Effects of Parity and Stage of Lactation on Trend and Variability of Metabolic Markers in Dairy Cows
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. General Information & Population
2.2. Laboratory Analyses
2.3. Statistical Analyses
2.4. Graphical Presentation
3. Results and Discussion
3.1. Metabolic Parameters
3.1.1. NEFA Serum Concentrations

3.1.2. BHB Serum Concentrations
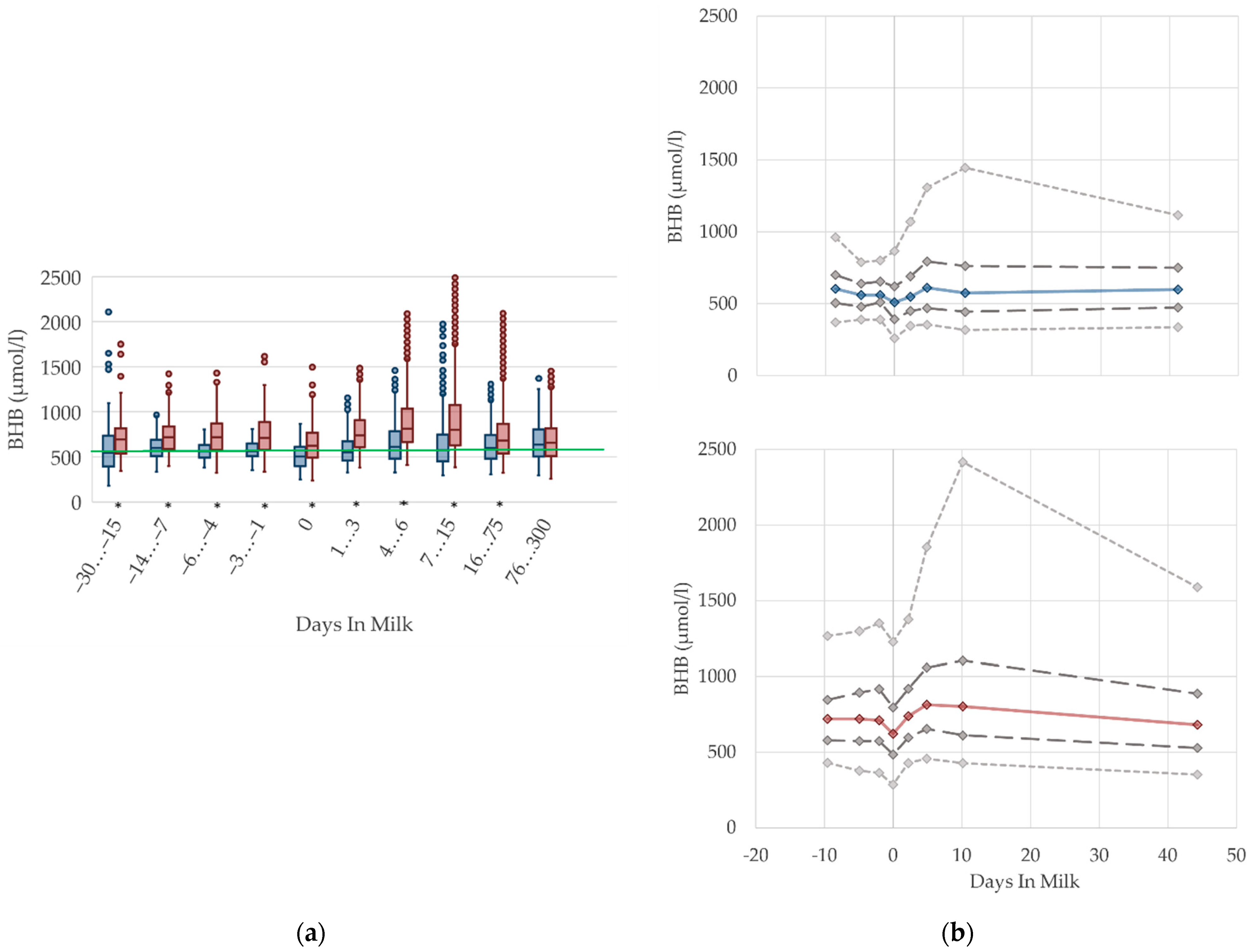
3.1.3. Bilirubin Serum Concentrations

3.1.4. Cholesterol Serum Concentrations
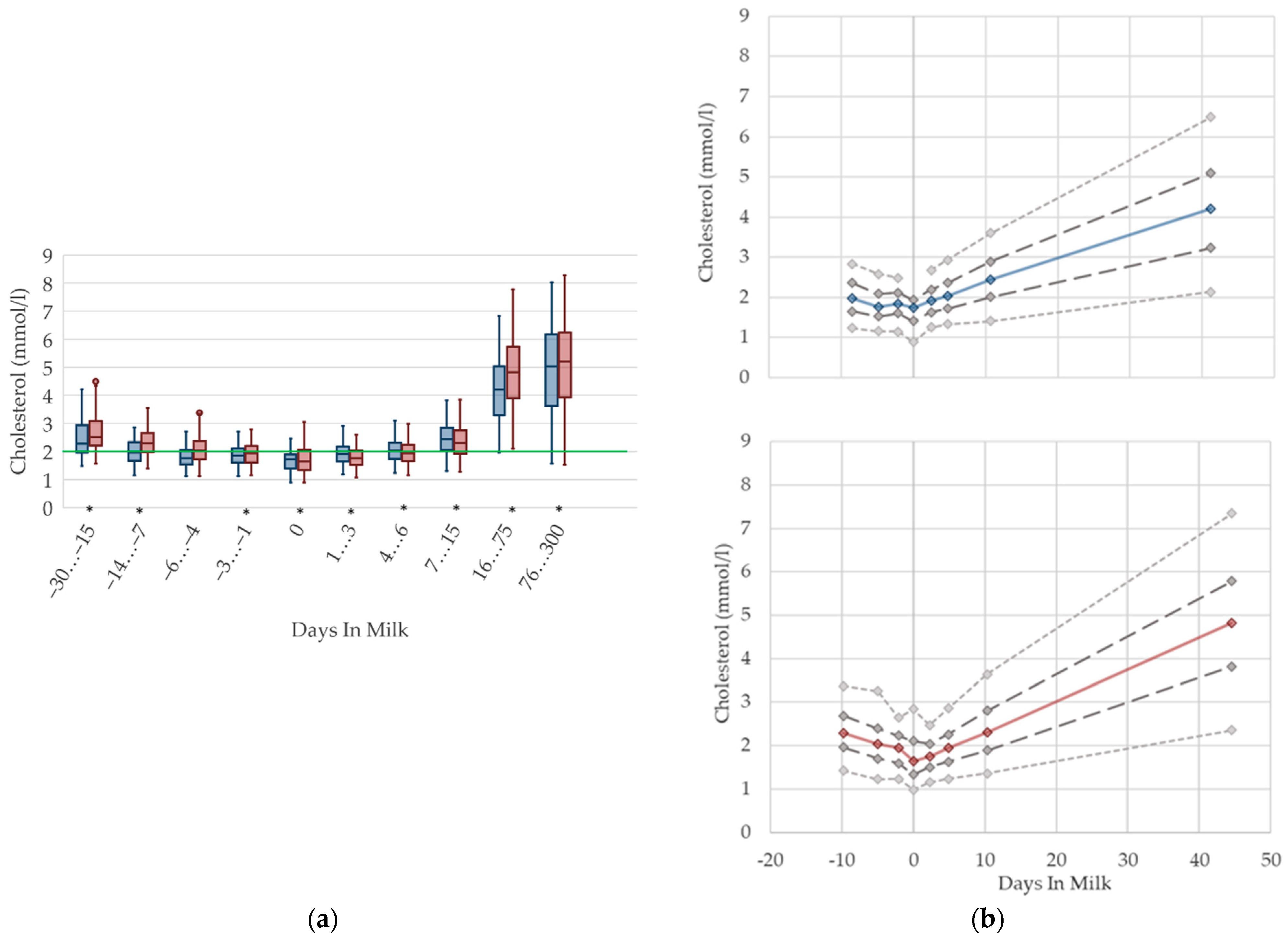
3.1.5. Urea Serum Concentrations
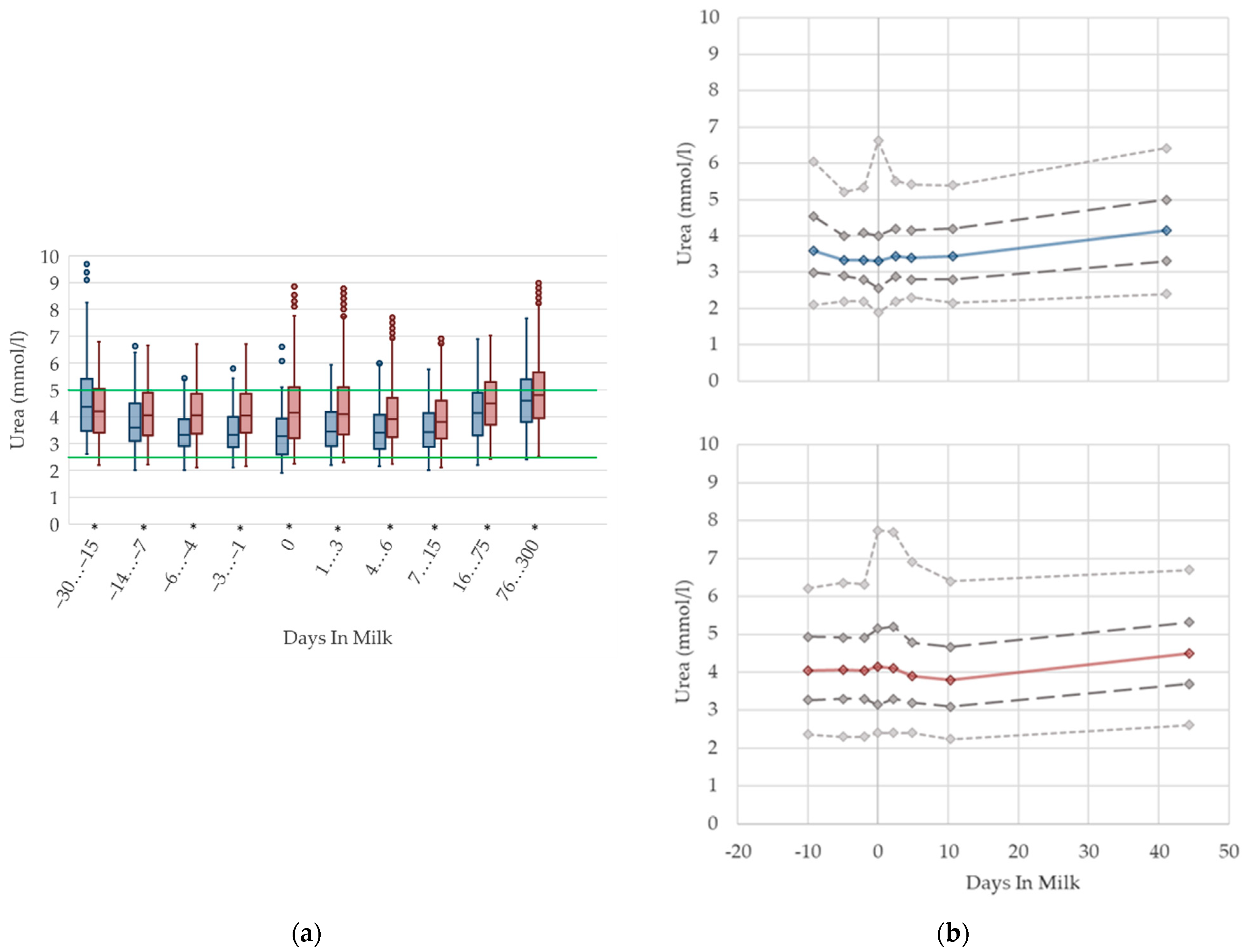
3.1.6. AST Serum Activities
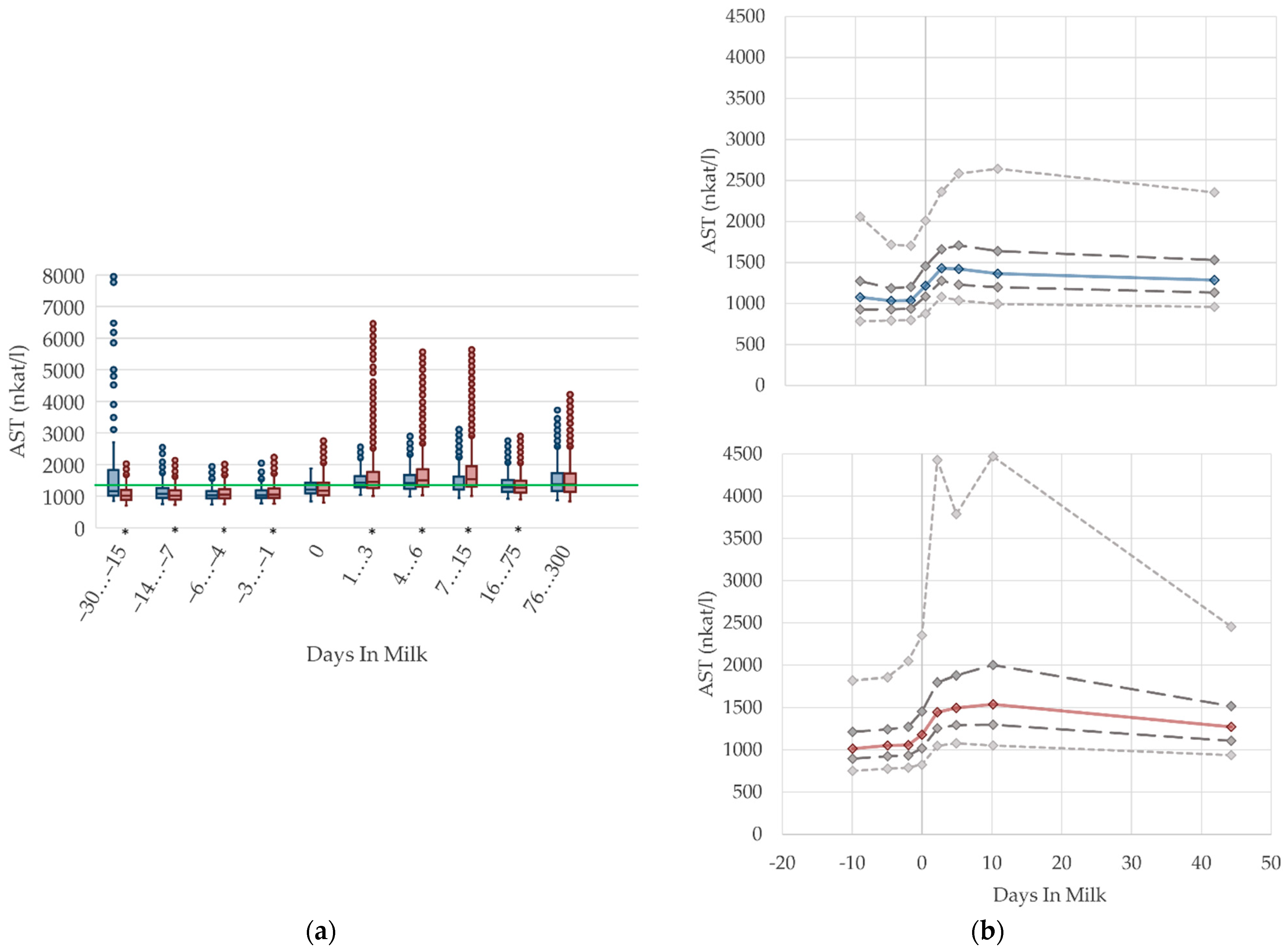
3.1.7. CK Serum Activities
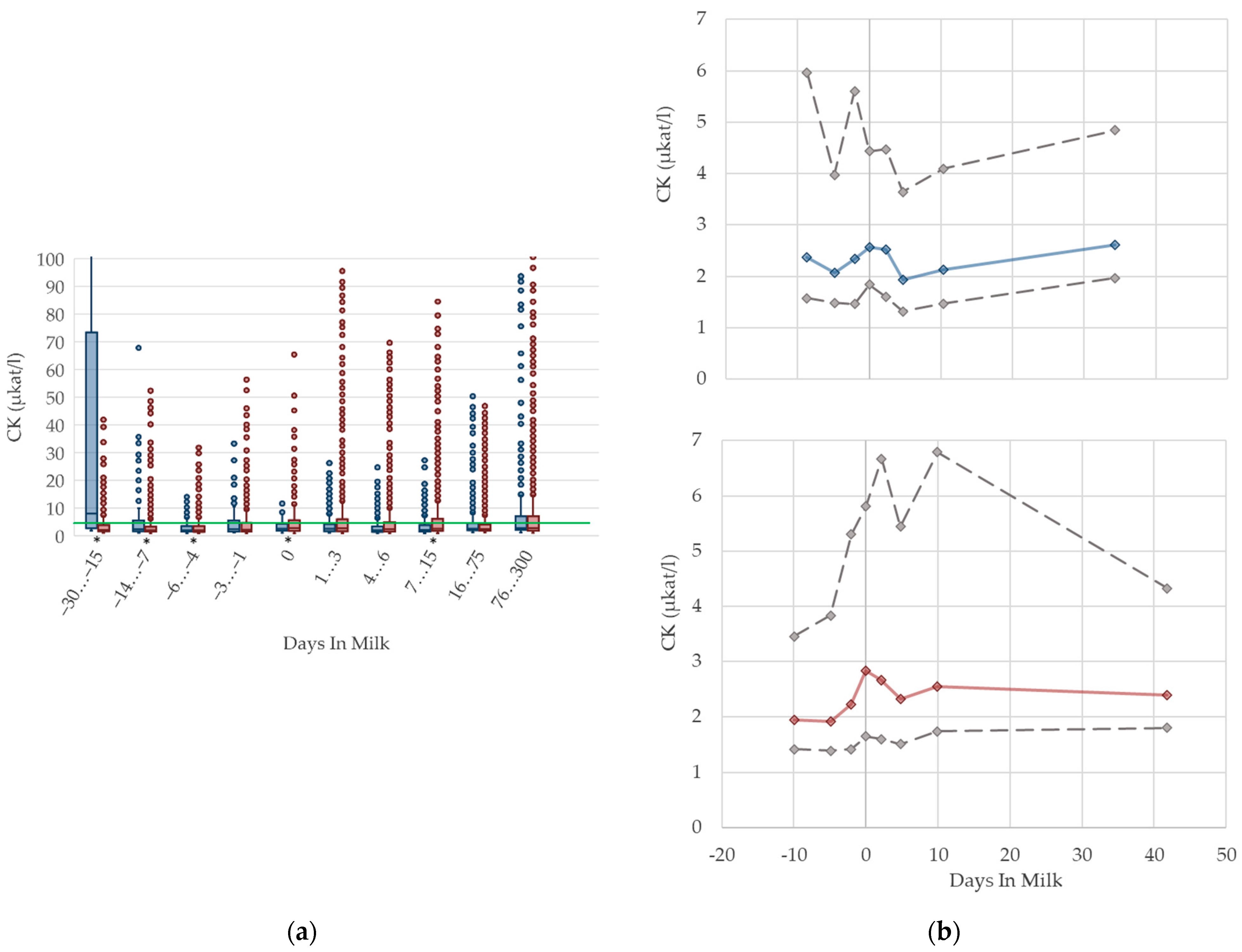
3.1.8. GLDH Serum Activities
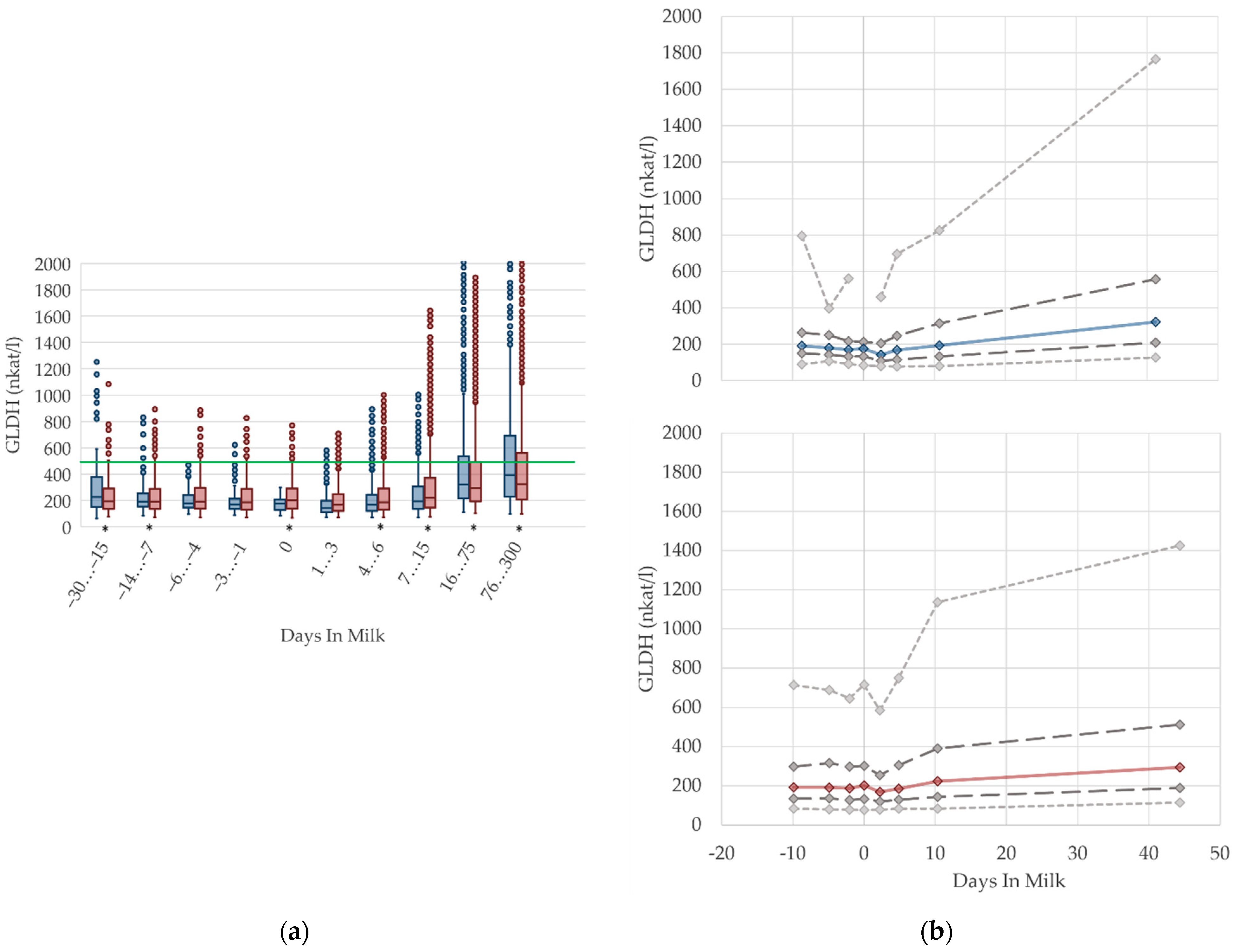
3.1.9. Calcium Serum Concentrations

3.1.10. Phosphorus Serum Concentrations
