Productive Performance, Serum Antioxidant Status, Tissue Selenium Deposition, and Gut Health Analysis of Broiler Chickens Supplemented with Selenium and Probiotics—A Pilot Study
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals, Diets, and Experimental Design
2.2. Growth Performance Measurement
2.3. Sample Collection
2.4. Detection of Tissue Se Content
2.5. Serum Antioxidant Capacity Analysis
2.6. Intestinal Morphometry and 16S RNA-Based Microbiota Analysis
2.7. Statistical Analysis
3. Results
3.1. Growth Performance
3.2. Tissue Se Content
3.3. Antioxidant Activities
3.4. Intestinal Morphometry
3.5. Effects of Se and Probiotics on the Composition of the Cecal Microbiota
3.5.1. Alpha Diversity Analysis
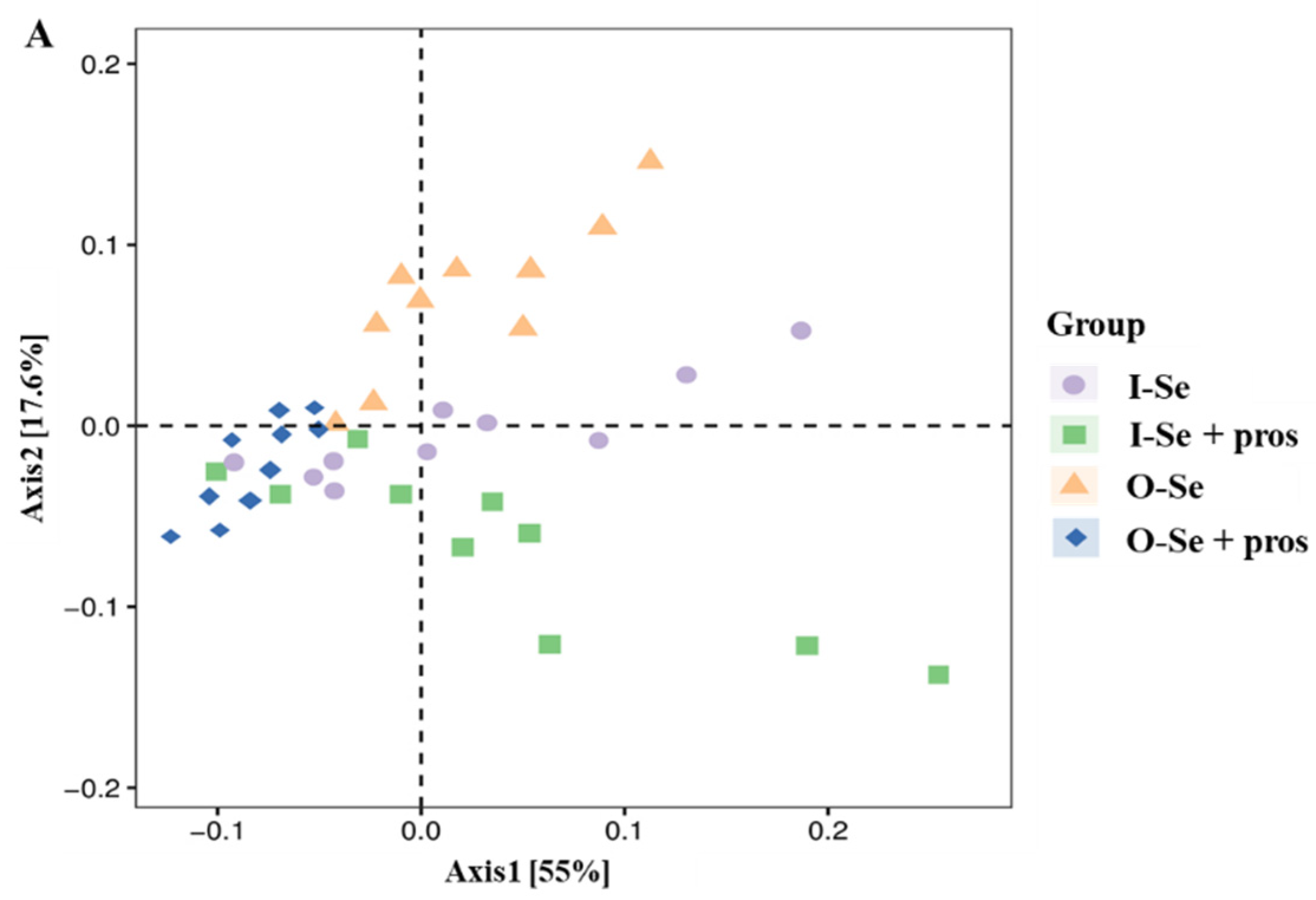
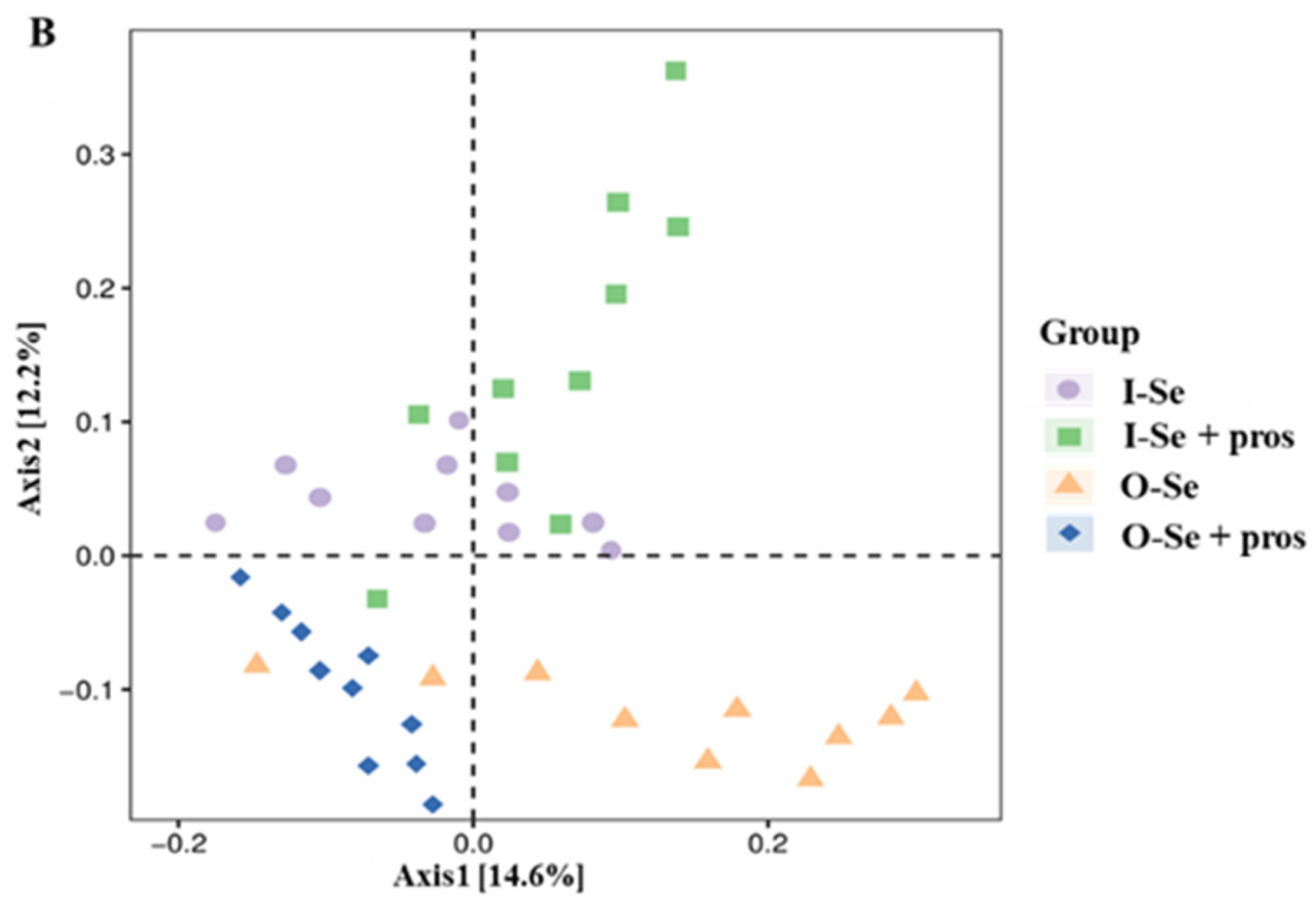
3.5.2. Beta Diversity Analysis
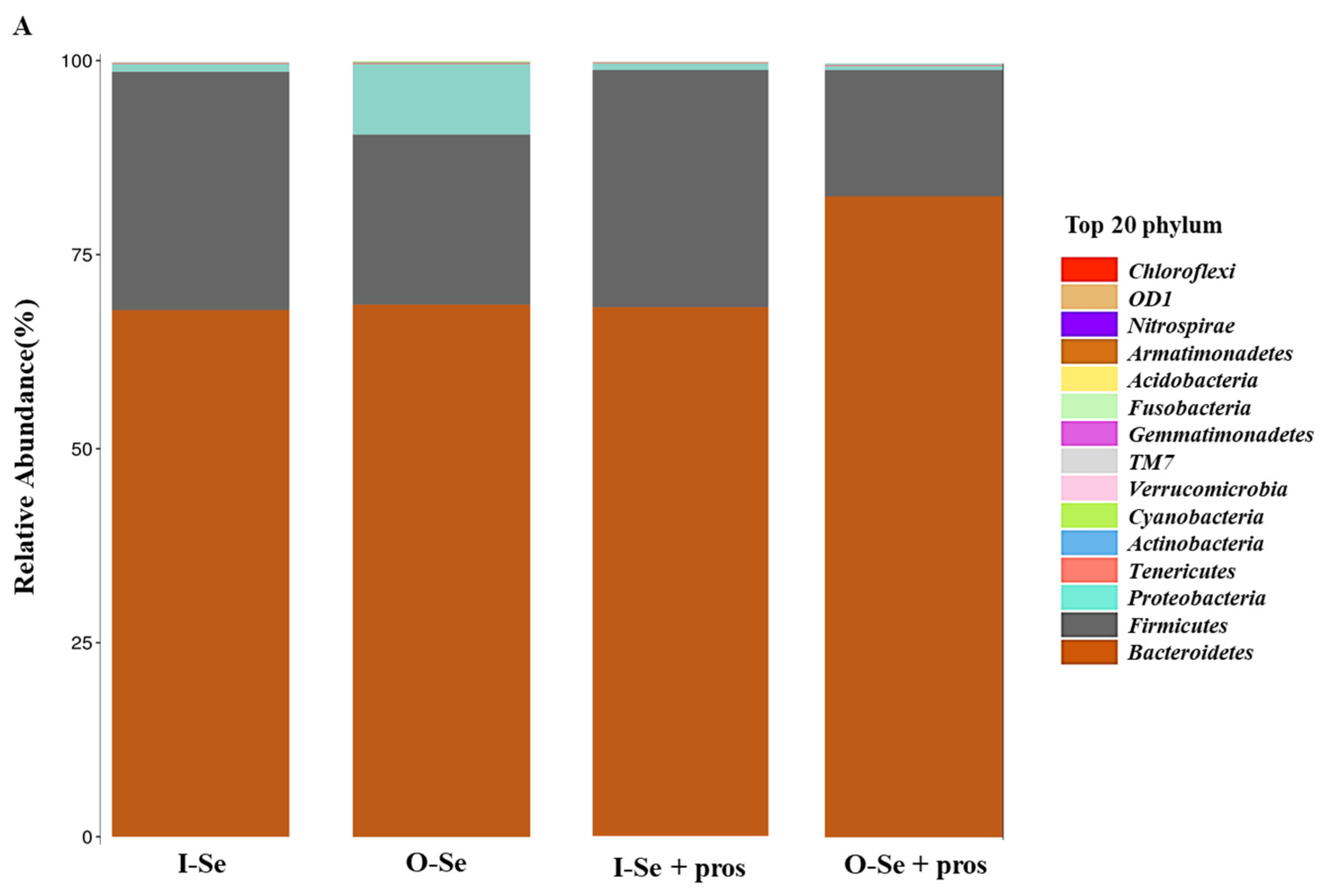
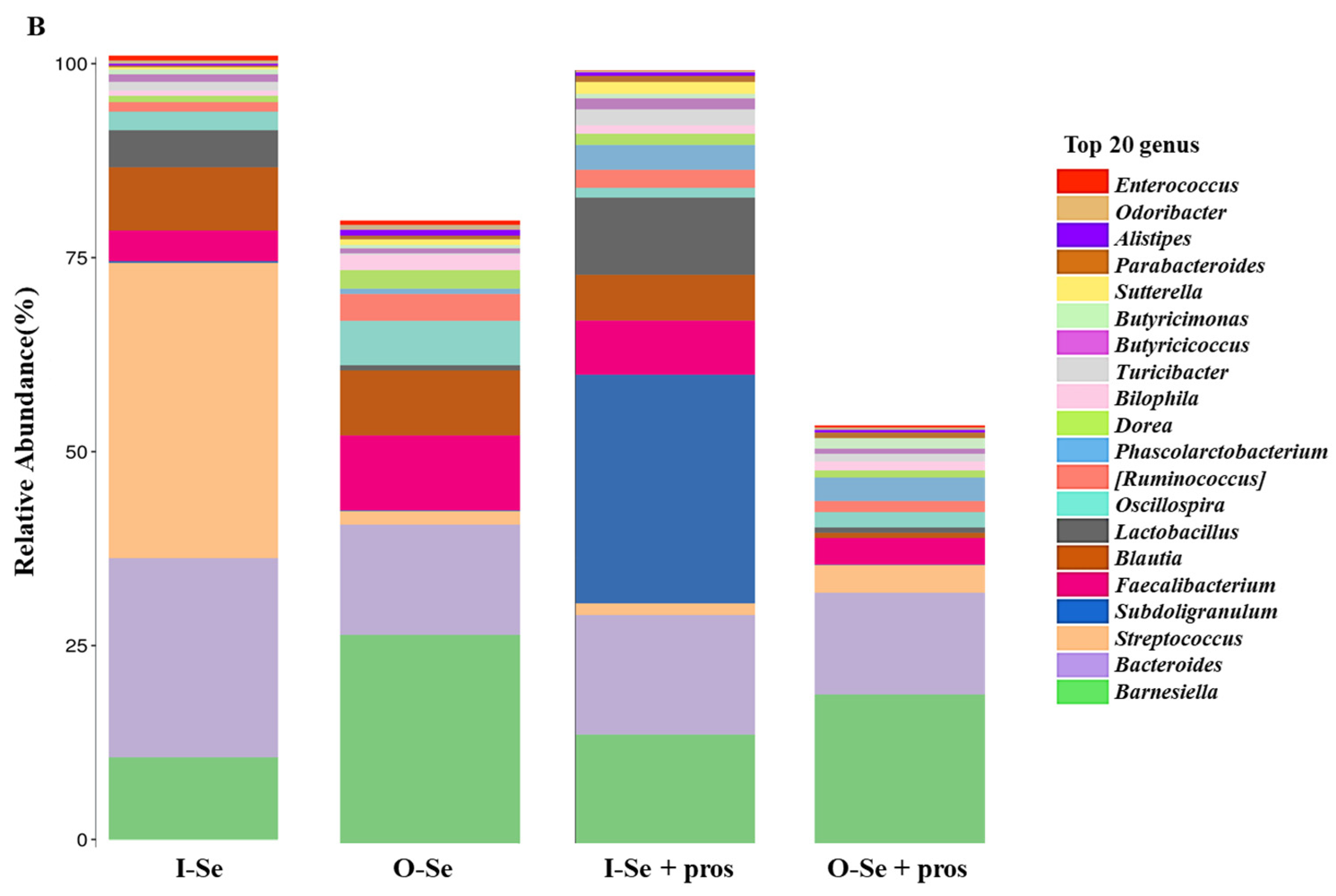
3.5.3. Taxonomic Assignments
- Taxonomic Assignment at the Phylum Level
- Taxonomic Assignment at the Genus Level
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Surai, P.F.; Kochish, I.I. Nutritional modulation of the antioxidant capacities in poultry: The case of selenium. Poult. Sci. 2018, 98, 4231–4239. [Google Scholar] [CrossRef]
- Zoidis, E.; Seremelis, I.; Kontopoulos, N.; Danezis, G.P. Selenium-Dependent Antioxidant Enzymes: Actions and Properties of Selenoproteins. Antioxidants 2018, 7, 66. [Google Scholar] [CrossRef]
- Li, J.L.; Sunde, R.A. Selenoprotein Transcript Level and Enzyme Activity as Biomarkers for Selenium Status and Selenium Requirements of Chickens (Gallus gallus). PLoS ONE 2016, 11, e0152392. [Google Scholar] [CrossRef]
- Surai, P.F.; Fisinin, V.I. Selenium in poultry breeder nutrition: An update. Anim. Feed Sci. Technol. 2014, 191, 1–15. [Google Scholar] [CrossRef]
- Gan, F.; Chen, X.; Liao, S.F.; Lv, C.; Ren, F.; Ye, G.; Pan, C.; Huang, D.; Shi, J.; Shi, X.; et al. Selenium-enriched probiotics improve antioxidant status, immune function, and selenoprotein gene expression of piglets raised under high ambient temperature. J. Agric. Food Chem. 2014, 62, 4502–4508. [Google Scholar] [CrossRef]
- Rayman, M.P. The use of high-selenium yeast to raise selenium status: How does it measure up? Br. J. Nutr. 2004, 92, 557–573. [Google Scholar] [CrossRef]
- Li, K.; Wang, J.; Dong, Y.; Zhao, R.; Xiu, A. Effects of different selenium sources and levels on antioxidant status in broiler breeders. Asian-Australas J. Anim. Sci. 2018, 31, 1939. [Google Scholar] [CrossRef]
- Arnaut, P.R.; Viana, G.D.; da Fonseca, L.; Alves, W.; Muniz, J.C.L.; Pettigrew, J.E.; Silva, F.F.E.; Rostagno, H.S.; Hannas, M.I. Selenium source and level on performance, selenium retention and biochemical responses of young broiler chicks. BMC Vet. Res. 2021, 17, 13. [Google Scholar] [CrossRef]
- Khan, A.Z.; Kumbhar, S.; Liu, Y.; Hamid, M.; Pan, C.; Nido, S.A.; Parveen, F.; Huang, K. Dietary supplementation of selenium-enriched probiotics enhances meat quality of broiler chickens (Gallus gallus domesticus) raised under high ambient temperature. Biol. Trace Elem. Res. 2018, 182, 328–338. [Google Scholar] [CrossRef]
- Gangadoo, S.; Dinev, I.; Willson, N.-L.; Moore, R.J.; Chapman, J.; Stanley, D. Nanoparticles of selenium as high bioavailable and non-toxic supplement alternatives for broiler chickens. Environ. Sci. Pollut. Res. 2020, 27, 16159–16166. [Google Scholar] [CrossRef]
