Simple Summary
In an era of shrinking stocks and intensive fisheries production, pathogenic microorganisms are a serious threat to fish health. This study confirms that Vibrio metschnikovii—a pathogen mainly found in aquatic environments—was the pathogenic bacterium causing disease in hybrid sturgeon. The authors reveal the hazard of V. metschnikovii to hybrid sturgeon and the potential risks to the sturgeon farming industry.
Abstract
In July 2021, a disease with a high mortality rate broke out in freshwater cultured hybrid sturgeon in Zhengzhou, Henan Province. A dominant strain, H-701, was isolated from diseased fish; physiological changes in diseased fish were investigated and molecular identification, biochemical characterization, and pathogenicity and drug sensitivity tests of H-701 were performed. The 16S rRNA gene sequence of H-701 was 99.86% homologous with that of Vibrio metschnikovii in GenBank. The 50% lethal dose of H-701 was 3.72 ± 0.929 × 104 CFU/g fish weight. The proportion of monocytes, neutrophils, and eosinophils in the blood of diseased sturgeon increased significantly, whereas the proportion of lymphocytes decreased. In diseased fish, the serum levels of total protein, albumin, globulin, and alkaline phosphatase decreased significantly, and those of aspartate aminotransferase, alanine aminotransferase, and complement C3 increased significantly. There were obvious pathological changes in several tissues of the diseased fish. H-701 was sensitive to antibiotics such as florfenicol, enrofloxacin, and doxycycline. This study not only demonstrated that V. metschnikovii was the cause of death of a large number of hybrid sturgeon but also revealed its potential risk in hybrid sturgeon aquaculture. The results provide a basis for the diagnosis and prevention of this disease.
1. Introduction
Sturgeons, belonging to the order Acipenseriformes, are among the most primitive fishes in existence [1]. Sturgeons have great research value and extremely high economic value [2]. Owing to the changes in their living environment and the overexploitation and utilization of their resources, most sturgeon species in nature are classified as endangered [3]. They are protected and managed by the International Union for Conservation of Nature (IUCN; www.iucn.org) and the Convention on International Trade in Endangered Species (CITES) [4]. Considering the lengthy maturation period of sturgeon species under natural conditions, it takes a long time for their populations to recover after being destroyed; however, the reproductive ability of hybrid sturgeon is higher than that of the parents which signifies the advantages of hybridization [5,6]. Hybrid sturgeon species (Huso dauricus ♀ × Acipenser schrenckii ♂, Acipenser schrenckii ♀ × Huso dauricus ♂, and Acipenser baerii ♀ × Acipenser schrenckii ♂) constitute the main commercial breeding varieties in China and are associated with remarkable economic benefits [3].
At present, bacterial and viral diseases are the most important diseases in sturgeon culture. The main bacterial pathogens are Aeromonas hydrophila [7], Streptococcus iniae [8], Streptococcus dysgalactiae [9], Edwardsiella tarda [10], and Mycobacterium ulcerans [11]. The viral pathogens common in sturgeon are Acipenser Iridovirus European [12], sturgeon nucleocytoplasmatic large DNA virus [13], herpesviruses, and mimiviruses [14]. Bacterial infections are considered one of the main sources of sturgeon disease in China [7,8,9,10].
In July 2021, a large number of hybrid sturgeon died at a sturgeon breeding company in Zhengzhou, Henan Province. Initially, some sick hybrid sturgeon in a pond stopped eating and swam slowly. After 5 days, the diseased hybrid sturgeon began to die, with a gradual increase in mortality, resulting in more than 50 fish deaths per day. The death of the sick fish lasted for 15 days, and the condition was controlled after drug treatment. The present study aimed to investigate the etiology of this disease. To this end, a bacterial strain was isolated from a naturally diseased hybrid sturgeon and identified by phenotypic and molecular analysis, biochemical analysis, and a pathogenicity test. Blood analysis of diseased hybrid sturgeon and histopathological injury observations were performed to investigate the changes in blood parameters and pathological characteristics of the injury.
2. Materials and Methods
2.1. Fish
The diseased hybrid sturgeon (Acipenser baerii ♀ × Acipenser schrenckii ♂) (body length approximately 65 ± 10 cm) came from an aquaculture company in Zhengzhou city, Henan Province. We made a preliminary diagnosis of the diseased hybrid sturgeon (n = 6) on site, recorded the clinical symptoms, and collected fresh blood and tissue samples. Some of the diseased hybrid sturgeon (n = 6) were transported to the laboratory in oxygenated bags to isolate the pathogen. Healthy hybrid sturgeon (n = 600) with no disease history (body length approximately 15 ± 2 cm) were purchased from a sturgeon farm in Yichang city, Hubei Province. Healthy hybrid sturgeon were kept in a recirculating aquaculture system for 14 days to allow them to acclimatize to the environment. During the acclimatization period, the water temperature was 20 ± 1 °C and the fish were fed with commercial feed twice a day. All animal experiments were approved by the Animal Experimental Ethical Inspection of Laboratory Animal Centre, Yangtze River Fisheries Research Institute, Chinese Academy of Fishery Sciences (ID Number: YFI 2021-zhouyong-06).
2.2. Isolation
Diseased sturgeon were euthanized in 0.04% tricaine methane sulfonate (MS-222; Sigma, Saint Louis, MO, USA). The liver and kidney tissues of diseased sturgeon were sampled with a sterile inoculation loop, streaked on Brain Heart Infusion (BHI; HopeBio, Qingdao, China) agar medium, and cultured at 28 °C for 24 h. Single colonies were selected and streaked on BHI agar to obtain pure culture colonies. Two dominant colonies were picked on each plate, and a total of 12 bacteria were obtained. Picked colonies were streaked on BHI agar to obtain pure cultures. After 16S rRNA gene sequencing of 12 isolates, the isolates were identified as the same species. The isolated strain was named H-701.