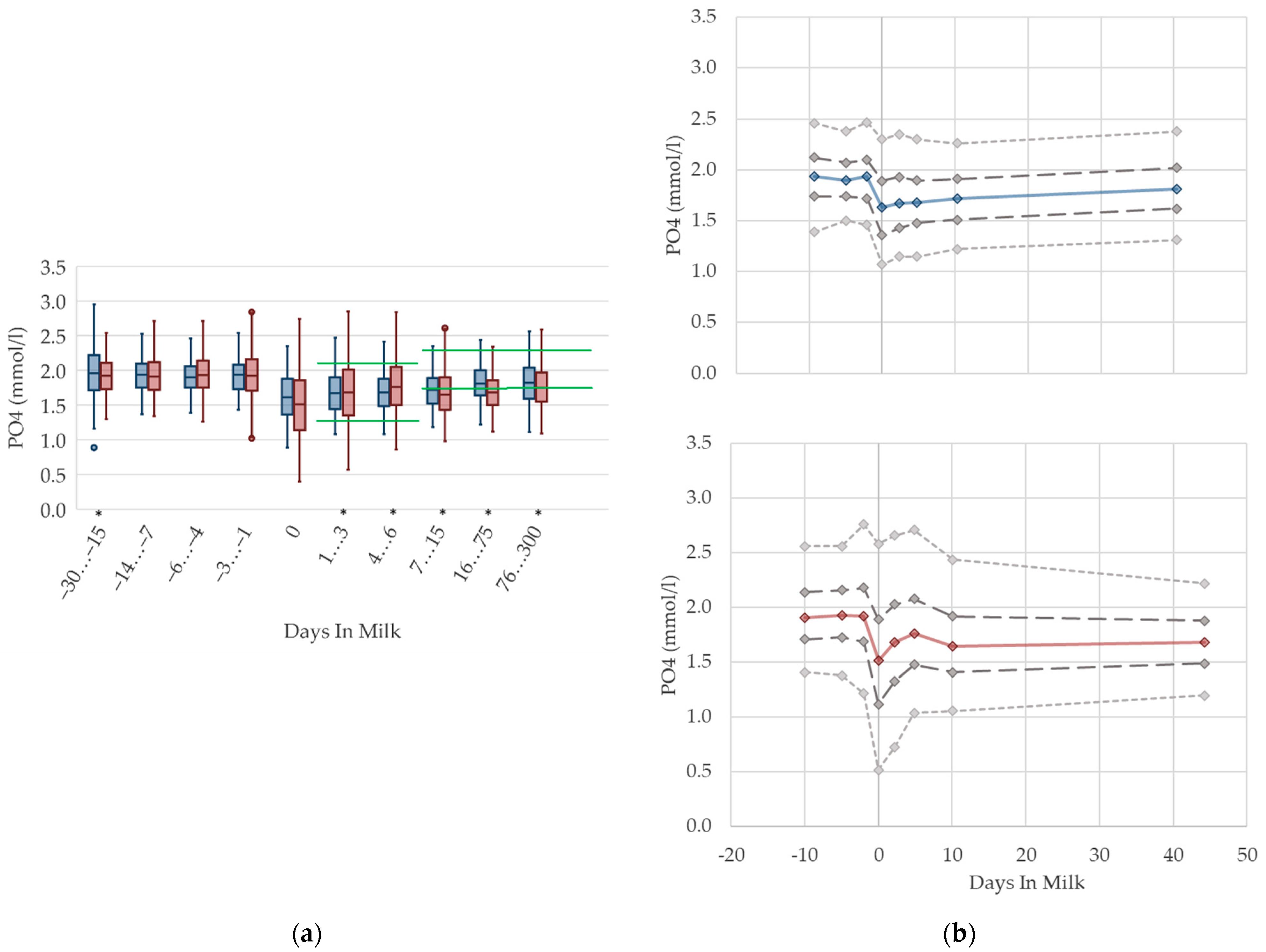
3.1.11. NABE Urine Concentrations
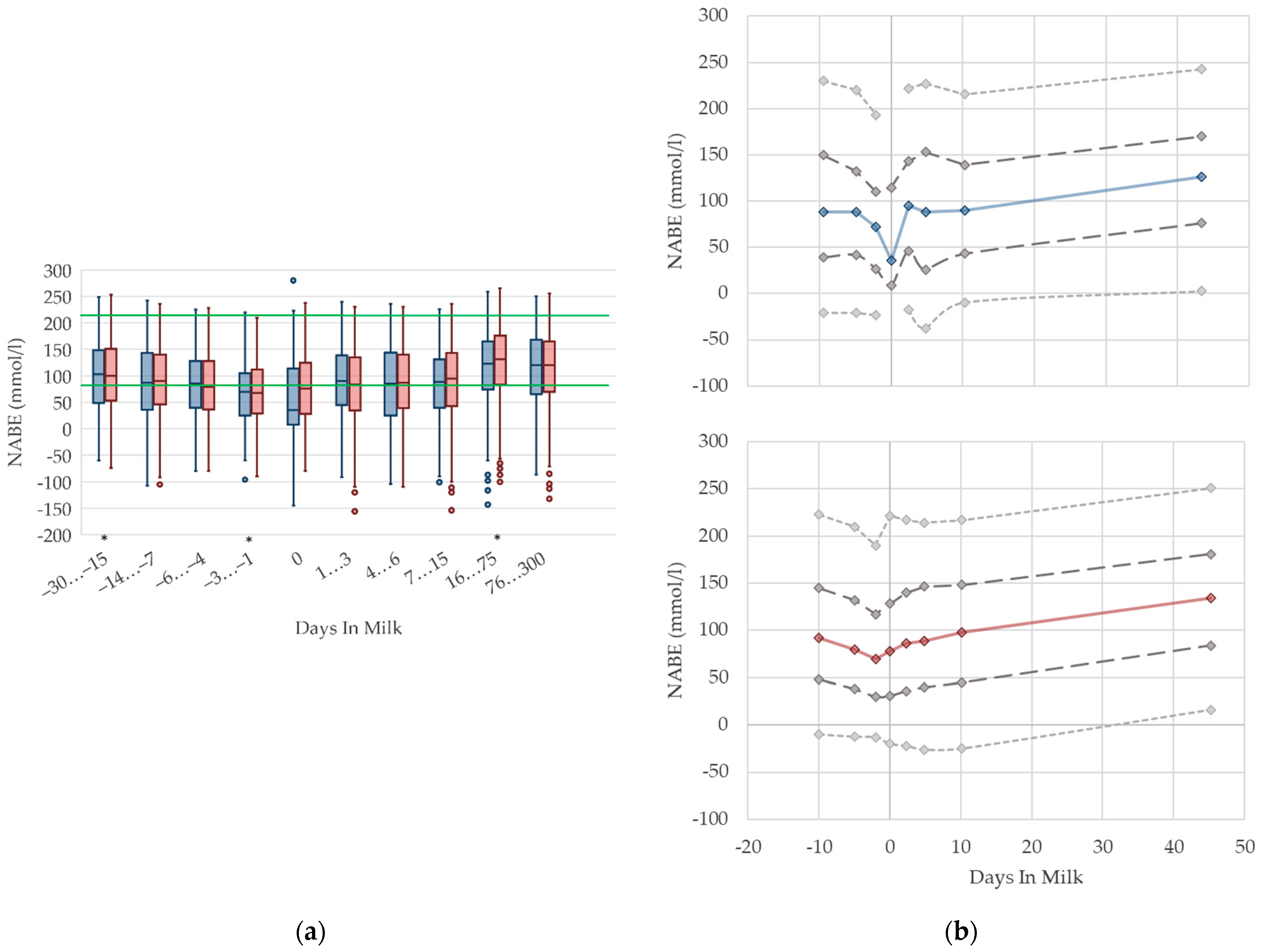
3.1.12. Potassium Urine Concentrations

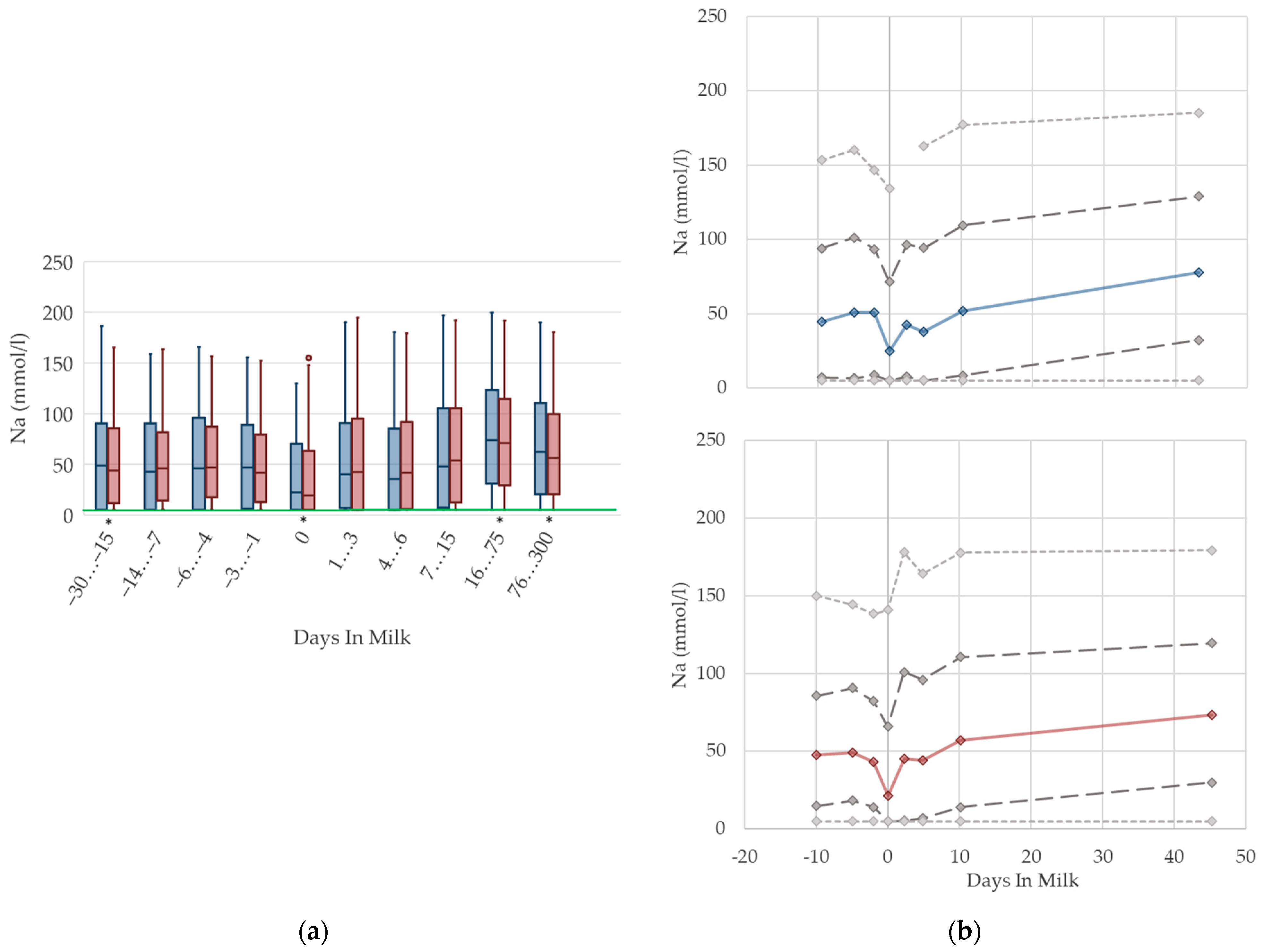
3.1.13. Sodium Urine Concentrations
3.2. General Discussion
3.3. Strengths and Limitations of the Study
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Parameter | Days in Milk Classes | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| From −30 | From −14 | From −6 | From −3 | 0 | From 1 | From 4 | From 7 | From 16 | From 76 | ||
| to −15 | to −7 | to −4 | to −1 | to 3 | to 6 | to 15 | to 75 | to 300 | |||
| NEFA 1 (mmol/L) | median | 0.27 | 0.31 | 0.34 | 0.35 | 0.62 | 0.58 | 0.61 | 0.49 | 0.33 | 0.30 |
| n | 104 | 304 | 321 | 299 | 32 | 493 | 525 | 593 | 589 | 616 | |
| DIM 3 | −19.7 | −9.3 | −4.9 | −2.1 | 0 | 2.5 | 4.6 | 10.6 | 31.1 | 398.9 | |
| significance | * | * | * | * | * | * | |||||
