Fecal Glucocorticoid Metabolites as a Noninvasive Indicator of Stress in the Tsushima Leopard Cats (Prionailurus bengalensis euptilurus): Application to Health Care
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Sample Collection
2.2. Fecal Glucocorticoid Analysis
2.3. High-Performance Liquid Chromatography (HPLC)
2.4. Data Analyses
3. Results
3.1. Fecal Glucocorticoid Associated with Physical Examination and Disease
3.2. Comparison of Healthy and Diseased Animals
3.3. Identification of Fecal Glucocorticoid Metabolites
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Masuda, R.; Yoshida, M.C. Two Japanese wildcats, the Tsushima cat and the Iriomote cat, show the same mitochondrial DNA lineage as the leopard cat Felis bengalensis. Zool. Sci. 1995, 12, 655–659. [Google Scholar] [CrossRef] [PubMed]
- Frankham, R. Genetics and extinction. Biol. Conserv. 2005, 126, 131–140. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Urata, A. Tsushima leopard cat. In Creatures in Tsushima; The Nagasaki Biological Society: Nagasaki, Japan, 1976; pp. 167–180. (In Japanese) [Google Scholar]
- Kyushu Regional Environment Office, Ministry of the Environment in Japan. Press Release, 30 March 2020: Summary of the Latest Results of the Tsushima Leopard Cat Habitat Survey (5th Round Survey). Available online: http://kyushu.env.go.jp/pre_2020/327.html (accessed on 1 March 2022). (In Japanese)
