1. Introduction
Antimicrobial resistance (AMR) is a global human and animal health threat [
1]. Inappropriate or excessive use of antimicrobials (AMU) may result in the development of resistance to these substances, and to the subsequent inefficacy of the treatments administered to tackle infectious diseases. In humans, AMR is causing over 33,000 deaths every year just in the EU [
2], and it is already a leading cause of death worldwide [
3]. Thus, although antimicrobials are fundamental for the health of humans and animals, their misuse poses a paramount risk to the development of resistant bacteria. This link has been widely confirmed by the European Medicines Agency (EMA) and the European Food Safety Authority (EFSA) in their joint report [
4].
The use of medications in veterinary medicine together with human medicine [
4], are major contributors to the development of AMR. The role played by the veterinary sector has been mainly reported in studies on farm animals [
5] which, among all the categories of animals raised and/or managed by humans, are likely to be the highest consumers of antimicrobials [
6]. However, evidence of resistant bacteria has been described in all captive species (i.e., companion, laboratory (lab), and zoo animals) [
7,
8,
9] making them reservoirs of AMR. For instance, an investigation by Álvarez-Pérez et al. [
10] reported that zoo species such as chimpanzees and Iberian ibex carried strains of
Clostridioides difficile exhibiting resistance to antimicrobials commonly used in both veterinary and human medicine. The study of Ishihara et al. [
11] also identified an association of AMU with the spread of resistant
Escherichia coli among zoo animals. One of the roles of modern zoos is to promote wildlife conservation through breeding and reintroduction programs [
12]. However, these practices may become a potential route of dissemination of resistant bacteria not only among zoos worldwide but also into the wild. Indeed, reintroduction of zoo species to their natural environment can contribute to the spread of AMR to the wildlife [
13]. A similar scenario was also observed in studies on companion and lab animals such as that of Loncaric and colleagues [
14] where different companion animals (e.g., dogs, cats, rabbits) receiving antibiotic therapy had higher chance to develop resistant bacteria. The authors also observed that ‘hospitalized animals’ had higher risk to carry methicillin-resistant
Staphylococcus sp. Another example is the study of Yamanaka et al. [
15] who found laboratory mice showing resistance to several classes of antibiotics such as macrolides and fluoroquinolones.
In the companion, laboratory, and zoo animal groups, AMU seems low when compared to farm animals [
16]. For instance, Joosten et al. [
17], who investigated AMU and AMR in companion animals across three EU countries, reported that 81% of the animals included in their study did not receive any antimicrobial treatment. Thus, in these species the issue to address regarding AMU is not a matter of ‘quantity’ but of ‘quality’, since the most common medications used were critically important antimicrobials [
17]. Despite this, a more prudent antimicrobial stewardship in all group species is needed and should rely on the development of effective strategies that can help to address an inappropriate AMU [
18]. Greater knowledge on AMU and on potential risk factors for its use in all captive animal groups is then pivotal to achieve this goal.
At the same time, animal welfare is nowadays an ethical and societal demand. Indeed, providing appropriate welfare standards is considered a priority for animals living in captivity including those in the agriculture sector, in zoo institutions, those used in research, and pet animals. A set of rules are in place, both in EU and internationally [
19,
20], for the protection of all animal categories from farm [
21], to companion [
22], to laboratory [
23], and to zoo species [
24]. These rules/legal frameworks establish the minimum welfare standards to be respected.
The relationship between animal health, animal welfare, and productivity is well acknowledged and scientifically recognized [
25], as stated by the OIE in its Guiding Principles for Animal Welfare, which declared ‘
a critical relationship between animal health and animal welfare’ and emphasised that ‘
improvements in animal welfare can often improve productivity and food safety’ [
26]. Yet, animal health can still be perceived as separated from animal welfare, with the latter being considered more as a cost than a benefit to exploit. Instead, they depend on each other and can be considered as ‘two faces of the same coin’, thus making the concept of ‘One Welfare’ the natural extension of the ‘One Health’ approach [
27,
28]. Both concepts recognise the interconnection between humans, animals, environment, and conservation to support a more global sustainable development [
27]. Integrating these two approaches in research studies allows for a more holistic perspective towards certain areas of interest. In particular, it will permit to gather more evidence on direct and indirect benefits of incorporating the field of animal welfare to other disciplines to untangle the AMR threat [
27,
28].