| BHB 2 (µmol/L) | median | 544 | 605 | 560 | 560 | 509 | 549 | 610 | 576 | 598 | 636 |
| n | 60 | 22 | 183 | 161 | 78 | 1065 | 984 | 1131 | 1762 | 1337 | |
| DIM | −22.4 | −8.6 | −4.9 | −2.1 | 0 | 2.3 | 4.8 | 10.3 | 41.2 | 271.5 | |
| significance | * | * | * | * | * | * | * | ||||
| Bilirubin (µmol/L) | median | 3.33 | 2.99 | 2.82 | 2.99 | 4.11 | 5.37 | 5.27 | 3.86 | 2.82 | 2.97 |
| n | 156 | 354 | 346 | 324 | 100 | 1074 | 987 | 1142 | 1778 | 1547 | |
| DIM | −20.7 | −9.3 | −4.9 | −2.1 | 0 | 2.3 | 4.8 | 10.3 | 41.0 | 303.7 | |
| significance | * | * | * | * | * | * | * | * | |||
| Cholesterol (mmol/L) | median | 2.31 | 1.97 | 1.77 | 1.85 | 1.74 | 1.91 | 2.03 | 2.44 | 4.20 | 5.03 |
| n | 34 | 118 | 184 | 156 | 15 | 578 | 621 | 940 | 1706 | 1221 | |
| DIM | −21.8 | −8.5 | −4.9 | −2.1 | 0 | 2.5 | 4.7 | 10.7 | 41.2 | 277.2 | |
| significance | * | * | * | * | * | * | |||||
| Urea (mmol/L) | median | 4.38 | 3.60 | 3.33 | 3.34 | 3.30 | 3.44 | 3.40 | 3.44 | 4.15 | 4.60 |