- Azumano, A.; Ueda, M.; Nomura, M.; Usui, M.; Ichinose, M.; Yanagawa, Y.; Kusuda, S.; Matsumoto, Y.; Murata, K. Successful laparoscopic oviductal artificial insemination in the endangered Tsushima leopard cat (Prionailurus bengalensis euptilurus). Animals 2022, 12, 777. [Google Scholar] [CrossRef] [PubMed]
- Selye, H.; Collip, J. Fundamental factors in the interpretation of stimuli influencing endocrine glands. Endocrinology 1936, 20, 667–672. [Google Scholar] [CrossRef]
- Weissman, C. The metabolic response to stress: An overview and update. Anesthesiology 1990, 73, 308–327. [Google Scholar] [CrossRef] [PubMed]
- Stratakis, C.A.; Chrousos, G.P. Neuroendocrinology and pathophysiology of the stress system. Ann. N. Y. Acad. Sci. 1995, 771, 1–18. [Google Scholar] [CrossRef]
- Axelrod, J.; Reisine, T.D. Stress hormones: Their interaction and regulation. Science 1984, 224, 452–459. [Google Scholar] [CrossRef] [Green Version]
- Padgett, D.A.; Glaser, R. How stress influences the immune response. Trends Immunol. 2003, 24, 444–448. [Google Scholar] [CrossRef]
- Carlstead, K.; Shepherdson, D. Effects of environmental enrichment on reproduction. Zoo Biol. 1994, 13, 447–458. [Google Scholar] [CrossRef]
- Fraser, D. Stress. In Understanding Animal Welfare: The Science in Its Cultural Context; Wiley-Blackwell: West Sussex, UK, 2008; pp. 104–124. [Google Scholar]
- Sapolsky, R.M.; Romero, L.M.; Munck, A.U. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr. Rev. 2000, 21, 55–89. [Google Scholar] [CrossRef] [Green Version]