2.3. Morphological Analysis
The H-701 colony was diluted with phosphate buffer saline (PBS). The diluted solution was applied on glass slides, dried, fixed, and stained with Gram stain (Jiancheng, Nanjing, China), and the morphological characteristics of the cells were observed using an optical microscope (Olympus, Tokyo, Japan) [15]. The bacteria were fixed in 2.5% glutaraldehyde solution and dried by dehydration. The morphology of the bacteria was observed, and images were taken using a scanning electron microscope (Hitachi, Tokyo, Japan) [16].
2.4. 16S rRNA Gene Analysis
The H-701 strain DNA was extracted using a Bacterial Genomic Extraction Kit (Tiangen, China). PCR amplification was performed using 16S rRNA gene universal primers (F: 5′-AGAGTTTGATCATGGCTCAG-3′, R: 5′-TACGGTTACCTT GTTACGACTT-3′) [17]. PCR conditions were as follows: 30 s at 94 °C, 30 s at 57 °C, and 90 s at 72 °C for 32 cycles [18]. PCR products were resolved on 1.5% agarose gel electrophoresis. The gel recovery kit (OMEGA, GA, USA) was used for DNA recovery from agarose gels. The DNA was cloned into the pMD19-T vector (TaKaRa, Dalian, China) and sequenced (Huayu Gene, Inc., Wuhan, China).
2.5. Biochemical Identification
The biochemical identification was accomplished using a Biolog microbial identification system (Biolog, Hayward, CA, USA). The H-701 strain was picked with a sterile cotton swab and inoculated into the IF-A inoculation solution. The IF-A inoculum solution with strain H-701 was then added to the GEN III assay plate (100 µL per well). The GEN III identification plate was placed into the Biolog microbial identification system for automatic identification.
2.6. Blood Parameter Analysis
Differential leukocyte counts (DLC) were performed to classify and count leukocytes. Diseased hybrid sturgeon (n = 6) and healthy hybrid sturgeon (n = 6) were anesthetized, and blood was drawn from the tail vein using a disposable sterile syringe and immediately added to a slide to make a blood smear. Three blood smears were made per fish and stained with Wright–Giemsa stain (Baso, Zhuhai, China). White blood cells (n = 200) were observed on each blood smear under the oil lens of a microscope and classified and counted [19].
For the determination of serum biochemical indicators, the drawn blood was placed in a centrifuge tube to allow natural coagulation, left at 4 °C for 30 min, and centrifuged at 2000× g for 10 min. The upper serum layer was transferred to a new centrifuge tube for testing. The activity of aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (ALP) and content of total protein (TP), albumin (ALB), globulin (GLB), and complement C3 in the serum were determined by the automatic biochemical analyzer (Sysmex, Kobe, Japan).
2.7. Observation of Histopathology
The liver, spleen, kidney, gill, intestine, and heart tissues of diseased hybrid sturgeon were collected, fixed in 4% paraformaldehyde solution for 24 h, subjected to gradient alcohol dehydration, and embedded in paraffin. Continuous sectioning (5 µm) was performed using a rotary microtome (Leica, Wetzlar, Germany); the sections were stained with hematoxylin-eosin (HE, Solarbio, Beijing, China) and sealed with neutral resin [20]. Histopathological changes were observed with an optical microscope (Olympus, Tokyo, Japan).
2.8. Pathogenicity
Bacterial concentration was calculated using the plate colony counting method [21]. The healthy hybrid sturgeon were randomly divided into six groups with 30 fish per group. The experimental fish in each group were raised in an independent circulating water system, and the size of the water tank was 1 m × 0.8 m × 0.6 m. The water temperature was 20 ± 1 °C and the pH value was 7.5. The experimental groups were injected intraperitoneally with bacterial solutions of 103, 104, 105, 106, and 107 colony-forming units (CFU)/g fish weight. The control group fish were injected with sterile PBS. After infection, the inoculated fish were observed for 10 consecutive days, and the number of deaths in each group was counted every day. Bacteria were isolated from diseased fish and identified again. The experiment was repeated three times. The 50% lethal dose (LD50) value of the H-701 strain was calculated by the method of Reed and Muench [22].
2.9. Drug Sensitivity
The isolated strain was tested for drug susceptibility using the Kirby-Bauer disk diffusion method [23]. In a biological safety cabinet (Esco, Singapore), strain H-701 was inoculated into the BHI liquid medium and cultured with shaking at 200 rotations per min at 28 °C for 20 h. The culture was centrifuged at 3000× g for 5 min to remove the liquid. The bacterial precipitation was adjusted to 0.5 McFarland standard for turbidity using sterile normal saline. The bacterial suspension was evenly spread on BHI agar plates (100 µL/plate), and paper discs containing antibiotics were then placed on each plate (Hangwei, Hangzhou, China). The plates were placed at a constant temperature of 28 °C for 24 h, and then the diameters of the inhibition zones were measured. The isolates were classified as sensitive (S), moderately sensitive (M), or resistant (R) according to the National Committee for Clinical Laboratory Standards (NCCLS).
2.10. Statistics
The data were analyzed by SPSS software (SPSS, version 19.0). Statistical analysis was performed using analysis of variance (ANOVA). The significance of differences between means was evaluated by Duncan’s multiple range test. Differences were considered significant at a level of p < 0.05.