- Woods, S.; Sobolewska, S.; Rose, S.; Whiting, I.; Blanchard, A.; Ionescu, C.; Bravo, D.; Pirgozliev, V. Effect of feeding different sources of selenium on growth performance and antioxidant status of broilers. Br. Poult. Sci. 2020, 61, 274–280. [Google Scholar] [CrossRef]
- Tong, C.; Li, P.; Yu, L.H.; Li, L.; Long, M. Selenium-rich yeast attenuates ochratoxin A-induced small intestinal injury in broiler chickens by activating the Nrf2 pathway and inhibiting NF-KB activation. J. Funct. Foods 2020, 66, 103784. [Google Scholar] [CrossRef]
- Musa, H.H.; Wu, S.L.; Zhu, C.H.; Seri, H.I.; Zhu, G.Q.J.J.o.A.; Advances, V. The Potential Benefits of Probiotics in Animal Production and Health. J. Anim. Vet. Adv. 2012, 8, 313–321. [Google Scholar] [CrossRef][Green Version]
- Zhao, Y.; Zeng, D.; Wang, H.; Qing, X.; Sun, N.; Xin, J.; Luo, M.; Khalique, A.; Pan, K.; Shu, G.; et al. Dietary Probiotic Bacillus licheniformis H2 Enhanced Growth Performance, Morphology of Small Intestine and Liver, and Antioxidant Capacity of Broiler Chickens Against Clostridium perfringens-Induced Subclinical Necrotic Enteritis. Probiotics Antimicrob. Proteins 2020, 12, 883–895. [Google Scholar] [CrossRef]
- Rashidi, N.; Khatibjoo, A.; Taherpour, K.; Akbari-Gharaei, M.; Shirzadi, H. Effects of licorice extract, probiotic, toxin binder and poultry litter biochar on performance, immune function, blood indices and liver histopathology of broilers exposed to aflatoxin-B-1. Poult. Sci. 2020, 99, 5896–5906. [Google Scholar] [CrossRef]
- Abdel Baset, S.; Ashour, E.A.; Abd El-Hack, M.E.; El-Mekkawy, M.M. Effect of different levels of pomegranate peel powder and probiotic supplementation on growth, carcass traits, blood serum metabolites, antioxidant status and meat quality of broilers. Anim. Biotechnol. 2020, 1825965. [Google Scholar] [CrossRef]
- Atsbeha, A.T.; Hailu, T.G. The Impact of Effective Microorganisms (EM) on Egg Quality and Laying Performance of Chickens. Int. J. Food Sci. 2021, 2021, 8895717. [Google Scholar] [CrossRef]
- Gnanadesigan, M.; Isabella, S.; Saritha, P.; Ramkumar, L.; Manivannan, N.; Ravishankar, R. Quality evaluation of egg composition and productivity of layers in EM (effective microorganisms) treatments: A field report. Egypt. J. Basic Appl. Sci. 2014, 1, 161–166. [Google Scholar] [CrossRef][Green Version]
- Steczny, K.; Kokoszynski, D. Effect of probiotic preparations (EM) and sex on morphometric characteristics of the digestive system and leg bones, and caecal microflora in broiler chickens. J. Appl. Anim. Res. 2020, 48, 45–50. [Google Scholar] [CrossRef]
- Jwher, D.M.; Abd, S.; Mohammad, A. The study of using effective microorganisms (EM) on health and performance of broiler chicks. Iraqi J. Vet. Sci. 2013, 27, 73–78. [Google Scholar] [CrossRef]
- Simeamelak, M.; Solomon, D.; Taye, T. The effect of effective microorganisms on production and quality performance of Rhode Island Red layers. Int. J. Livest. Prod. 2013, 4, 22–29. [Google Scholar] [CrossRef][Green Version]
- Stęczny, K.; Kokoszynski, D. Effects of probiotics and sex on physicochemical, sensory and microstructural characteristics of broiler chicken meat. J. Appl. Anim. Res. 2019, 18, 1385–1393. [Google Scholar] [CrossRef]
