faster, and cheaper.
Both males and females produce androgens and estrogens due to the steroidogenic pathway (i.e., testosterone is a precursor of estradiol) [45,46]. The males of some species (e.g., pigs [47] and horses [48]) produce large amounts of estrogen compared with females of the same species. Although female neotropical otters (Lontra longicaudis) have higher levels of male hormones in fecal samples during the breeding season [40], in this study the urine T concentration of the male CGSs was significantly higher than that of the females, and the concentration of urine E1G of the males was less pronounced than that of the female salamanders. This differs from the Lontra longicaudis [40] and is consistent with the findings on bell frogs (Litoria castanea) [25].
Previous studies found that T and estradiol could be detected in the blood of both breeding and non-breeding CGSs, but there was no significant difference in the blood levels of the two hormones [32], which was different from the results of this study. This result may be caused by different tissues, or it may be related to the growth of CGSs, which needs further study.
As the breeding season of CGSs is during the flood season from June to July of every year, flood disasters affect the survival of artificially released CGSs. Consequently, the release of CGSs usually occurs in March to May before the flood season [49], which is during the non-breeding season of the salamanders. Therefore, the sex identification of the salamanders is particularly important at this time.
This is the first study to characterize the relationship between the T and E1G concentrations in the urine of CGSs. The findings should be further verified by increasing the sample size. In the future, this minimally invasive sampling method could be used to determine the sex of CGSs by measuring the urine sex hormones and also could provide a new approach for the sex identification of other vertebrates with no obvious secondary sex characteristics. We will increase the number of samples to verify the findings in this study in the future, especially CGS juveniles aged 2–3 years, during both the breeding and non-breeding seasons.
5. Conclusions
In conclusion, an interesting pattern of urine T and E1G was identified for CGSs. In other words, the sex of CGSs can be accurately determined by T/E1G in urine hormones; when there is a log10(T/E1G) value greater than 1, the salamander is male, and when the log10(T/E1G) value is less than 1, the salamander is female. This is applicable to both sub-adults and adults, as well as breeding and non-breeding salamanders.
Author Contributions
Conceptualization, J.Z., J.H. and H.Z. (Hu Zhao); methodology, H.Z. (Hongxing Zhang) and F.K.; formal analysis, J.D. and J.H.; writing—original draft preparation, J.Z.; writing—review and editing, F.K. and Q.W.; supervision, H.Z. (Hongxing Zhang) and Q.W.; funding acquisition, Q.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was jointly supported by the Shaanxi Academy of Sciences (2015K-03), Shaanxi Science and Technology Department (2018NY-108), and the Natural Science Foundation of Shaanxi Province of China (2020JQ-972).
Institutional Review Board Statement
The animal study protocol was approved by the Animal Ethics Committee of the Shaanxi Institute of Zoology (protocol code: L22D001A51, date of approval: 28 March 2022) for studies involving animals.
Informed Consent Statement
Not applicable, as this research did not involve any humans.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
Our sincere thanks go to Hong Liu and Hong Ji for their technical guidance in this study. We also thank Jing-jing Tian and Cai-xia Lei for their help in conducting the EIA analysis.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Browne, R.K.; Wang, Z.H.; Okada, S.; Hime, P.; McMillan, A.; Wu, M.Y.; Diaz, R.; McGinnity, D.; Briggler, J.T. The giant salamanders (Cryptobranchidae): Part B. Biogeography, ecology and reproduction. Amphib. Reptile Conserv. 2014, 5, 30–50. [Google Scholar]
- Liang, G.; Geng, B.R.; Zhao, E.M. Andrias Davidianus. In The IUCN Red List of Threatened Species; IUCN: Gland, Switzerland, 2004; Available online: https://www.iucnredlist.org/species/1272/3375181 (accessed on 10 March 2022).