| n | 156 | 349 | 342 | 318 | 37 | 589 | 627 | 966 | 1784 | 1528 | |
| DIM | −20.7 | −9.3 | −4.9 | −2.1 | 0 | 2.5 | 4.7 | 10.7 | 41.1 | 301.7 | |
| significance | * | * | * | * | |||||||
| Parameter | Days in Milk Classes | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| From −30 | From −14 | From −6 | From −3 | 0 | From 1 | From 4 | From 7 | From 16 | From 76 | ||
| to −15 | to −7 | to −4 | to −1 | to 3 | to 6 | to 15 | to 75 | to 300 | |||
| NEFA 1 (mmol/L) | median | 0.23 | 0.25 | 0.28 | 0.32 | 0.60 | 0.62 | 0.62 | 0.57 | 0.37 | 0.32 |
| n | 1096 | 2575 | 1539 | 1591 | 338 | 1324 | 1383 | 1828 | 1216 | 1247 | |
| DIM 3 | −19.2 | −10 | −5 | −2 | 0 | 2.3 | 4.9 | 10 | 35.3 | 331.4 | |
| significance | * | * | * | * | * | * | |||||
| BHB 2 (µmol/L) | median | 694.0 | 718.5 | 720.0 | 711.0 | 622.0 | 738.0 | 814.0 | 802. | 681.0 | 655.0 |
| n | 90 | 226 | 221 | 287 | 401 | 2754 | 2486 | 3099 | 7158 | 3859 | |