- Liu, H.; Yu, Q.F.; Tang, X.P.; Fang, C.K.; Chen, S.J.; Fang, R.J. Effect of selenium on performance, egg quality, egg selenium content and serum antioxidant capacity in laying hens. Pak. J. Zool. 2020, 52, 635–640. [Google Scholar] [CrossRef]
- Bai, K.; Feng, C.; Jiang, L.; Zhang, L.; Zhang, J.; Zhang, L.; Wang, T. Dietary effects of Bacillus subtilis fmbj on growth performance, small intestinal morphology, and its antioxidant capacity of broilers. Poult. Sci. 2018, 97, 2312–2321. [Google Scholar] [CrossRef]
- Nkukwana, T.; Muchenje, V.; Masika, P.J.; Mushonga, B. Intestinal morphology, digestive organ size and digesta pH of broiler chickens fed diets supplemented with or without Moringa oleifera leaf meal. S. Afr. J. Anim. Sci. 2015, 45, 362–370. [Google Scholar] [CrossRef]
- Choct, M.; Naylor, A.; Reinke, N. Selenium supplementation affects broiler growth performance, meat yield and feather coverage. Br. Poult. Sci. 2004, 45, 677–683. [Google Scholar] [CrossRef]
- Bai, K.; Huang, Q.; Zhang, J.; He, J.; Zhang, L.; Wang, T. Supplemental effects of probiotic Bacillus subtilis fmbJ on growth performance, antioxidant capacity, and meat quality of broiler chickens. Poult. Sci. 2017, 96, 74–82. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, M.; Zhou, Y. Effects of selenium-enriched Bacillus sp. compounds on growth performance, antioxidant status, and lipid parameters breast meat quality of Chinese Huainan partridge chicks in winter cold stress. Lipids Health Dis. 2019, 18, 63. [Google Scholar] [CrossRef]
- Yang, J.; Wang, J.; Huang, K.; Zhu, M.; Liu, Q.; Liu, G.; Chen, F.; Zhang, H.; Qin, S.J.F. Selenium enriched Bacillus subtilis yb-1 114246 activated the TLR2–NF-κB1 signaling pathway to regulate chicken intestinal β-defensin 1 expression. Food Funct. 2021, 12, 5913–5926. [Google Scholar] [CrossRef]
- Wondmeneh, E.; Getachew, T.; Dessie, T. Effect of Effective Microorganisms (EM®) on the growth parameters of fayoumi and Horro chicken. Int. J. Poult. Sci. 2011, 10, 185–188. [Google Scholar] [CrossRef]
- Gul, F.; Ahmad, B.; Afzal, S.; Ullah, A.; Khan, S.; Aman, K.; Khan, M.; Hadi, F.; Kiran, K.; Zahra, M. Comparative analysis of various sources of selenium on the growth performance and antioxidant status in broilers under heat stress. Braz. J. Biol. 2021, 83, 2023. [Google Scholar] [CrossRef] [PubMed]
- Schrauzer, G.N. The nutritional significance, metabolism and toxicology of selenomethionine. J. Nutr. 2003, 47, 73–112. [Google Scholar] [CrossRef]
- Chen, G.; Wu, J.; Li, C. Effect of different selenium sources on production performance and biochemical parameters of broilers. J. Anim. Physiol. Anim. Nutr. 2014, 98, 747–754. [Google Scholar] [CrossRef] [PubMed]
- Foster, L.; Sumar, S.J.N.; Science, F. Selenium in the environment, food and health. Nutr. Food Sci. 1995, 95, 17–23. [Google Scholar] [CrossRef]
- Wang, Y.-B.; Xu, B.-H. Effect of different selenium source (sodium selenite and selenium yeast) on broiler chickens. Anim. Feed. Sci. Technol. 2008, 144, 306–314. [Google Scholar] [CrossRef]
- Wang, Y.; Heng, C.; Zhou, X.; Cao, G.; Jiang, L.; Wang, J.; Li, K.; Wang, D.; Zhan, X. Supplemental Bacillus subtilis DSM 29784 and enzymes, alone or in combination, as alternatives for antibiotics to improve growth performance, digestive enzyme activity, anti-oxidative status, immune response and the intestinal barrier of broiler chickens. Br. J. Nutr. 2021, 125, 494–507. [Google Scholar] [CrossRef] [PubMed]