- Gao, K.Q.; Shubin, N.H. Earliest known crown-group salamanders. Nature 2003, 422, 424–428. [Google Scholar] [CrossRef]
- Pyron, R.A.; Wiens, J.J. A Large-Scale Phylogeny of Amphibia Including Over 2800 Species, and a Revised Classification of Extant Frogs, Salamanders, and Caecilians. Mol. Phylogenet. Evol. 2011, 61, 543–583. [Google Scholar] [CrossRef] [PubMed]
- Luo, Q.H.; Tong, F.; Song, Y.J.; Wang, H.; Du, M.L.; Ji, H.B. Observation of the breeding behavior of the Chinese giant salamander (Andrias davidianus) using a digital monitoring system. Animals 2018, 8, 161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ijspeert, A.J.; Crespi, A.; Ryczko, D.; Cabelguen, J.M. From swimming to walking with a salamander robot driven by a spinal cord model. Science 2007, 315, 1416–1420. [Google Scholar] [CrossRef] [Green Version]
- CITES. Convention on International Trade in Endangered Species of Wild Fauna and Flora. Appendices I, II and III. 2014. Available online: http://cites.org/eng/app/appendices.php (accessed on 11 March 2022).
- IUCN. IUCN Red List of Threatened Species v. 2012. Available online: http://www.iucnredlist.org (accessed on 10 March 2022).
- Zhang, P.; Chen, Y.Q.; Liu, Y.F.; Zhou, H.; Qu, L.H. The Complete Mitochondrial Genome of the Chinese giant salamander, Andrias Davidianus (Amphibia: Caudata). Gene 2003, 311, 93–98. [Google Scholar] [CrossRef]
- Meng, Y.; Ma, J.; Jiang, N.; Zeng, L.B.; Xiao, H.B. Pathological and microbiological findings from mortality of the Chinese giant salamander (Andrias Davidianus). Arch. Virol. 2014, 159, 1403–1412. [Google Scholar] [CrossRef]
- Jiang, N.; Fan, Y.D.; Zhou, Y.; Liu, W.Z.; Ma, J.; Meng, Y.; Xie, C.X.; Zeng, L.B. Characterization of Chinese giant salamander iridovirus tissue tropism and inflammatory response after infection. Dis. Aquat. Organisms. 2015, 114, 229–237. [Google Scholar] [CrossRef] [Green Version]
- Cunningham, A.A.; Turvey, S.T.; Zhou, F.; Meredith, H.M.R.; Guan, W.; Liu, X.L.; Sun, C.M.; Wang, Z.Q.; Wu, M.Y. Development of the Chinese giant salamander Andrias davidianus farming industry in Shaanxi Province, China: Conservation threats and opportunities. Oryx 2015, 50, 265–273. [Google Scholar] [CrossRef] [Green Version]
- Murphy, R.W.; Fu, J.; Upton, D.E.; Lema, T.D.; Zhao, E.M. Genetic variability among endangered Chinese giant salamanders, Andrias davidianus. Mol. Ecol. 2010, 9, 1539–1547. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.M.; Zhang, K.J.; Wang, Z.H.; Ding, Y.Z.; Wu, W.; Huang, S. The decline of the Chinese giant salamander Andrias davidianus and implications for its conservation. Oryx 2004, 38, 197–202. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.J.; Zhang, L.; Zhao, H.; Zhao, Q.; Deng, J.; Kong, F.; Jiang, W.; Zhang, H.X.; Liu, H.; Kouba, A. Abiotic and biotic influences on the movement of reintroduced Chinese giant salamanders (Andrias davidianus) in two montane rivers. Animals 2021, 11, 1480. [Google Scholar] [CrossRef] [PubMed]
- Stuart, S.N.; Chanson, J.S.; Cox, N.A.; Young, B.E.; Rodrigues, A.S.L.; Fischman, D.L.; Willers, R.W. Status and trends of amphibian declines and extinctions world-wide. Science 2004, 306, 1783–1786. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, L.; Jiang, W.; Wang, Q.J.; Zhao, H.; Zhang, H.X.; Marcec, R.M.; Willard, S.T.; Kouba, A.J. Reintroduction and post-release survival of a living fossil: The Chinese giant salamander. PLoS ONE 2016, 11, e0156715. [Google Scholar] [CrossRef]