3. Results
3.1. Clinical Signs
Diseased hybrid sturgeon floated on the water surface and rolled over as they could not keep their balance. Hemorrhages on the body surface and no feeding were observed. After dissection, the gill filaments were observed to be dark red. A small number of ascites were found in the abdominal cavity; the spleen was hyperemic, and the liver was white (Figure 1).
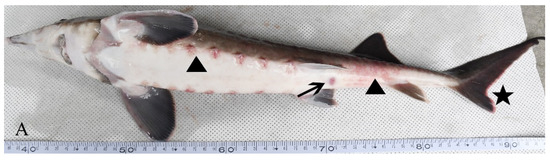
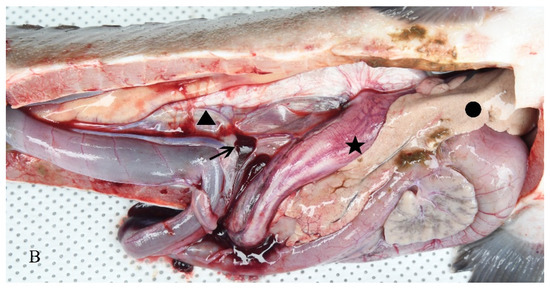
Figure 1.
Clinical symptoms of an H-701-infected hybrid sturgeon: (A) Body surface (triangle), cloacal orifice (arrow), and fin (asterisk); (B) Ascites (triangle), liver whitening (dot), intestinal wall bleeding (asterisk), and spleen redness (arrow).
3.2. Colony Morphology
The H-701 strain was grown on a BHI agar medium, and the colony surface was smooth, moist, and grayish white. Optical microscopy showed that the cells were stained red (Gram-negative) after Gram staining and were curved, short, and rod-shaped (Figure 2). Scanning electron microscopy showed that the bacterial cells were arc-shaped and approximately 1.5 µm long (Figure 2).
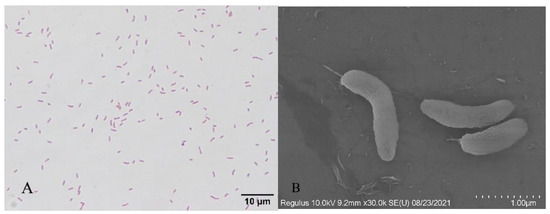
Figure 2.
Gram staining and scanning electron microscopy of bacterium H-701: (A) Gram staining (bar scale: 10 µm); (B) Scanning electron microscopy (bar scale: 1 µm).
3.3. Phylogenetic Analysis
The nucleotide sequence of the H-701 strain was compared with the NCBI database (http://blast.ncbi.nlm.nih.gov), and it showed >99.86% homology with the 16S rRNA gene sequence of Vibrio metschnikovii included in GenBank (GenBank accession no. KT986183.1). Using MEGA 6.0 software, the nucleotide sequences of the isolated strains were analyzed for homology with the nucleotide sequences of similar strains downloaded from NCBI. A phylogenetic tree was constructed using the neighbor-joining method [24]. The H-701 strain was clustered in the same clade as V. metschnikovii (Figure 3).
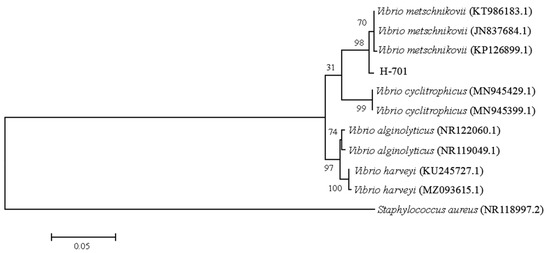
Figure 3.
Phylogenetic tree of strain H-701 based on 16S rRNA gene sequences. The values at the node indicate the percentage of trees in which this grouping occurred after bootstrapping the data (1000 replicates). The scale bar indicates the number of substitutions per site.
3.4. Bacterial Biochemical Identification
The Biolog microbial identification system compared the H-701 strain with the database on the basis of its biochemical reaction and determined that the strain was V. metschnikovii (Table 1).

Table 1.
Biochemical identification results of strain H-701.
3.5. Differential Leukocyte Counts
In terms of the number of white blood cells (Figure 4), the most numerous cells in diseased hybrid sturgeon were lymphocytes and neutrophils, followed by monocytes, and the least numerous were eosinophils. The most numerous white blood cells in healthy hybrid sturgeon were lymphocytes, followed by neutrophils and monocytes, and the least numerous were eosinophils. Diseased hybrid sturgeon had a significantly higher proportion of neutrophils (p < 0.01), monocytes (p < 0.01), and eosinophils (p < 0.05) and a significantly lower proportion of lymphocytes (p < 0.01) than healthy hybrid sturgeon.
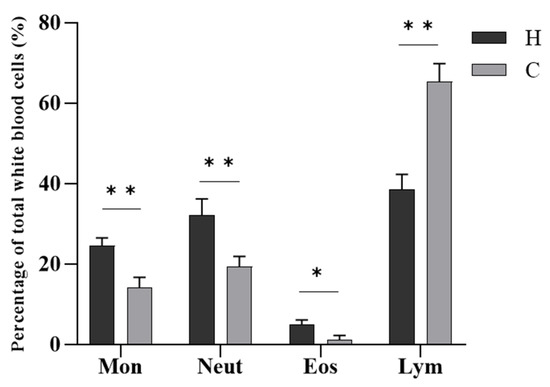
Figure 4.