- Heindl, J.; Ledvinka, Z.; Englmaierova, M.; Zita, L.; Tumova, E. The effect of dietary selenium sources and levels on performance, selenium content in muscle and glutathione peroxidase activity in broiler chickens. Czech J. Anim. Sci. 2010, 55, 572–578. [Google Scholar] [CrossRef]
- Huang, Y.; Ren, J.; Qu, X. Nanozymes: Classification, catalytic mechanisms, activity regulation, and applications. Chem. Rev. 2019, 119, 4357–4412. [Google Scholar] [CrossRef]
- Skřivan, M.; Dlouha, G.; Mašata, O.; Ševčíková, S. Effect of dietary selenium on lipid oxidation, selenium and vitamin E content in the meat of broiler chickens. Czech J. Anim. Sci. 2008, 53, 306–311. [Google Scholar] [CrossRef]
- Aluwong, T.; Kawu, M.; Raji, M.; Dzenda, T.; Govwang, F.; Sinkalu, V.; Ayo, J. Effect of yeast probiotic on growth, antioxidant enzyme activities and malondialdehyde concentration of broiler chickens. Antioxidants 2013, 2, 326–339. [Google Scholar] [CrossRef]
- Xu, Y.; Yu, Y.; Shen, Y.; Li, Q.; Lan, J.; Wu, Y.; Zhang, R.; Cao, G.; Yang, C. Effects of Bacillus subtilis and Bacillus licheniformis on growth performance, immunity, short chain fatty acid production, antioxidant capacity, and cecal microflora in broilers. Poult. Sci. 2021, 100, 101358. [Google Scholar] [CrossRef] [PubMed]
- Deraz, S.F.; Elkomy, A.E.; Khalil, A.A. Assessment of probiotic-supplementation on growth performance, lipid peroxidation, antioxidant capacity, and cecal microflora in broiler chickens. J. Appl. Pharm. Sci. 2019, 9, 30–39. [Google Scholar] [CrossRef]
- Ye, Y.; Li, Z.; Wang, P.; Zhu, B.; Zhao, M.; Huang, D.; Ye, Y.; Ding, Z.; Li, L.; Wan, G. Effects of probiotic supplements on growth performance and intestinal microbiota of partridge shank broiler chicks. PeerJ 2021, 9, e12538. [Google Scholar] [CrossRef] [PubMed]
- Manning, T.; Gibson, G. Microbial-gut interactions in health and disease. Best Pract. Res. Clin. Gastroenterol. 2004, 18, 287–298. [Google Scholar] [CrossRef] [PubMed]
- Bami, M.K.; Afsharmanesh, M.; Espahbodi, M.; Esmaeilzadeh, E. Effects of dietary nano-selenium supplementation on broiler chicken performance, meat selenium content, intestinal microflora, intestinal morphology, and immune response. J. Trace Elem. Med. Biol. 2022, 69, 126897. [Google Scholar] [CrossRef]
- Sun, P.; Zhang, W.; Miao, Y.; Chen, Z. meta-analysis of prebiotics, probiotics, synbiotics and antibiotics in IBS. Aliment. Pharmacol. Ther. 2019, 49, 1253–1254. [Google Scholar] [CrossRef]
- Robinson, K.; Xiao, Y.; Johnson, T.J.; Chen, B.; Yang, Q.; Lyu, W.; Wang, J.; Fansler, N.; Becker, S.; Liu, J.; et al. Chicken intestinal mycobiome: Initial characterization and its response to bacitracin methylene disalicylate. Appl. Environ. Microbiol. 2020, 86, e00304-20. [Google Scholar] [CrossRef]
- Khan, S.; Moore, R.J.; Stanley, D.; Chousalkar, K.K. The gut microbiota of laying hens and its manipulation with prebiotics and probiotics to enhance gut health and food safety. Biol. Trace Elem. Res. 2020, 86, e00600-20. [Google Scholar] [CrossRef]
- Tarradas, J.; Tous, N.; Esteve-Garcia, E.; Brufau, J. The control of intestinal inflammation: A major objective in the research of probiotic strains as alternatives to antibiotic growth promoters in poultry. Microorganisms 2020, 8, 148. [Google Scholar] [CrossRef]