- Palme, R.; Rettenbacher, S.; Touma, C.; El-Bahr, S.M.; Möstl, E. Stress hormones in mammals and birds: Comparative aspects regarding metabolism, excretion, and noninvasive measurement in fecal samples. Ann. N. Y. Acad. Sci. 2005, 1040, 162–171. [Google Scholar] [CrossRef] [PubMed]
- Przkop, F.; Stupnicka, E.; Wolińska, W.E.; Mateusiak, K.; Sadowski, B.; Domański, E. Changes in circadian rhythm and suppression of the plasma cortisol level after prolonged stress in the sheep. Acta Endocrinol. 1985, 110, 540–545. [Google Scholar] [CrossRef] [PubMed]
- Ladewig, J.; Smidt, D. Behavior, episodic secretion of cortisol, and adrenocortical reactivity in bulls subjected to tethering. Horm. Behav. 1989, 23, 344–360. [Google Scholar] [CrossRef]
- Möstl, E.; Palme, R. Hormones as indicators of stress. Domest. Anim. Endocrinol. 2002, 23, 67–74. [Google Scholar] [CrossRef]
- Graham, L.H.; Brown, J.L. Cortisol metabolism in the domestic cat and implications for noninvasive monitoring of adrenocortical function in endangered felids. Zoo Biol. 1996, 15, 71–82. [Google Scholar] [CrossRef]
- Schatz, S.; Palme, R. Measurement of faecal cortisol metabolites in cats and dogs: A non-invasive method for evaluating adrenocortical function. Vet. Res. Commun. 2001, 25, 271–287. [Google Scholar] [CrossRef]
- Young, K.M.; Walker, S.L.; Lanthier, C.; Waddell, W.T.; Monfort, S.L.; Brown, J.L. Noninvasive monitoring of adrenocortical activity in carnivores by fecal glucocorticoid analyses. Endocrinology 2004, 137, 148–165. [Google Scholar] [CrossRef]