- Li, P.Q.; Zhu, B.C.; Wang, Y.F.; Xiang, X.J. Sex identification of Chinese giant salamanders (Andrias davidianus) by Doppler B-ultrasoud method. J. Biol. 2010, 27, 94–96. (In Chinese) [Google Scholar]
- Zang, K.J.; Wang, X.M.; Wu, W.; Wang, Z.H.; Huang, S. Advances in conservation biology of Chinese giant salamander. Biodivers. Sci. 2002, 3, 291–297. (In Chinese) [Google Scholar]
- Bauer, B.; Palme, R.; Machatschke, I.H.; Dittami, J.; Huber, S. Noninvasive measurement of adrenocortical and gonadal activity in male and female guinea pigs (Cavia aperea f. porcellus). Gen. Comp. Endocrinol. 2008, 156, 482–489. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, J.L.; Wasser, S.K.; Wildt, D.E.; Graham, L.H. Comparative aspects of steroid hormone metabolism and ovarian activity in felids, measured noninvasively in feces. Biol. Reprod. 1994, 51, 776–786. [Google Scholar] [CrossRef] [Green Version]
- Kirkpatrick, J.F.; Bancroft, K.; Kincy, V. Pregnancy and ovulation detection in bison (Bison bison) assessed by means of urinary and fecal steroids. J. Wildlife Dis. 1992, 28, 590–597. [Google Scholar] [CrossRef]
- Cry, N.E.; Romero, L.M. Fecal glucocorticoid metabolites of experimentally stressed captive and free-living starlings: Implications for conservation research. Gen. Comp. Endocrinol. 2008, 158, 20–28. [Google Scholar] [CrossRef]
- Goymann, W. Noninvasive monitoring of hormones in bird droppings: Physiological validation, sampling, extraction, sex differences, and the influence of diet on hormone metabolite levels. Ann. N. Y. Acad. Sci. 2005, 1046, 35–53. [Google Scholar] [CrossRef] [PubMed]
- Germano, J.M.; Molinia, F.C.; Bishop, P.J.; Cree, A. Urinary hormone analysis assists reproductive monitoring and sex identification of bell frogs (Litoria raniformis). Theriogenology 2009, 72, 663–671. [Google Scholar] [CrossRef]
- Narayan, E.J.; Molinia, F.C.; Christi, K.S.; Morley, C.G.; Cockrem, J.F. Annual cycles of urinary reproductive steroid concentrations in wild and captive endangered Fijian ground frogs (Platymantis vitiana). Gen. Comp. Endocrinol. 2009, 166, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Narayan, E.J.; Molinia, F.C.; Kindermann, C.; Cockrem, J.F.; Hero, J. Urinary corticosterone responses to capture and toe-clipping in the cane toad (Rhinella marina) indicate that toe-clipping is a stressor for amphibians. Gen. Comp. Endocrinol. 2011, 174, 238–245. [Google Scholar] [CrossRef]
- Szymanski, D.C.; Gist, D.H.; Roth, T.L. Anuran gender identification by fecal steroid analysis. Zoo Biol. 2006, 25, 35–46. [Google Scholar] [CrossRef]
- Guntrum, E.B.; Haley, A.M.; Margulis, S.W. Characterization of cycling in a Hoolock Gibbon (Hoolock leuconedys). Folia Primatol. 2021, 92, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Margulis, S.W.; Hálfdanardóttir, M.R. Hormones and color change in female White-Cheeked Gibbons, Nomascus leucogenys. Int. J. Primatol. 2021, 42, 201–219. [Google Scholar] [CrossRef]
- Sattler, R.; Bishop, A.; Woodie, K.; Polasek, L. Characterizing estrus by trans-abdominal ultrasounds, fecal estrone-3-glucuronide, and vaginal cytology in the Steller sea lion (Eumetopias jubatus). Theriogenology 2018, 120, 25–32. [Google Scholar] [CrossRef]
- Li, P.Q.; Xiang, X.J.; Zhu, B.C. Preliminary study on the reproductive hormones of Chinese giant salamander Andrias davidianus. J. Biol. 2008, 03, 30–32. (In Chinese) [Google Scholar]
- Jiang, W.; Wang, Q.J.; Zhao, H.; Zhang, H.X.; Andy, K.; Zhang, L.; Ruth, M. Effectiveness of MS-222 as anesthetic agents for Andrias davidianus. Freshwater Fisheries 2014, 44, 94–97. (In Chinese) [Google Scholar]