- Li, Z.; Dong, Y.; Chen, S.; Jia, X.; Jiang, X.; Che, L.; Lin, Y.; Li, J.; Feng, B.; Fang, Z. Organic Selenium Increased Gilts Antioxidant Capacity, Immune Function, and Changed Intestinal Microbiota. Front. Microbiol. 2021, 12, 723190. [Google Scholar] [CrossRef]
- Wei, S.; Morrison, M.; Yu, Z. Bacterial census of poultry intestinal microbiome. Poult. Sci. Sympos. 2013, 92, 671–683. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.; Zhang, Y.; Xiao, K.P.; Jiang, F.; Wang, H.C.; Tang, D.Z. The chicken gut metagenome and the modulatory effects of plant-derived benzylisoquinoline alkaloids. Micobiome 2018, 6, 211. [Google Scholar] [CrossRef] [PubMed]
- Ballou, A.L.; Ali, R.A.; Mendoza, M.A.; Ellis, J.C.; Hassan, H.M.; Croom, W.J. Development of the chick microbiome: How early exposure influences future microbial diversity. Front. Vet. Sci. 2016, 3, 2. [Google Scholar] [CrossRef] [PubMed]
- Recharla, N.; Kim, K.; Park, J.; Jeong, J.; Jeong, Y.; Lee, H.; Hwang, O.; Ryu, J.; Baek, Y.; Oh, Y.; et al. Effects of amino acid composition in pig diet on odorous compounds and microbial characteristics of swine excreta. J. Anim. Sci. Technol. 2017, 59, 28. [Google Scholar] [CrossRef]
- Ubeda, C.; Bucci, V.; Caballero, S.; Djukovic, A.; Toussaint, N.C.; Equinda, M.; Lipuma, L.; Ling, L.; Gobourne, A.; No, D.; et al. Intestinal microbiota containing Barnesiella species cures vancomycin-resistant Enterococcus faecium colonization. Infect. Immun. 2013, 81, 965–973. [Google Scholar] [CrossRef]
- Steinway, S.N.; Biggs, M.B.; Loughran Jr, T.P.; Papin, J.A.; Albert, R. Inference of network dynamics and metabolic interactions in the gut microbiome. PLoS Comput. Biol. 2015, 11, e1004338. [Google Scholar] [CrossRef]
- Wei, X.; Tao, J.; Xiao, S.; Jiang, S.; Shang, E.; Zhu, Z.; Qian, D.; Duan, J. Xiexin Tang improves the symptom of type 2 diabetic rats by modulation of the gut microbiota. Sci. Rep. 2018, 8, 3685. [Google Scholar] [CrossRef]
| Ingredient | Content, % | Nutrient Level 3 | Content | ||
|---|---|---|---|---|---|
| 1 to 35 d | 36 to 70 d | 1 to 35 d | 36 to 70 d | ||
| Corn | 59.00 | 65.00 | DE (Mcal/kg) | 3.00 | 3.12 |
| Soybean meal | 30.28 | 20.00 | Crude protein (%) | 21.26 | 16.32 |
| Cottonseed meal | 2.00 | 4.50 | Crude lipid (%) | 4.35 | 4.56 |
| Fish meal | 3.20 | - | Crude ash (%) | 6.08 | 5.93 |
| Wheat bran | - | 3.15 | Ca (%) | 0.97 | 0.88 |
| Soybean oil | 1.45 | 3.00 | Total P | 0.72 | 0.65 |
| Ca(H2PO4)2 | 1.05 | 1.10 | Available P (%) | 0.46 | 0.36 |
| Limestone | 1.50 | 1.70 | Lys (%) | 1.11 | 0.86 |
| Choline chloride | 0.10 | 0.10 | Met (%) | 0.53 | 0.41 |
| DL-Met | 0.18 | 0.15 | Met + Cys (%) | 0.86 | 0.69 |
| NaCl | 0.24 | 0.20 | Thr | 0.80 | 0.69 |
| NaHCO3 | - | 0.10 | |||
| Mineral premix 1 | 0.98 | 0.98 | |||
| Vitamin premix 2 | 0.02 | 0.02 | |||
| Total | 100.00 | 100.00 | |||
| Items 2 | Se, mg/kg | Pros, % | IW 5, g | FBW 6, g | Starter (0–35 days) | Finisher (36–70 days) | Whole Term (0–70 d) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ADG 7, g | ADF 8, g | F/G 9 | ADG, g | ADFI, g | F/G | ADG, g | ADFI, g | F/G | |||||
| I-Se | 0.2 | 0 | 35.80 | 1909.01 | 19.23 | 36.78 | 1.91 ab | 34.14 | 98.82 | 2.89 | 26.61 | 67.50 | 2.54 |
| O-Se | 0.2 | 0 | 35.76 | 1898.09 | 19.43 | 36.69 | 1.89 b | 33.26 | 96.79 | 2.91 | 26.30 | 66.55 | 2.53 |
| I-Se + pros | 0.2 | 0.5 | 35.65 | 1912.58 | 18.96 | 37.19 | 1.96 a | 34.46 | 97.97 | 2.85 | 26.65 | 67.36 | 2.53 |