- Rozhnov, V.V.; Lukarevskiy, V.S.; Hemandez, H.A.; Sorokin, P.A.; Litvinov, M.N.; Kotlyar, A.K.; Udin, V.G.; Naydenko, S.V. Noninvasive approach to the assessment of activity of the hypothalamic-pituitary-adrenal system of the Amur tigers. Dokl. Biol. Sci. 2009, 430, 57–59. [Google Scholar] [CrossRef]
- Wielebnowski, N.C.; Fletchall, N.; Carlstead, K.; Busso, J.M.; Brown, J.L. Noninvasive assessment of adrenal activity associated with husbuandry and behavioral factors in the North American clouded leopard population. Zoo Biol. 2002, 21, 77–98. [Google Scholar] [CrossRef]
- Moreira, N.; Brown, J.L.; Moraes, W.; Swanson, W.F.; Monteiro-Filho, E.L.A. Effect of housing and environmental enrichment on adrenocortical activity, behavior and reproductive cyclicity in the female tigrina (Leopardus tigrinus) and margay (Leopardus wiedii). Zoo Biol. 2007, 26, 441–460. [Google Scholar] [CrossRef]
- Adachi, I.; Kusuda, S.; Nagao, E.; Taira, Y.; Asano, M.; Tsubota, T.; Doi, O. Fecal steroid metabolites and reproductive monitoring in a female Tsushima leopard cat (Prionailurus bengalensis euptilurus). Theriogenology 2010, 74, 1499–1503. [Google Scholar] [CrossRef] [PubMed]
- Adachi, I.; Kusuda, S.; Kawai, H.; Ohazawa, M.; Taniguchi, A.; Kondo, N.; Yoshihara, M.; Okuda, R.; Ishikawa, T.; Kanda, I.; et al. Fecal progestagens to detect and monitor pregnancy in captive female cheetahs (Acinonyx jubatus). J. Reprod. Dev. 2011, 57, 