- Klein, S.S.; Bogart, K. Achieving sex equity in education: A comparison at Pre-and Post-Secondary levels. Equity Excell. Educ. 1987, 23, 114–122. [Google Scholar] [CrossRef]
- Nakamura, M. The mechanism of sex determination in vertebrates-are sex steroids the key-factor? J. Exp. Zool. A Ecol. Genet. Physiol. 2010, 313, 381–398. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, M. Is a sex-determining gene(s) necessary for sex-determination in amphibians? Steroid hormones may be the key factor. Sex. Dev. 2013, 7, 104–114. [Google Scholar] [CrossRef] [PubMed]
- Blackwell, L.F.; Cooke, D.G.; Brown, S. The Use of Estrone-3-Glucuronide and Pregnanediol-3-Glucuronide Excretion Rates to Navigate the Continuum of Ovarian Activity. Front. Public. Health 2018, 31, 153. [Google Scholar] [CrossRef]
- Zou, Z.Y.; Wang, H.W.; Ou, D.S.; He, W. Seasonal changes of sexual hormone level in Andrias davidianus urinarys and its relationship with breeding. J. Hydroecol. 2012, 33, 128–131. (In Chinese) [Google Scholar]
- Kalz, B.; Jewgenow, K.; Fickel, J. Structure of an otter (Lutra lutra) population in Germany-results of DNA and hormone analyses from faecal samples. Mamm. Biol. 2006, 71, 321–335. [Google Scholar] [CrossRef]
- Prado-Ortiz, L.E.; Valdespino, C.; Romano, M.; González-Romero, A. Quantification of immunoreactive testosterone and estradiol-17ß metabolites to identify the sex of Neotropical otters (Lontra longicaudis annectens) in the field. Anim. Reprod. Sci. 2020, 222, 106607. [Google Scholar] [CrossRef]
- Lynch, K.S.; Wilczynski, W. Gonadal steroids vary with reproductive stage in a tropically breeding anuran. Gen. Comp. Endocrinol. 2005, 143, 51–56. [Google Scholar] [CrossRef]
- Medina, M.F.; Ines, R.; Crespo, C.A.; Gonzalez-Calvar, S.; Fenandez, S.L. Changes in serum sex steroid levels throught the reproductive cycle of Bufo arenarum females. Gen. Comp. Endocrinol. 2004, 136, 143–151. [Google Scholar] [CrossRef]
- Mendoca, M.T.; Licht, P.; Ryan, M.J.; Barnes, R. Changes in hormone levels in relation to breeding behavior in male bullfrogs (Rana catesbeiana) at the individual and population levels. Gen. Comp. Endocrinol. 1985, 58, 270–279. [Google Scholar] [CrossRef]
- Rastogi, R.K.; Iela, L.; Delrio, G.; Bagnara, J.T. Reproduction in the Mexican leaf frog, Pachymedusa dacnicolor II. The Male. Gen. Comp. Endocrinol. 1986, 62, 23–35. [Google Scholar] [CrossRef]
- Dehnhard, M.; Naidenko, S.; Frank, A.; Braun, B.; Goritz, F.; Jewgenow, K. Non-invasive monitoring of hormones: A tool to improve reproduction in captive breeding of the Eurasian lynx hormone monitoring in breeding programmes of mammals. Reprod. Dom. Anim. 2008, 43, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Pribbenow, S.; Wachter, B.; Ludwig, C.; Weigold, A.; Dehnhard, M. Validation of an enzyme-immunoassay for the non-invasive monitoring of faecal testosterone metabolites in male cheetahs (Acinonyx jubatus). Gen. Compar. Endocrinol. 2016, 228, 40–47. [Google Scholar] [CrossRef]
- Anderson, L.L. Pigs. In Reproduction in Farm Animals; Hafez, E.S.E., Ed.; Lea & Febiger: Philadelphia, PA, USA, 1992; pp. 343–360. [Google Scholar]
- Hafez, E.S.E. (Ed.) Horses. In Reproduction in Farm Animals; Lea & Febiger: Philadelphia, PA, USA, 1992; pp. 361–384. [Google Scholar]
- Lin, Y.; Gan, C.; Wang, Y. Evaluation of the Effect of releasing Giant Salamanders into the Shuaishui River. J. Hydroecol. 2017, 38, 88–96. (In Chinese) [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).