| O-Se + pros | 0.2 | 0.5 | 35.68 | 1883.30 | 19.68 | 37.32 | 1.90 b | 33.07 | 96.40 | 2.92 | 26.36 | 66.80 | 2.54 |
| SEM | 0.05 | 11.10 | 0.12 | 0.23 | 0.01 | 0.71 | 0.69 | 0.22 | 0.16 | 0.39 | 0.01 | ||
| Main effect means 3 | |||||||||||||
| Se-S | Inorganic | 35.73 | 1910.80 | 19.09 | 36.99 | 1.94 a | 34.30 | 98.40 | 2.87 | 26.63 | 67.43 | 2.53 | |
| Organic | 35.72 | 1890.69 | 19.56 | 37.00 | 1.89 b | 33.16 | 96.60 | 2.92 | 26.33 | 66.67 | 2.54 | ||
| SEM | 0.15 | 33.24 | 0.32 | 0.69 | 0.02 | 0.90 | 2.01 | 0.06 | 0.48 | 1.18 | 0.04 | ||
| Pros | 0 | 35.78 | 1903.55 | 19.32 | 37.25 | 1.93 a | 33.70 | 97.81 | 2.90 | 26.45 | 67.02 | 2.54 | |
| 0.5 | 35.67 | 1897.94 | 19.33 | 36.74 | 1.90 b | 33.76 | 97.19 | 2.88 | 26.51 | 67.08 | 2.53 | ||
| SEM | 0.15 | 33.24 | 0.32 | 0.69 | 0.02 | 0.90 | 2.01 | 0.06 | 0.48 | 1.18 | 0.04 | ||
| p-value | |||||||||||||
| Treatment | 0.735 | 0.815 | 0.171 | 0.759 | 0.011 | 0.375 | 0.620 | 0.650 | 0.843 | 0.832 | 0.994 | ||
| Se-S | 0.963 | 0.405 | 0.055 | 0.973 | 0.001 | 0.095 | 0.223 | 0.324 | 0.386 | 0.381 | 0.884 | ||
| Pros | 0.294 | 0.814 | 0.972 | 0.303 | 0.022 | 0.920 | 0.668 | 0.664 | 0.873 | 0.946 | 0.942 | ||
| Se-S × Pros 4 | 0.746 | 0.206 | 0.256 | 0.206 | 0.063 | 0.697 | 0.875 | 0.516 | 0.701 | 0.970 | 0.816 | ||
| Items 2 | Se, mg/kg | Pros, % | Liver, mg/kg | Kidney, mg/kg | Pancreas, mg/kg | Breast Muscles, mg/kg | Thigh Muscle, mg/kg | Serum, mg/L |
|---|---|---|---|---|---|---|---|---|
| I-Se | 0.2 | 0 | 0.62 b | 0.70 | 0.21 c | 0.16 b | 0.16 c | 0.12 b |
| O-Se | 0.2 | 0 | 0.79 a | 0.72 | 0.39 b | 0.38 a | 0.33 a | 0.12 b |
| I-Se + pros | 0.2 | 0.5 | 0.75 a | 0.75 | 0.18 c | 0.16 b | 0.26 b | 0.19 a |
| O-Se + pros | 0.2 | 0.5 | 0.80 a | 0.61 | 0.62 a | 0.34 a | 0.34 a | 0.12 b |
| SEM | 0.02 | 0.02 | 0.04 | 0.03 | 0.02 | 0.01 | ||
| Main effect means 3 | ||||||||
| Se-S | Inorganic | 0.68 b | 0.72 | 0.20 b | 0.16 b | 0.21 b | 0.15 a | |
| Organic | 0.79 a | 0.68 | 0.49 a | 0.36 a | 0.33 a | 0.12 b | ||
| SEM | 0.05 | 0.05 | 0.04 | 0.02 | 0.02 | 0.02 | ||
| Pros | 0 | 0.70 b | 0.71 | 0.30 b | 0.28 | 0.24 b | 0.12 b | |
| 0.5 | 0.78 a | 0.70 | 0.37 a | 0.25 | 0.30 a | 0.16 a | ||
| SEM | 0.05 | 0.06 | 0.04 | 0.02 | 0.02 | 0.02 | ||
| p-value | ||||||||
| Treatment | 0.004 | 0.236 | <0.001 | <0.001 | <0.001 | 0.007 | ||
| Se-S | 0.004 | 0.160 | 0.001 | 0.001 | 0.001 | 0.040 | ||
| Pros | 0.041 | 0.457 | 0.006 | 0.202 | 0.002 | 0.037 | ||
| Se-S × Pros 4 | 0.094 | 0.101 | 0.001 | 0.367 | 0.010 | 0.022 |
| Items 2 | Se, mg/kg | Pros, % | T-AOC 5, U/mL | GSH-Px 6, U/mL | SOD 7, U/mL | MDA 8, nmol/mL |
|---|---|---|---|---|---|---|
| I-Se | 0.2 | 0 | 11.83 c | 761.40 b | 191.36 | 3.25 |
| O-Se | 0.2 | 0 | 14.64 bc | 738.30 b | 192.00 | 3.32 |
| I-Se + pros | 0.2 | 0.5 | 16.44 ab | 914.40 a | 199.27 | 2.40 |
| O-Se + pros | 0.2 | 0.5 | 19.86 a | 953.25 a | 173.60 | 3.20 |
| SEM | 0.87 | 28.87 | 3.80 | 0.19 | ||
| Main effect means 3 | ||||||
| Se-S | Inorganic | 14.13 b | 837.90 | 194.33 | 2.83 | |
| Organic | 16.96 a | 833.83 | 185.10 | 3.26 | ||
| SEM | 1.70 | 57.45 | 10.26 | 0.53 | ||
| Pros | 0 | 13.23 b | 749.85 b | 191.68 | 3.29 | |
| 0.5 | 17.96 a | 931.67 a | 186.44 | 2.80 | ||
| SEM | 1.80 | 60.93 | 10.26 | 0.53 | ||
| p-value | ||||||
| Treatment | 0.004 | 0.005 | 0.193 | 0.296 | ||
| Se-S | 0.024 | 0.853 | 0.110 | 0.079 | ||
| Pros | 0.001 | 0.001 | 0.483 | 0.096 | ||