262–266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carlstead, K.; Brown, J.L.; Monfort, S.L.; Killens, R.; Wildt, D.E. Urinary monitoring of adrenal responses to psychological stressors in domestic and nondomestic felids. Zoo Biol. 1992, 11, 165–176. [Google Scholar] [CrossRef]
- Polzin, D.J.; Cowgill, L.D. Management of glomerulopathies. In BSAVA Manual of Canine and Feline Nephrology and Urology, 2nd ed.; Elliott, J., Grauer, G.F., Westropp, J.L., Eds.; British Small Animal Veterinary Association: Gloucester, UK, 2007; pp. 278–290. [Google Scholar]
- Jepson, R.; Syme, H. Management of chronic kidney disease. In BSAVA Manual of Canine and Feline Nephrology and Urology, 2nd ed.; Elliott, J., Grauer, G.F., Westropp, J.L., Eds.; British Small Animal Veterinary Association: Gloucester, UK, 2007; pp. 263–277. [Google Scholar]
- Rojko, J.L.; Hoover, E.A.; Mathes, L.E.; Krakowka, S.; Olsen, R.G. Influence of adrenal corticosteroids on the susceptibility of cats to feline leukemia virus infection. Cancer Res. 1979, 39, 3789–3791. [Google Scholar]
- Willett, B.; Flynn, N.; Hosic, M. FIV infection of the domestic cat: An animal model for AIDS. Immunol. Today 1997, 18, 182–189. [Google Scholar] [CrossRef]
- Biglino, A.; Limone, P.; Forno, B.; Pollono, A.; Cariti, G.; Molinatti, G.M.; Gioannini, P. Altered adrenocorticotropin and cortisol response to corticotrophin-releasing hormone in HIV-1 infection. Eur. J. Endocrinol. 1995, 133, 173–179. [Google Scholar] [CrossRef]
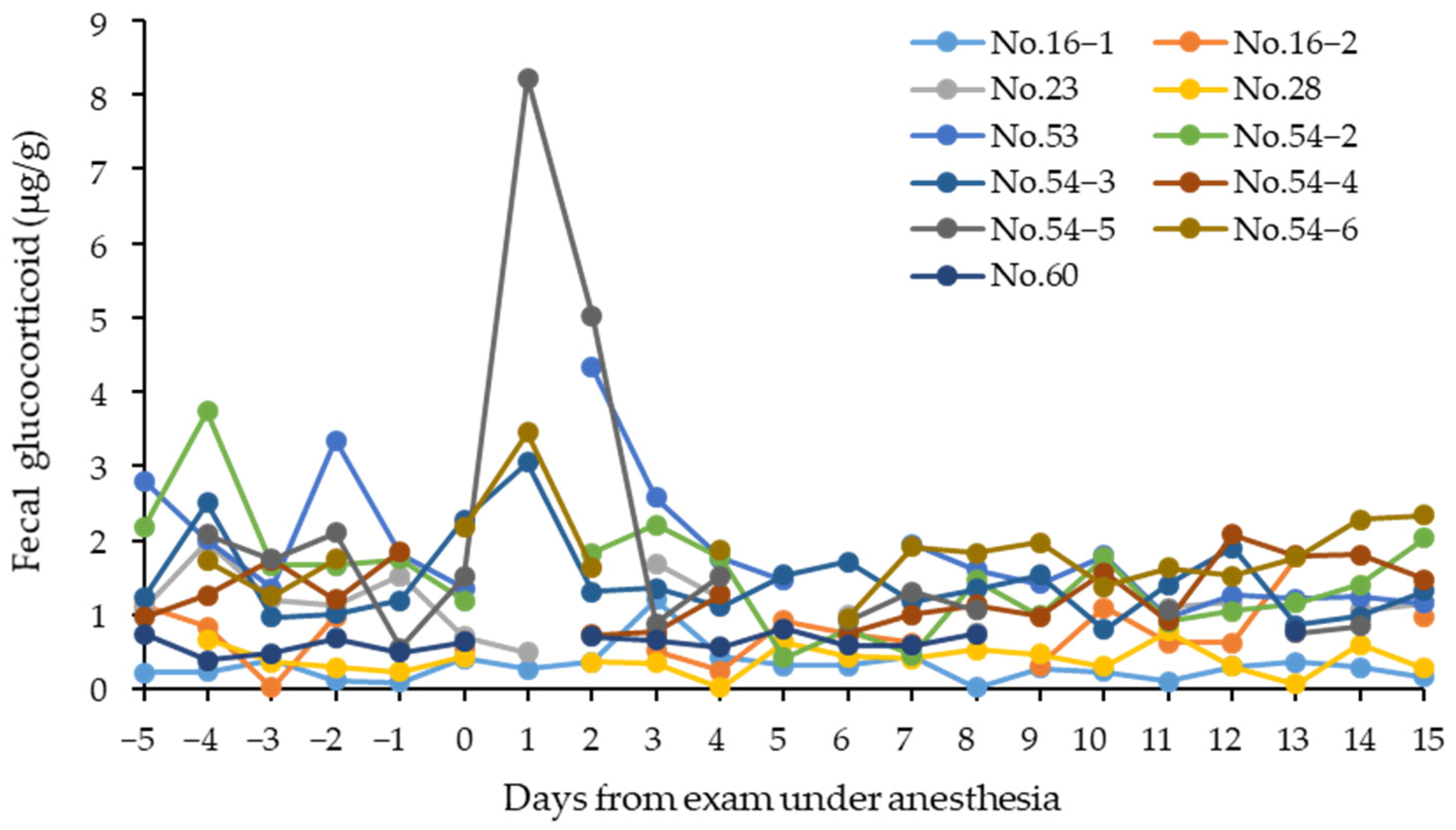
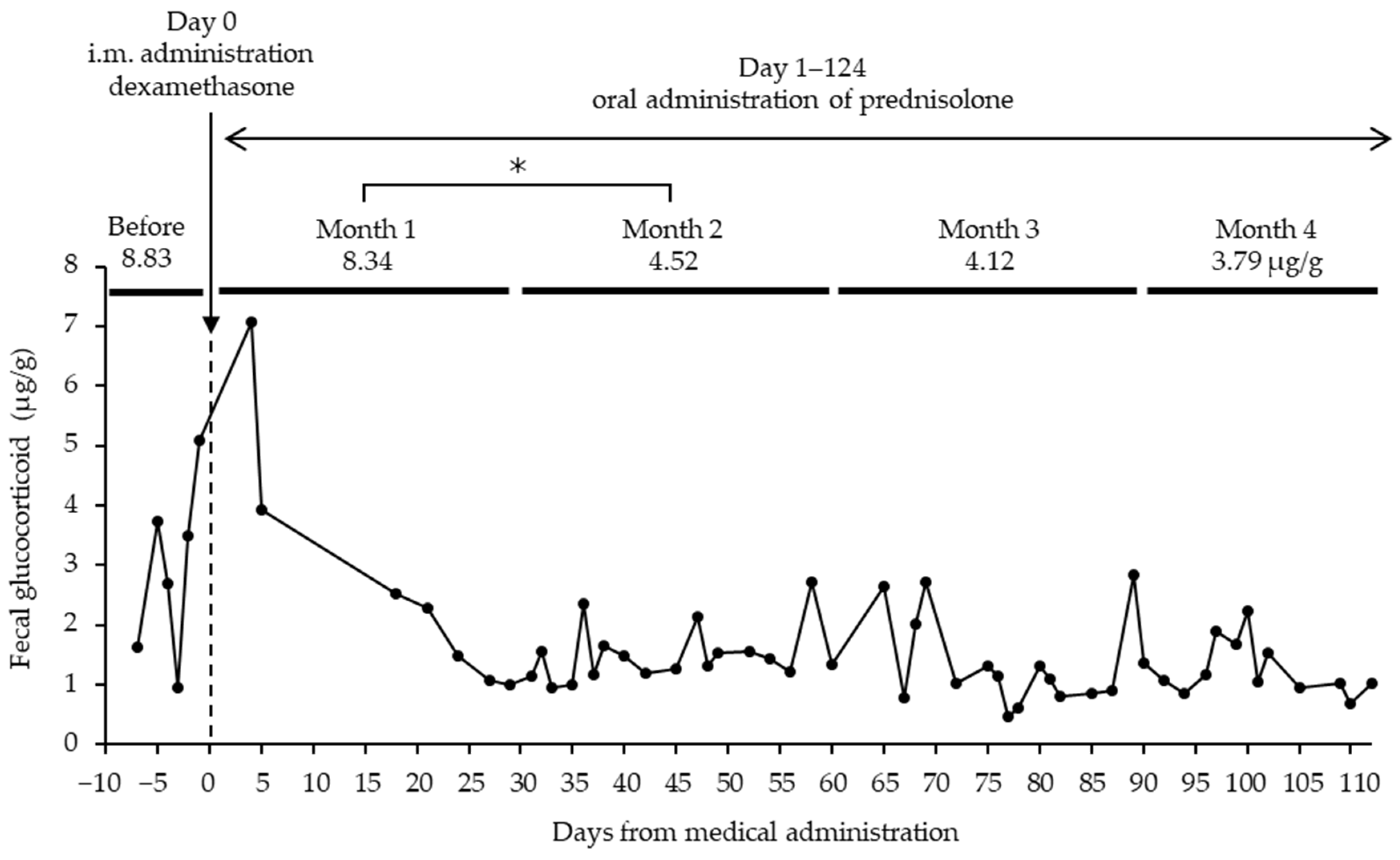