Differential leukocyte counts of hybrid sturgeon. Mon, monocytes; Neut, neutrophils; Eos, eosinophils; Lym, lymphocytes; H, diseased hybrid sturgeon (H-701); C, healthy hybrid sturgeon (Control); * p < 0.05; ** p < 0.01.
3.6. Serum Biochemical Analysis
The serum biochemical analysis showed (Figure 5) that the levels of TP, ALB, and GLB in diseased hybrid sturgeon were significantly lower (p < 0.05) than those in healthy hybrid sturgeon. In diseased hybrid sturgeon, the AST and ALT levels were significantly higher and ALP levels were significantly lower (p < 0.01) than those in healthy hybrid sturgeon. Complement C3 was significantly higher in diseased hybrid sturgeon than in healthy hybrid sturgeon (p < 0.01).
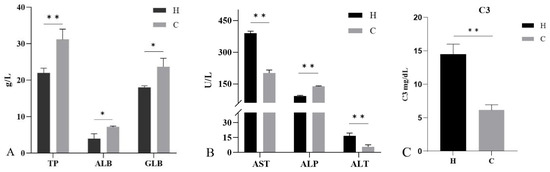
Figure 5.
Serum biochemical indices of hybrid sturgeon: (A) Total protein (TP), albumin (ALB), and globulin (GLB); (B) Aspartate aminotransferase (AST), alkaline phosphatase (ALP), and alanine aminotransferase (ALT); (C) Complement C3 (C3). H, diseased hybrid sturgeon (H-701); C, healthy hybrid sturgeon (Control); * p < 0.05; ** p < 0.01.
3.7. Histopathological Changes
The histopathological observations revealed pathological changes in several tissues of diseased hybrid sturgeon (Figure 6). The epithelial cells of the gill lamellae proliferated and formed a hyperplastic fusion. The liver showed pathological damage with hepatocytes enlarged, nuclei shifted, and inflammatory cells infiltrated. The spleen was cytopathic and vacuolized, and a large number of eosinophils appeared. Glomerular atrophy, renal tubular epithelial cell necrosis, nuclear lysis, and renal interstitial inflammatory cell infiltration also occurred. Moreover, epicardial cysts, adipose tissue hyperplasia, and nuclear deformation and dissolution were observed in diseased hybrid sturgeon. The epidermal cells of intestinal villi were exfoliated, and the submucosa was loose.
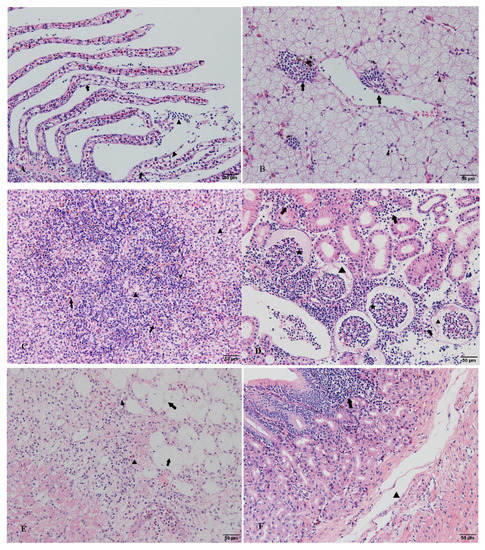
Figure 6.
Histopathological observation of diseased hybrid sturgeon (scale: 50 µm): (A) Hyperplasia of epithelial cells in gill lamellae, hyperplastic fusion (arrow), inflammatory cell infiltration (triangle); (B) Hepatocyte swelling and degeneration, nuclear concentration and migration (triangle), and inflammatory cell infiltration (arrow); the presence of phagocytes in liver tissue (asterisk); (C) The white and red pulp structures of the spleen were blurred, cytopathic, and vacuolated (triangle) with numerous eosinophils (arrow); (D) Renal tubular epithelial cell necrosis, nuclear lysis, and inflammatory cell infiltration (lymphocytes, monocytes, eosinophils, etc.) of renal interstitial tissue (arrow). (E) Epicardial cyst, the proliferation of adipose tissue at the cyst site (arrow), nuclear deformation, dissolution, and extensive inflammatory cell infiltration (triangle). (F) The epidermal cells of the intestinal villi were exfoliated; the mucosal layer was infiltrated by inflammatory cells (arrow), the submucosa was loose, and the gap was enlarged (triangle).
3.8. Pathogenicity
In the pathogenicity test, death occurred in all fish groups treated with different concentrations of bacterial suspension, and none of the fish in the 107 CFU/g fish weight experimental group survived (Figure 7). Dead hybrid sturgeon showed obvious bleeding on the body surface, redness and swelling of the anus, and visceral hyperemia. According to the experimental results, the LD50 of H-701 was 3.72 ± 0.929 × 104 CFU/g fish weight. V. metschnikovii was again isolated from artificially infected hybrid sturgeon.
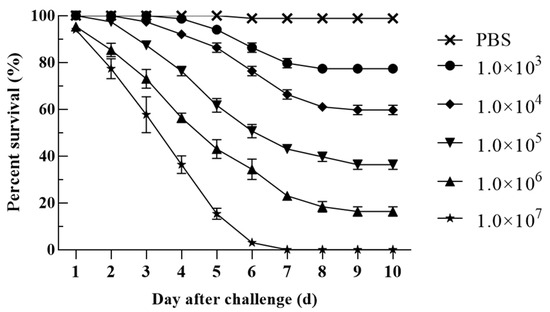
Figure 7.
Pathogenicity of different concentrations of H-701 bacterial solution to hybrid sturgeon (Injection concentration: CFU/g fish weight).