| Se-S × Pros 4 | 0.808 | 0.471 | 0.095 | 0.056 | ||
| Items 2 | Se, mg/kg | Pros, % | Duodenum | Jejunum | Ileum | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| V 5, µm | C 6, µm | V/C 7 | V, µm | C, µm | V/C | V, µm | C, µm | V/C | |||
| I-Se | 0.2 | 0 | 1208.20 | 255.87 a | 4.80 b | 885.37 b | 135.23 | 6.55 | 878.51 | 167.50 | 5.27 |
| O-Se | 0.2 | 0 | 1188.05 | 198.12 b | 6.05 ab | 1155.08 a | 150.87 | 7.85 | 982.20 | 167.53 | 5.87 |
| I-Se + pros | 0.2 | 0.5 | 1336.74 | 184.87 b | 7.26 a | 1306.01 a | 144.01 | 9.09 | 1001.96 | 153.33 | 6.59 |
| O-Se + pros | 0.2 | 0.5 | 1387.05 | 222.78 ab | 6.37 ab | 1206.51 a | 143.89 | 8.40 | 973.29 | 165.49 | 6.04 |
| SEM | 38.51 | 10.92 | 0.38 | 58.69 | 2.64 | 0.39 | 19.40 | 3.37 | 0.22 | ||
| Main effect means 3 | |||||||||||
| Se-S | Inorganic | 1272.47 | 220.37 | 6.03 | 1095.69 | 139.62 | 7.82 | 940.23 | 160.42 | 5.93 | |
| Organic | 1287.55 | 210.45 | 6.21 | 1185.94 | 146.68 | 8.18 | 977.74 | 166.51 | 5.96 | ||
| SEM | 61.70 | 11.38 | 0.44 | 102.38 | 7.39 | 0.87 | 43.30 | 9.47 | 0.57 | ||
| Pros | 0 | 1200.14 | 232.77 | 5.30 b | 993.25 b | 141.49 | 7.07 b | 930.36 | 167.52 | 5.57 | |
| 0.5 | 1356.87 | 200.04 | 6.90 a | 1256.26 a | 143.95 | 8.75 a | 987.62 | 159.41 | 5.95 | ||
| SEM | 75.57 | 13.94 | 0.55 | 91.58 | 6.61 | 0.78 | 43.30 | 9.47 | 0.57 | ||
| p-value | |||||||||||
| Treatment | 0.232 | 0.021 | 0.042 | 0.013 | 0.279 | 0.065 | 0.082 | 0.427 | 0.216 | ||
| Se-S | 0.834 | 0.465 | 0.724 | 0.255 | 0.162 | 0.622 | 0.255 | 0.390 | 0.955 | ||
| Pros | 0.055 | 0.119 | 0.032 | 0.011 | 0.861 | 0.033 | 0.098 | 0.261 | 0.099 | ||
| Se-S × Pros 4 | 0.628 | 0.009 | 0.075 | 0.031 | 0.156 | 0.132 | 0.063 | 0.392 | 0.189 | ||
| Items 2 | Effective Sequence | OTU | Unique OTU |
|---|---|---|---|
| I-Se | 163,325 | 2362 | 1135 |
| O-Se | 149,415 | 2515 | 1308 |
| I-Se + pros | 121,335 | 2858 | 1557 |
| O-Se + pros | 170,332 | 2689 | 1342 |
| Items 2 | Se, mg/kg | Pros, % | Chao 1 Index | Simpson Index | Shanon Index | Pielou E Index | Observed_Species |
|---|---|---|---|---|---|---|---|
| I-Se | 0.2 | 0 | 965.99 | 0.56 | 3.70 | 0.37 | 865.07 |
| O-Se | 0.2 | 0 | 1009.06 | 0.61 | 4.08 | 0.40 | 937.60 |
| I-Se + pros | 0.2 | 0.5 | 1117.96 | 0.56 | 3.77 | 0.37 | 990.80 |
| O-Se + pros | 0.2 | 0.5 | 1124.41 | 0.48 | 3.26 | 0.32 | 986.37 |
| SEM | 75.57 | 0.05 | 0.29 | 0.03 | 67.66 | ||
| Main effect means 3 | |||||||
| Se-S 3 | Inorganic | 1041.98 | 0.56 | 3.73 | 0.37 | 927.93 | |
| Organic | 1066.73 | 0.54 | 3.67 | 0.36 | 961.98 | ||
| SEM | 55.80 | 0.05 | 0.37 | 0.03 | 50.24 | ||
| Pros | 0 | 987.53 | 0.59 | 3.89 | 0.38 | 901.33 | |
| 0.5 | 1121.18 | 0.52 | 3.51 | 0.34 | 988.58 | ||
| SEM | 55.80 | 0.05 | 0.37 | 0.03 | 50.24 | ||
| p-value | |||||||
| Treatment | 0.438 | 0.584 | 0.737 | 0.682 | 0.590 | ||
| Se-S | 0.762 | 0.760 | 0.899 | 0.879 | 0.645 | ||
| Pros | 0.129 | 0.344 | 0.488 | 0.414 | 0.254 | ||
| Se-S × Pros 4 | 0.822 | 0.357 | 0.414 | 0.402 | 0.603 | ||
| Items 2 | Se, mg/kg | Pros, % | Bacteroidetes | Firmicutes | Proteobacteria | Actinobacteria |
|---|---|---|---|---|---|---|
| I-Se | 0.2 | 0 | 67.84 | 30.70 | 1.00 | 0.06 |
| O-Se | 0.2 | 0 | 68.51 | 21.88 | 9.06 | 0.10 |
| I-Se + pros | 0.2 | 0.5 | 68.20 | 30.57 | 0.83 | 0.06 |
| O-Se + pros | 0.2 | 0.5 | 82.60 | 16.25 | 0.51 | 0.17 |
| SEM | 7.35 | 7.20 | 1.17 | 0.17 | ||
| Main effect means 3 | ||||||