| DIM | −19.2 | −9.6 | −4.9 | −2 | 0 | 2.2 | 4.9 | 10.1 | 44.3 | 205.1 | |
| significance | * | * | * | * | * | * | * | ||||
| Bilirubin (µmol/L) | median | 2.82 | 2.82 | 3.04 | 3.28 | 5.48 | 5.67 | 5.52 | 4.77 | 3.07 | 3.06 |
| n | 1270 | 2925 | 1749 | 1843 | 679 | 2800 | 2494 | 3116 | 7181 | 4249 | |
| DIM | −19.3 | −10 | −5 | −2 | 0 | 2.2 | 4.9 | 10 | 44.3 | 231.7 | |
| significance | * | * | * | * | * | * | * | * | |||
| Cholesterol (mmol/L) | median | 2.52 | 2.29 | 2.04 | 1.94 | 1.64 | 1.76 | 1.94 | 2.30 | 4.82 | 5.21 |
| n | 189 | 521 | 324 | 329 | 128 | 1563 | 1705 | 2672 | 6772 | 3257 | |
| DIM | −19.6 | −9.8 | −4.9 | −2.1 | 0 | 2.3 | 4.9 | 10.3 | 44.6 | 208.2 | |
| significance | * | * | * | * | * | * | * | ||||
| Urea (mmol/L) | median | 4.20 | 4.05 | 4.06 | 4.04 | 4.15 | 4.10 | 3.90 | 3.80 | 4.50 | 4.80 |
| n | 1271 | 2933 | 1752 | 1835 | 491 | 1868 | 1841 | 2850 | 7190 | 4050 | |