Nevertheless, despite such promising benefits, the role that animal welfare can play in the reduction of AMU has been poorly investigated especially with regards to empirical evidence. In their recent report, the Food and Agriculture Organization of the United Nations (FAO) stated that improved health and welfare would make animals less prone to contract infectious diseases, thus minimising the need for antimicrobials [
29]. The necessity for more research on this argument is then evident. However, despite several publications that widely emphasised and theoretically discussed the importance of exploring such a relationship in animals kept in captivity [
29,
30,
31,
32], the extent of scientific work where this link has been demonstrated and/or studied in the literature is unclear. The importance of involving multiple disciplines when investigating this topic seems also to be a returning argument of discussion, with it (i.e., multi-disciplinarity) having been identified as a key tool to better understand such a relationship while also providing additional information on AMU among captive species. Deeper knowledge on the link between animal welfare and AMU, will greatly contribute to the development of effective strategies for a more judicious AMU in veterinary medicine.
In this systematic review, we aimed to gather research that explores the link between animal welfare and AMU in captive species. In particular, we focused on those studies that investigated either the impact of improved/poor animal welfare on AMU or vice versa—i.e., that of reduced/increased AMU on welfare indicators. This work is paramount to synthetize the empirical knowledge available so far, to compare the state-of-the-art between different captive animal groups, and to generate valuable information to target gaps in the literature regarding the aforesaid topic.
The research question framed was “Does animal welfare have an impact on antimicrobial use, or vice versa, in captive species (farm, zoo, companion and laboratory animals)?”. The population targeted were all captive animals within those four groups and the outcomes expected were empirical evidence of the effect of animal welfare on antimicrobial use or vice versa.
2. Materials and Methods
This systematic review aimed to explore the link between animal welfare and antimicrobial use in captive animals. This work was framed in the context of a special issue entitled ‘A Multidisciplinary Approach to Unveil the Link between Animal Welfare and Antimicrobial Use in Captive Animals’ for the journal Animals. The methods employed were based on the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) statement [
33]. PRISMA’s checklist for systematic reviews is included as
Supplementary Materials (Table S1).
Captive animals were grouped into: Farm animals, Zoo animals, Companion animals, and Laboratory (Lab) animals. The search string built for the searches was composed of three segments. Each segment assembled relevant keywords and synonyms. An ‘OR’ operator was used within segments whereas an ‘AND’ operator was used between segments. The structure of the search strings follows example 1:
The animal welfare and antimicrobial use segments (keywords used of the search) were common to all groups. The last segment referred to the animal group (i.e., farm animals, zoo animals, etc.) and to a list of the most representative animals included in that group. Two online databases, PubMed
® and Web of Science
®, were selected to conduct the literature searches. All searches were conducted in April 2021. The searches were restricted to the title and abstract and included only peer-reviewed studies in English. No time limit was imposed. The detailed search strings employed in each database are available in the
Supplementary Materials (Table S2).
The quality of the search was assessed by checking whether previously (manually) identified papers of interest (sentinel papers) were retained in the systematic searches. The search results in both databases were imported using EndNote
®. The same reference manager was used to remove duplicates.
Table 1 presents the inclusion and exclusion criteria defined to screen titles and abstracts and for the full text evaluation.
An initial sampling of 80 records (20 records in each animal group) for training purposes was performed. These records’ titles and abstracts were screened by the two assessors (the two authors) in parallel to practice the application of the inclusion and exclusion criteria. After this step, the authors discussed the results and refined the established criteria. Additionally, 20 records (5 in each group) were assessed in conjunction in real time to further validate the criteria. After this session, the assessors conducted the title/abstract evaluation of all records blindingly and independently. Any disagreements were discussed. Once the paper selection for full text analysis was finalised, the papers selected were retrieved and the two co-authors (in parallel and blinded to each other’s decisions) read the full texts using the same eligibility criteria (
Table 1). Exclusion of records had to be agreed by both authors.