| Se-S 3 | Inorganic | 68.02 | 30.63 | 0.92 | 0.06 | |
| Organic | 75.56 | 19.06 | 4.79 | 0.13 | ||
| SEM | 13.98 | 13.00 | 4.90 | 0.08 | ||
| Pros | 0 | 68.18 | 26.29 | 5.03 | 0.08 | |
| 0.5 | 75.40 | 23.41 | 0.67 | 0.11 | ||
| SEM | 13.98 | 13.00 | 4.90 | 0.08 | ||
| p-value | ||||||
| Treatment | 0.673 | 0.638 | 0.304 | 0.505 | ||
| Se-S | 0.468 | 0.244 | 0.296 | 0.226 | ||
| Pros | 0.486 | 0.762 | 0.244 | 0.539 | ||
| Se-S × Pros 4 | 0.507 | 0.772 | 0.261 | 0.539 | ||
| Items 2 | Se, mg/kg | Pros 2, % | Barnesiellaceae | Bacteroides | Barnesiella | Lactobacillus | Ruminococcaceae | Clostridiales | Lachnospiraceae | Faecalibacterium | Blautia |
|---|---|---|---|---|---|---|---|---|---|---|---|
| I-Se | 0.2 | 0 | 55.79 | 7.21 | 2.99 b | 0.18 | 3.47 | 4.24 | 2.23 | 1.11 | 2.29 |
| O-Se | 0.2 | 0 | 54.66 | 3.75 | 7.10 a | 1.34 | 3.08 | 6.53 | 0.77 | 2.55 | 2.21 |
| I-Se + pros | 0.2 | 0.5 | 57.86 | 4.06 | 3.99 b | 0.18 | 4.4 | 3.6 | 1.9 | 1.84 | 1.56 |
| O-Se + pros | 0.2 | 0.5 | 71.51 | 3.47 | 5.13 a | 2.62 | 3.68 | 4.7 | 1.03 | 0.91 | 0.18 |
| SEM | 4.85 | 0.75 | 0.51 | 0.44 | 0.58 | 0.76 | 0.33 | 0.37 | 0.45 | ||
| Main effect means 3 | |||||||||||
| Se-S3 | Inorganic | 56.83 | 5.64 | 3.49 b | 0.18 b | 3.94 | 3.92 | 2.07 | 1.47 | 1.92 | |
| Organic | 63.09 | 3.61 | 6.12 a | 1.98 a | 3.38 | 5.61 | 0.90 | 0.91 | 1.19 | ||
| SEM | 10.31 | 1.40 | 0.54 | 0.76 | 1.32 | 1.60 | 0.66 | 0.73 | 0.87 | ||
| Pros | 0 | 55.22 | 5.48 | 5.05 | 0.76 | 3.28 | 5.38 | 1.50 | 1.83 | 2.25 | |
| 0.5 | 64.69 | 3.76 | 4.56 | 1.40 | 4.04 | 4.15 | 1.47 | 1.37 | 0.87 | ||
| SEM | 10.31 | 1.40 | 0.54 | 0.76 | 1.32 | 1.60 | 0.66 | 0.73 | 0.87 | ||
| p-value | |||||||||||
| Treatment | 0.648 | 0.274 | 0.004 | 0.146 | 0.909 | 0.623 | 0.399 | 0.424 | 0.354 | ||
| Se-S | 0.561 | 0.185 | 0.001 | 0.044 | 0.683 | 0.321 | 0.114 | 0.737 | 0.428 | ||
| Pros | 0.386 | 0.253 | 0.393 | 0.418 | 0.577 | 0.463 | 0.955 | 0.552 | 0.152 | ||
| Se-S × Pros 4 | 0.494 | 0.335 | 0.025 | 0.423 | 0.906 | 0.719 | 0.666 | 0.144 | 0.478 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deng, S.; Hu, S.; Xue, J.; Yang, K.; Zhuo, R.; Xiao, Y.; Fang, R. Productive Performance, Serum Antioxidant Status, Tissue Selenium Deposition, and Gut Health Analysis of Broiler Chickens Supplemented with Selenium and Probiotics—A Pilot Study. Animals 2022, 12, 1086. https://doi.org/10.3390/ani12091086
Deng S, Hu S, Xue J, Yang K, Zhuo R, Xiao Y, Fang R. Productive Performance, Serum Antioxidant Status, Tissue Selenium Deposition, and Gut Health Analysis of Broiler Chickens Supplemented with Selenium and Probiotics—A Pilot Study. Animals. 2022; 12(9):1086. https://doi.org/10.3390/ani12091086
Chicago/Turabian StyleDeng, Shengting, Shengjun Hu, Junjing Xue, Kaili Yang, Ruiwen Zhuo, Yuanyuan Xiao, and Rejun Fang. 2022. "Productive Performance, Serum Antioxidant Status, Tissue Selenium Deposition, and Gut Health Analysis of Broiler Chickens Supplemented with Selenium and Probiotics—A Pilot Study" Animals 12, no. 9: 1086. https://doi.org/10.3390/ani12091086
APA StyleDeng, S., Hu, S., Xue, J., Yang, K., Zhuo, R., Xiao, Y., & Fang, R. (2022). Productive Performance, Serum Antioxidant Status, Tissue Selenium Deposition, and Gut Health Analysis of Broiler Chickens Supplemented with Selenium and Probiotics—A Pilot Study. Animals, 12(9), 1086. https://doi.org/10.3390/ani12091086