3.9. Drug Sensitivity Analysis
The H-701 strain isolated from diseased hybrid sturgeon was sensitive to the antibiotics florfenicol, enrofloxacin, doxycycline, ampicillin, penicillin, minocycline, and compound sulfamethoxazole; moderately sensitive to neomycin sulfate, gentamicin and amikacin; and resistant to polymyxin B (Table 2).

Table 2.
Detection of drug sensitivity of the H-701 strain.
4. Discussion
V. metschnikovii mainly exists in the aquatic environment in nature [25]. As a zoonotic agent, it has been recognized as one of the pathogenic Vibrio species [26]. It mainly causes sepsis and diarrhea in humans and infection in aquatic animals [27,28,29]. V. metschnikovii produces hemolytic toxins and has often been isolated from seafood [30,31]. Exposure to wounds or consumption of contaminated seafood increases the risk of infection [31]. Vibrio parahemolyticus [32], Vibrio mimicus [33], and Vibrio vulnificus [34] have been reported in freshwater-cultured fish in China; however, to date, no infection of V. metschnikovii had been reported. In the present study, the pathogenic strain H-701 isolated from diseased hybrid sturgeon was identified as V. metschnikovii. The pathogenicity test showed that the LD50 was 3.72 ± 0.929 × 104 CFU/g fish weight, and the H-701 strain had strong virulence to hybrid sturgeon. The symptoms of the artificially infected hybrid sturgeon were similar to those observed in naturally infected sturgeon. In addition, V. metschnikovii could be isolated from the artificially infected hybrid sturgeon again. These findings indicate that V. metschnikovii was the pathogenic bacterium that caused the disease in hybrid sturgeon.
In teleosts, white blood cells are key components of innate immune defense and are involved in the regulation of fish immune function [35]. The number of white blood cells in the blood is often used as a response to the health of fish [36]. White blood cells in sturgeon blood are divided into lymphocytes, granulocytes, and monocytes [36]. Among them, the proportion of lymphocytes was the highest, followed by neutrophils, with fewer eosinophils and monocytes [37]. Lymphocytes are an important component of the cellular immune response of the body, and a decrease in lymphocytes indicates poor immune function [38]. In the present study, the proportion of lymphocytes in the blood of diseased hybrid sturgeon decreased significantly, indicating a weakened immune system. Monocytes and granulocytes are the main phagocytes that kill pathogens chiefly by phagocytosis [39]. The proportion of monocytes, neutrophils, and eosinophils in the blood of hybrid sturgeon infected with V. metschnikovii was significantly increased, which enhanced phagocytosis.
The serum biochemical indices of fish signify their health status, and crowding stress leads to significant changes in the blood physiological indices of hybrid sturgeon species [40]. The liver is an important site for protein synthesis in the fish body [41]. The levels of serum ALB and GLB are indicators of hepatocyte injury and immune response, respectively [42]. The serum ALB and GLB contents of diseased hybrid sturgeon were significantly reduced, indicating that hepatocytes were damaged and liver function decreased. ALT and AST exist in healthy hepatocytes, and when the hepatocytes are damaged, they escape the cells and enter the bloodstream [42,43]. In diseased hybrid sturgeon, the serum levels of ALT and AST increased, whereas the serum levels of ALP decreased, which is similar to the results in diseased Pseudosciaena crocea [44]. Complement C3 is an integral part of the innate immune system and has a role in protecting the host from invasion by foreign pathogens [45]. The increased content of complement C3 in the diseased hybrid sturgeon indicates the aggravation of the body’s inflammatory response.
Histopathological observation is an important method for fish pathology research [46,47]. Various pathological changes occurred in the tissues of hybrid sturgeon infected with V. metschnikovii. Among them, liver, kidney, and spleen tissue damage were the most serious, including cytopathic changes, nuclear lysis, and infiltration of a large number of inflammatory cells. Histopathological changes may be caused by bacterial toxin-induced cytopathic effects. Similar histopathological changes have been observed in fish infected with different Vibrio species [33,34,48].
Drug sensitivity tests can provide a basis for the identification of candidate drugs for the effective treatment of disease [49]. The H-701 strain isolated from diseased hybrid sturgeon was sensitive to various antibiotics such as florfenicol, enrofloxacin, doxycycline, and compound sulfamethoxazole. The test results were similar to those reported by Wei et al., but H-701 showed stronger sensitivity to penicillin and compound sulfamethoxazole [50]. This may be related to different origin strains or different resistance to antibiotics. Therefore, in the treatment of bacterial diseases, highly sensitive drugs should be selected according to the results of drug susceptibility tests for pathogenic bacteria.
This study confirmed that V. metschnikovii is the pathogen causing the disease of hybrid sturgeon. Whether V. metschnikovii is pathogenic to other freshwater fish and infectious in freshwater culture needs to be further verified. The differences in virulence genes between V. metschnikovii and other Vibrio that cause fish diseases need to be further studied.
5. Conclusions
In this study, a V. metschnikovii strain was isolated and identified from diseased hybrid sturgeon. A virulence test showed that it was pathogenic to sturgeon, with an LD50 of 3.72 ± 0.929 × 104 CFU/g fish weight. The diseased hybrid sturgeon showed serious histopathological changes and significant changes in hematological parameters. The strain was sensitive to florfenicol, enrofloxacin, doxycycline, ampicillin, penicillin, minocycline, and compound sulfamethoxazole. In conclusion, V. metschnikovii was the pathogenic bacterium that caused a large number of deaths in hybrid sturgeon; the study highlights its potential risk in hybrid sturgeon aquaculture.