| DIM | −19.3 | −10 | −5.0 | −2.0 | 0 | 2.2 | 4.9 | 10.3 | 44.3 | 228.6 | |
| significance | * | * | * | * | |||||||
| Parameter | Days in Milk Classes | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| From −30 | From −14 | From −6 | From −3 | 0 | From 1 | From 4 | From 7 | From 16 | From 76 | ||
| to −15 | to −7 | to −4 | to −1 | to 3 | to 6 | to 15 | to 75 | to 300 | |||
| AST 1 (nkat/L) | median | 1158.0 | 1075.5 | 1034.5 | 1036.0 | 1214.0 | 1428.0 | 1423.0 | 1362.5 | 1286.0 | 1380.0 |
| n | 155 | 352 | 340 | 324 | 100 | 1073 | 990 | 1136 | 1789 | 1541 | |
| DIM 4 | −20.7 | −9.9 | −4.9 | −2.1 | 0 | 2.3 | 4.8 | 10.3 | 41.2 | 303.1 | |
| significance | * | * | * | * | * | * | |||||
| CK 2 (µkat/L) | median | 9.73 | 2.38 | 2.07 | 2.35 | 2.57 | 2.53 | 1.93 | 2.13 | 2.61 | 2.77 |
| n | 55 | 129 | 178 | 170 | 76 | 799 | 603 | 457 | 585 | 448 | |
| DIM | −22.5 | −8.7 | −4.9 | −2.1 | 0 | 2.3 | 4.7 | 10.3 | 34.3 | 311.9 | |