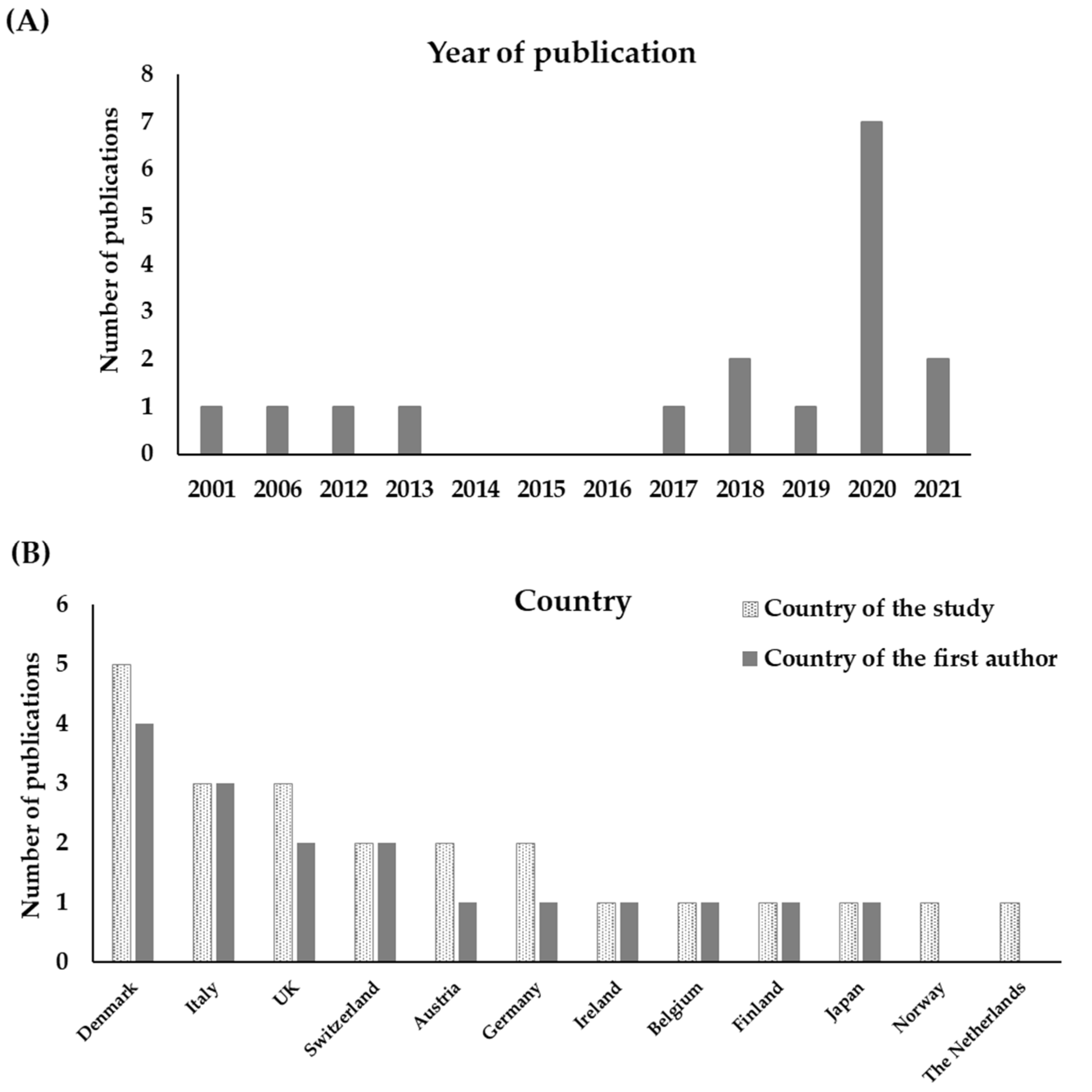
Study quality was ensured through the methods applied. First, this systematic review targeted only peer-reviewed publications, narrowing down the body of research to include only scientifically sound articles previously assessed by peers. Second, the article screening steps were devised to minimise bias of selection and ensure assessors were in agreement during different stages of the process. Risk of bias was not formally assessed in studies included in this review. Since the results were qualitative, a narrative description supported by graphs and tables was the preferred method of synthesis. Therefore, the risk of bias was addressed on a group basis, anchored in individual examples, and reported in the discussion.
The data within the final records included in this work was extracted onto a database (stored in a Microsoft Office Excel® spreadsheet). Data extraction accounted for the year of publication, the journal name, and the topic (the latter information was retrieved from ‘Scimago JR’ by selecting the first subject area of the journal), the country where the study was carried out, the country of the institution where the studied was developed (regarding first author), and the animal group studied. Other information, such as welfare indicators used, the route of antimicrobial administration, the direction of the study (i.e., whether it tested the impact of welfare on AMU or vice versa), and whether there was an effect, was also included. Graphical, tabular, and narrative commentary were the methods of synthesis used in the results’ section.
5. Conclusions
Despite several papers superficially invoking the link between animal welfare and AMU were originally retrieved (n = 6610), most of them did not investigate the topic empirically nor delved into the characteristics of the link, leading to a small number of final publications retained in this systematic review (n = 17). We conclude that evidence for this link remains scarce in the literature. As hypothesised, this work suggests that better animal welfare often leads to lower AMU, and this was especially the case reported for farm animals. Accordingly, some studies demonstrated that poor animal welfare was associated with higher AMU. However, judicious AMU may be necessary and inclusively lead to better welfare (i.e., having a protective effect) when animals are reared under intensive or conventional settings (i.e., minimum/legal welfare standards met). At the same time, AMU restrictions in organic farm systems may prevent animals from receiving treatments, when necessary, likely posing an extra risk of affecting their welfare. Therefore, more research is needed to corroborate these findings, especially with regards to the link between animal welfare and AMU in other captive species (i.e., zoo, companion, and laboratory), going beyond farm animals.