Author Contributions
Conceptualization, Z.X., X.L., M.X. and Y.Z.; methodology, Z.X., M.X., M.Z. and Z.C.; investigation, W.L., X.C., L.Z. and F.G.; data curation, M.X., Z.C. and M.Z.; writing—original draft preparation, Z.X. and X.L.; writing—review and editing, L.Z., X.C. and Y.Z.; supervision, Y.F. and L.Z.; project administration, Y.F., W.L. and L.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Key Research Development Program of China, grant number 2019YFD0900105, the Central Public-interest Scientific Institution Basal Research Fund, grant number 2020TD44, and the Earmarked Fund for China Agriculture Research System, grant number CARS-46.
Institutional Review Board Statement
All experimental procedures were conducted according to guidelines of the appropriate Animal Experimental Ethical Inspection of Laboratory Animal Centre, Yangtze River Fisheries Research Institute, Chinese Academy of Fishery Sciences (ID Number: YFI2021-zhouyong-06).
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
We would like to thank Hubei Qingjiang Sturgeon Valley Technology Co., Ltd. for their support in carrying out this study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bemis, W.E.; Findeis, E.K.; Grande, L. An overview of acipenseriformes. Environ. Biol. Fishes 1997, 48, 25–71. [Google Scholar] [CrossRef]
- Bakhshalizadeh, S.; Zykov, L.A.; Nasibulina, B.M.; Kurochkina, T.F.; Fazio, F. Influence of growth on the biological and commercial productivity of Caspian great sturgeon Huso huso. Aquaculture 2021, 533, 736139. [Google Scholar] [CrossRef]
- Chen, X.H.; Li, C.J.; Yang, C.G.; Zhang, S.H.; Wu, J.P. Status and prospects of techniques in the sturgeon aquaculture industry in China. Freshw. Fish. 2017, 47, 108–112. [Google Scholar]
- Raymakers, C. CITES, the convention on international trade in endangered species of wild Fauna and Flora: Its role in the conservation of Acipenseriformes. J. Appl. Ichthyol. 2006, 22, 53–65. [Google Scholar] [CrossRef]
- Zhang, H.; Kang, M.; Wu, J.; Wang, C.; Li, J.; Du, H.; Yang, H.; Wei, Q. Increasing river temperature shifts impact the Yangtze ecosystem: Evidence from the endangered Chinese sturgeon. Animals 2019, 9, 583. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Qu, Q.Z.; Wang, B.; Xia, Y.T.; Xu, S.J.; Sun, D.J. Comparison of reproductive characteristics among Amur sturgeon (Acipenser schrenckii), Kaluga sturgeon (Huso dauricus) and Their hybrid (Kaluga Sturgeon ♀ × Amur Sturgeon ♂). Chin. J. Fish. 2016, 29, 25–29,34. [Google Scholar]
- Zhou, Y.; Fan, Y.D.; Jiang, N.; Liu, W.Z.; Shi, Y.H.; Zhao, J.Q.; Zheng, L.B. Molecular characteristics and virulence analysis of eight Aeromonas hydrophila isolates obtained from diseased amur sturgeon Acipenser schrenckii Brandt, 1869. J. Vet. Med. Sci. 2018, 80, 421–426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pierezan, F.; Shahin, K.; Heckman, T.I.; Ang, J.; Byrne, B.A.; Soto, E. Outbreaks of severe myositis in cultured white sturgeon (Acipenser transmontanus L.) associated with Streptococcus iniae. J. Fish Dis. 2020, 43, 485–490. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.M.; Li, A.H. Isolation and characterization of Streptococcus dysgalactiae from diseased Acipenser schrenckii. Aquaculture 2009, 294, 14–17. [Google Scholar] [CrossRef]
- Yang, R.; Liu, Y.; Wang, Y.; Lei, M.K.; Pan, G.Y.; Wen, J.F.; Gong, Q.; Ren, L.; Huang, J.; Wen, X.T.; et al. Pathogenesis and pathological analysis of Edwardsiella tarda from Dabry’s sturgeon (Acipenser dabryanus) in China. Aquaculture 2018, 495, 637–642. [Google Scholar] [CrossRef]
- Zhang, S.H.; Huang, J.; Di, J.; Du, H.; Xu, Q.Q.; Zhou, Q.; Congiu, L.; Wei, Q.W. The genome sequence of a new strain of Mycobacterium ulcerans ecovar liflandii, emerging as a sturgeon pathogen. Aquaculture 2018, 489, 141–147. [Google Scholar] [CrossRef]
- Mugetti, D.; Pastorino, P.; Menconi, V.; Messina, M.; Masoero, L.; Ceresa, L.; Pedron, C.; Prearo, M. Two new sturgeon species are susceptible to Acipenser Iridovirus European (AcIV-E) Infection. Pathogens 2020, 9, 156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ciulli, S.; Volpe, E.; Sirri, R.; Passalacqua, P.L.; Bianchi, F.C.; Serratore, P.; Mandrioli, L. Outbreak of mortality in Russian (Acipenser gueldenstaedtii) and Siberian (Acipenser baerii) sturgeons associated with sturgeon nucleo-cytoplasmatic large DNA virus. Vet. Microbiol. 2016, 191, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Stachnik, M.; Matras, M.; Borzym, E.; Maj-Paluch, J.; Reichert, M. Emerging viral pathogens in sturgeon aquaculture in poland: Focus on Herpesviruses and Mimivirus detection. Viruses 2021, 13, 1496. [Google Scholar] [CrossRef]