| significance | * | * | * | * | * | ||||||
| GLDH 3 (nkat/L) | median | 235.7 | 191.5 | 180.2 | 171.2 | 177.2 | 143.2 | 167.9 | 194.7 | 322.9 | 393.8 |
| n | 64 | 131 | 183 | 162 | 16 | 584 | 623 | 951 | 1743 | 1288 | |
| DIM | −22.3 | −8.9 | −4.9 | −2.1 | 0 | 2.5 | 4.7 | 10.7 | 41.2 | 273.2 | |
| significance | * | * | * | * | |||||||
| Parameter | Days in Milk Classes | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| From −30 | From −14 | From −6 | From −3 | 0 | From 1 | From 4 | From 7 | From 16 | From 76 | ||
| to −15 | to −7 | to −4 | to −1 | to 3 | to 6 | to 15 | to 75 | to 300 | |||
| AST 1 (nkat/L) | median | 1023.0 | 1015.0 | 1052.0 | 1058.0 | 1181.0 | 1448.0 | 1498.0 | 1541.5 | 1273.0 | 1375.5 |
| n | 1265 | 2928 | 1751 | 1844 | 678 | 2806 | 2498 | 3112 | 7216 | 4278 | |
| DIM 4 | −19.3 | −10.0 | −5.0 | −2.0 | 0 | 2.2 | 4.9 | 10.1 | 44.3 | 229.8 | |