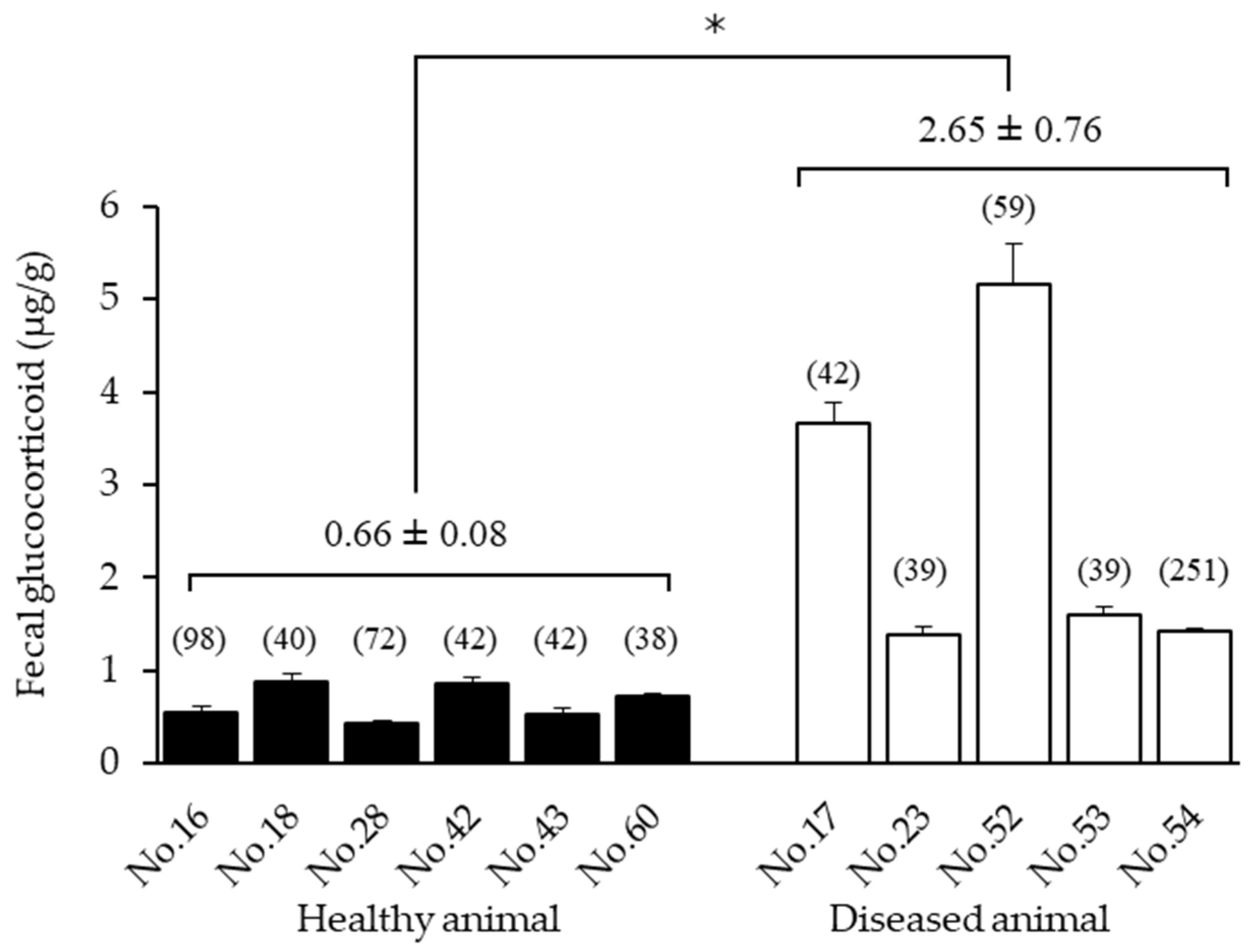
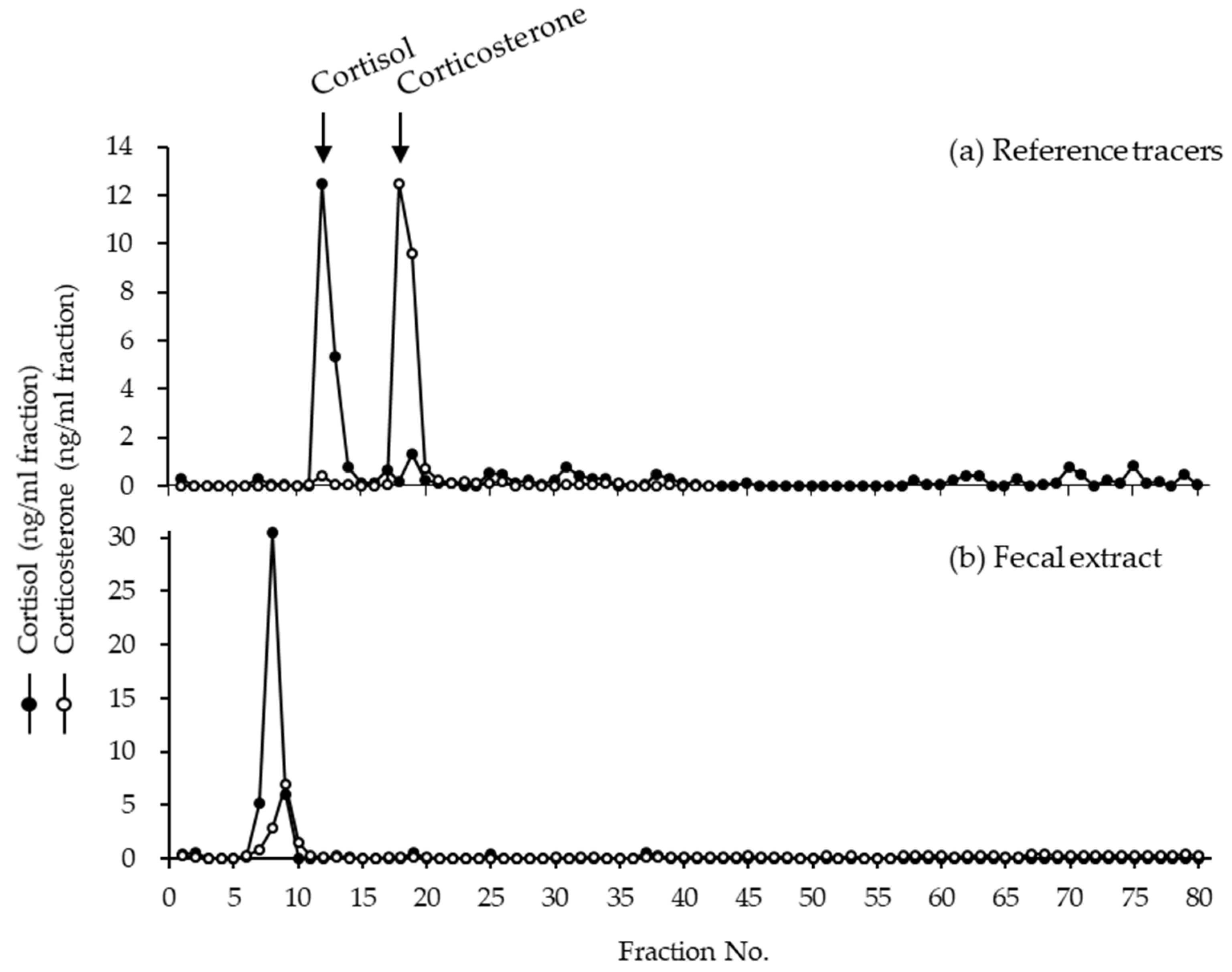
| Studbook No. | Sex | Birth/Caught Date | Institute | Months of GC Measurement (Age Range) | No. of Samples Collected | Physical Exam Date |
|---|---|---|---|---|---|---|
| Healthy animals | ||||||
| No.16 | ♂ | CB: 7 April 2003 | YZG | 1.8 months (3.6–3.7) 1.0 month (7.0–7.1) 2.0 months (7.5–7.6) | 38 28 40 | - 1: 10 May 2010 2: 19 October 2010 |
| No.18 | ♀ | CB: 5 May 2003 | FZG | 6.7 months (3.0–3.6) | 42 | - |
| No.28 | ♂ | CB: 19 April 2004 | YZG | 1.8 months (3.5–3.6) 1.2 months (6.0–6.1) | 42 36 | - 16 May 2010 |
| No.42 | ♀ | CB: 9 May 2007 | TFPZ | 4.6 months (2.6–2.9) | 42 | - |
| No.43 | ♂ | WC: 12 May 2002 | FZG | 4.0 months (unknown) | 42 | - |
| No.60 (Mk−45) | ♂ | WC: 28 December 2009 | FZG | 4.8 months (unknown) | 41 | 15 February 2010 |
| Diseased animals | ||||||
| No.17; renal dysfunction | ♀ | CB: 7 April 2003 | TWCC | 36.0 months (3.7–6.7) | 42 | - |
| No.23; renal dysfunction | ♂ | CB: 3 April 2004 | TWCC | 2.1 months (5.2–5.3) | 42 | 29 June 2009 |
| No.52 (Mt−09); FIV, hernia | ♂ | WC: 20 December 2000 | TWCC | 2.9 months (unknown) | 59 | 31 January 2007 |
| No.53 (CFT−17); FIV, adrenal tumor | ♀ | WC: 22 September 2002 | TWCC | 1.8 months (unknown) | 42 | 31 October 2008 |