- Xiao, Z.D.; Xue, M.M.; Wu, X.B.; Zeng, L.B.; Zhu, Y.J.; Jiang, N.; Fan, Y.D.; Zhou, Y. Isolation and identification of Staphylococcus warneri from diseased Coreius guichenoti. Aquacult. Rep. 2022, 22, 100988. [Google Scholar] [CrossRef]
- Arroyo, E.; Enríquez, L.; Sánchez, A.; Ovalle, M.; Olivas, A. Scanning electron microscopy of bacteria Tetrasphaera duodecadis. Scanning 2014, 36, 547–550. [Google Scholar] [CrossRef] [PubMed]
- Jensen, S.; Bergh, O.; Enger, O.; Hjeltnes, B. Use of PCR-RFLP for genotyping 16S rRNA and characterizing bacteria cultured from halibut fry. Can. J. Microbiol. 2002, 48, 379–386. [Google Scholar] [CrossRef]
- Pei, C.; Song, H.L.; Zhu, L.; Qiao, D.; Yan, Y.; Li, L.; Zhao, X.L.; Zhang, J.; Jiang, X.Y.; Kong, X.H. Identification of Aeromonas veronii isolated from largemouth bass Micropterus salmoides and histopathological analysis. Aquaculture 2021, 540, 736707. [Google Scholar] [CrossRef]
- Yao, D.D.; Chai, Y.; Wang, Y.; Guo, K.; Hang, J. Microscopic structures of peripheral blood cells in Chinese sturgeon (Acipenser sinensis). J. Yangtze Univ. 2015, 12, 22–26. [Google Scholar]
- Jiang, N.; Xu, J.; Ma, J.; Fan, Y.D.; Zhou, Y.; Liu, W.Z.; Zheng, L.B. Histopathology and ultrastructural pathology of cyprinid herpesvirus II(CyHV-2) infection in gibel carp, Carassius auratus gibelio. Wuhan Univ. J. Nat. Sci. 2015, 20, 413–420. [Google Scholar] [CrossRef]
- Snyder, T.L. The relative errors of bacteriological plate counting methods. J. Bacteriol. 1947, 54, 641–654. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reed, L.J.; Muench, H. A simple method for estimating 50 percent end points. Am. J. Hyg. 1838, 27, 493–497. [Google Scholar]
- Barry, A.L.; Coyle, M.B.; Thornsberry, C.; Gerlach, E.H.; Hawkinson, R.W. Methods of measuring zones of inhibition with the Bauer-Kirby disk susceptibility test. J. Clin. Microbiol. 1979, 10, 885–889. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saitou, N.; Nei, M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar] [PubMed]
- Austin, B. Vibrios as causal agents of zoonoses. Vet. Microbiol. 2010, 140, 310–317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ceccarelli, D.; Amaro, C.; Romalde, J.L.; Suffredini, E.R.; Vezzulli, L. Vibrio Species. Food Microbiol. 2019, 13, 347–388. [Google Scholar]
- Dalsgaard, A.; Alarcon, A.; Lanata, C.F.; Jensen, T.; Hansen, H.J.; Delgado, F.; Gil, A.I.; Penny, M.E.; Taylor, D. Clinical manifestations and molecular epidemiology of five cases of diarrhoea in children associated with Vibrio metschnikovii in Arequipa, peru. J. Med. Microbiol. 1996, 45, 494–500. [Google Scholar] [CrossRef] [PubMed]
- Choi, G.J.; Chun, H.J.; Jeon, D.S.; Kim, J.R. A case report of primary peritonitis and sepsis caused by Vibrio metschnikovii. Korean J. Clin. Pathol. 1999, 19, 329–332. [Google Scholar]
- Wallet, F.; Tachon, M.; Nseir, S.; Courcol, R.J.; Roussel-Delvallez, M. Vbrio metschnikovii pneumonia. Emerg. Infect. Dis. 2005, 11, 1641–1642. [Google Scholar] [CrossRef] [PubMed]
- Scoglio, M.E.; Pietro, A.; Picerno, I.; Delia, S.; Mauro, A.; Lagana, P. Virulence factors in Vibrios and Aeromonads isolated from seafood. New Microbiol. 2001, 24, 273–280. [Google Scholar] [PubMed]
- Matté, M.H.; Baldassi, L.; Barbosa, M.L.; Malucelli, M.; Nitrini, S.M.; Matté, G.R. Virulence factors of Vibrio metschnikovii strains isolated from fish in Brazil. Food Control 2007, 18, 747–751. [Google Scholar] [CrossRef]
- Lin, Y.; Pei, X.Y.; Zhang, X.L.; Guan, W.Y.; Chui, H.X.; Jia, H.Y.; Ma, G.Z.; Yang, S.R.; Li, Y.; Li, N.; et al. Occurrence of four pathogenic Vibrios in Chinese freshwater fish farms in 2016. Food Control 2019, 95, 85–89. [Google Scholar]
- Geng, Y.; Liu, D.; Han, S.; Zhou, Y.; Wang, K.Y.; Huang, X.L.; Chen, D.F.; Peng, X.; Lai, W.M. Outbreaks of vibriosis associated with Vibrio mimicus in freshwater catfish in China. Aquaculture 2014, 433, 82–84. [Google Scholar] [CrossRef]
- Liu, R.R.; Lian, Z.Y.; Hu, X.C.; Lü, A.J.; Sun, J.F.; Chen, C.X.; Liu, X.X.; Song, Y.J.; Yiksung, Y. First report of Vibrio vulnificus infection in grass carp Ctenopharyngodon idellus in China. Aquaculture 2019, 499, 283–289. [Google Scholar] [CrossRef]
- Mokhtar, D.M.; Abdelhafez, E.A. An overview of the structural and functional aspects of immune cells in teleosts. Histol. Histopath. 2021, 36, 399–414. [Google Scholar]
- Zarejabad, A.M.; Jalali, M.A.; Sudagar, M.; Pouralimotlagh, S. Hematology of great sturgeon (Huso huso linnaeus, 1758) juvenile exposed to brackish water environment. Fish Physiol. Biochem. 2010, 36, 655–659. [Google Scholar] [CrossRef] [PubMed]
- Rafatnezhad, S.; Falahatkar, B.; Gilani, M.H.T. Effects of stocking density on haematological parameters, growth and fin erosion of great sturgeon (Huso huso) juveniles. Aquac. Res. 2008, 39, 1506–1513. [Google Scholar] [CrossRef]
- Ye, W.J.; Lu, W.W.; Tang, Y.P.; Chen, G.X.; Li, X.P.; Ji, C.; Hou, M.; Zeng, G.W.; Lan, X.; Wang, Y.L.; et al. Identification of COVID-19 clinical phenotypes by principal component analysis-based cluster analysis. Front. Med. 2020, 12, 570614. [Google Scholar] [CrossRef] [PubMed]
- Döring, M.; Stanchi, K.M.C.; Erbacher, A.; Haufe, S.; Schwarze, C.P.; Handgretinger, R.; Hofbeck, M.; Kerst, G. Phagocytic activity of monocytes, their subpopulations and granulocytes during post-transplant adverse events after hematopoietic stem cell transplantation. Immunobiology 2015, 220, 605–613. [Google Scholar] [CrossRef] [PubMed]
- Dhang, M.; Li, J.F.; Wen, H.S.; Bu, Y.; Ni, M.; Ren, Y.Y.; Lai, C.Q.; Liu, C.Z. Effect of stocking density on bloodphysilogy and serum biochemistry indicators in hybrid sturgeon. Trans. Oceanol. Limnol. 2014, 2, 67–72. [Google Scholar]
- Javed, M.; Ahmad, M.I.; Usmani, N.; Ahmad, M. Multiple biomarker responses (serum biochemistry, oxidative stress, genotoxicity and histopathology) in Channa punctatus exposed to heavy metal loaded waste water. Sci. Rep. 2017, 7, 1675. [Google Scholar] [CrossRef]
- Sepúlveda, M.S.; Sutton, T.M.; Patrick, H.K.; Amberg, J.J. Blood Chemistry Values for Shovelnose and Lake Sturgeon. J. Aquat. Anim. Health 2012, 24, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Chuang, C.H.; Tsai, C.C.; Lin, E.S.; Huang, C.S.; Lin, Y.Y.; Lan, C.C.; Huang, C.C. Heat-Killed Lactobacillus salivarius and Lactobacillus johnsonii Reduce Liver Injury Induced by Alcohol In Vitro and In Vivo. Molecules 2016, 21, 1456. [Google Scholar] [CrossRef]
- Xu, X.J.; Xu, B.; Wang, J.; Su, Y.Q.; Zhang, Z.W.; Chen, X. Studies on blood chemistry indices and histopathology of Pseudosciaena crocea artificially challenged with Vibrio harveyi. J. Fish. China 2010, 34, 618–625. [Google Scholar] [CrossRef]
- Meng, X.Z.; Shen, Y.B.; Wang, S.T.; Xu, X.Y.; Dang, Y.F.; Zhang, M.; Li, L.S.; Zhang, J.H.; Wang, R.Q.; Li, J.L. Complement component 3 (C3): An important role in grass carp (Ctenopharyngodon idella) experimentally exposed to Aeromonas hydrophila. Fish Shellfish Immunol. 2019, 88, 189–197. [Google Scholar] [CrossRef]
- Sandlund, N.; Johansen, R.; Fiksdal, I.U.; Einen, A.C.B.; Modahl, I.; Gjerset, B.; Bergh, Ø. Susceptibility and pathology in juvenile Atlantic Cod Gadus morhua to a Marine Viral Haemorrhagic Septicaemia Virus isolated from diseased rainbow trout Oncorhynchus mykiss. Animals 2021, 11, 3523. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Li, Y.; Xue, M.; Yang, T.; Luo, X.; Fan, Y.; Meng, Y.; Liu, W.; Lin, G.; Li, B.; et al. Effects of dietary saccharomyces cerevisiae YFI-SC2 on the growth performance, intestinal morphology, immune parameters, intestinal microbiota, and disease resistance of Crayfish (Procambarus clarkia). Animals 2021, 11, 1963. [Google Scholar] [CrossRef]
- Liu, S.B.; Li, E.; Cai, Y.; Wang, S.F.; Ren, Z.L.; Li, Q.M.; Guo, W.L.; Wu, Y.; Zhou, Y.C. Isolation, identification and pathogenicity characterization of Vibrio ponticus from the golden pompano Trachinotus ovatus. Aquaculture 2018, 496, 285–290. [Google Scholar] [CrossRef]
- Smith, P. Accuracy, precision and meaning of antimicrobial agent susceptibility testing of bacteria associated with fish diseases. Aquaculture 2001, 196, 253–266. [Google Scholar] [CrossRef]
- Wei, C.; Zhou, H.; Luo, L.; Guo, L.A.; Dai, X.H.; Liu, W.; Qin, Q. Metabolism of Amino Acids and Susceptibility Test of Vibrio metschnikovii Isolated from Egg. Southw. China J. Agric. Sci. 2015, 28, 903–907. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).