| No.54 (Fm−28); FeLV | ♀ | WC: 14 March 2005 | TWCC | 1.8 months (unknown) 1.4 months (unknown) 2.0 months (unknown) 2.0 months (unknown) 1.7 months (unknown) 1.2 months (unknown) | 36 42 61 56 42 30 | 1: 29 June 2007 2: 2 June 2008 3: 31 October 2008 4: 30 May 2009 5: 4 August 2010 6: 26 October 2010 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kusuda, S.; Funahashi, T.; Adachi, I.; Yamamoto, H.; Nagao, E.; Matsui, K.; Akiba, Y. Fecal Glucocorticoid Metabolites as a Noninvasive Indicator of Stress in the Tsushima Leopard Cats (Prionailurus bengalensis euptilurus): Application to Health Care. Animals 2022, 12, 1072. https://doi.org/10.3390/ani12091072
Kusuda S, Funahashi T, Adachi I, Yamamoto H, Nagao E, Matsui K, Akiba Y. Fecal Glucocorticoid Metabolites as a Noninvasive Indicator of Stress in the Tsushima Leopard Cats (Prionailurus bengalensis euptilurus): Application to Health Care. Animals. 2022; 12(9):1072. https://doi.org/10.3390/ani12091072
Chicago/Turabian StyleKusuda, Satoshi, Takashi Funahashi, Itsuki Adachi, Hanae Yamamoto, Eiji Nagao, Kirito Matsui, and Yuki Akiba. 2022. "Fecal Glucocorticoid Metabolites as a Noninvasive Indicator of Stress in the Tsushima Leopard Cats (Prionailurus bengalensis euptilurus): Application to Health Care" Animals 12, no. 9: 1072. https://doi.org/10.3390/ani12091072
APA StyleKusuda, S., Funahashi, T., Adachi, I., Yamamoto, H., Nagao, E., Matsui, K., & Akiba, Y. (2022). Fecal Glucocorticoid Metabolites as a Noninvasive Indicator of Stress in the Tsushima Leopard Cats (Prionailurus bengalensis euptilurus): Application to Health Care. Animals, 12(9), 1072. https://doi.org/10.3390/ani12091072