| significance | * | * | * | * | * | * | * | ||||
| CK 2 (µkat/L) | median | 2.10 | 1.95 | 1.93 | 2.23 | 2.84 | 2.66 | 2.33 | 2.56 | 2.40 | 2.76 |
| n | 362 | 921 | 602 | 688 | 392 | 1782 | 1330 | 1183 | 2109 | 1443 | |
| DIM | −19.3 | −10.0 | −4.9 | −2.0 | 0 | 2.2 | 4.8 | 9.9 | 41.8 | 214.8 | |
| significance | * | * | * | * | * | * | |||||
| GLDH 3 (nkat/L) | median | 193.5 | 191.7 | 191.1 | 185.9 | 200.9 | 167.8 | 185.1 | 222.1 | 293.6 | 324.1 |
| n | 202 | 563 | 378 | 439 | 211 | 1778 | 1816 | 2815 | 7102 | 3690 | |
| DIM | −19.7 | −9.9 | −4.9 | −2.0 | 0 | 2.3 | 4.9 | 10.4 | 44.4 | 203.1 | |
| significance | * | * | * | * | * | ||||||
| Parameter | Days in Milk Classes | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| From −30 | From −14 | From −6 | From −3 | 0 | From 1 | From 4 | From 7 | From 16 | From 76 | ||
| to −15 | to −7 | to −4 | to −1 | to 3 | to 6 | to 15 | to 75 | to 300 | |||
| In Serum | |||||||||||
| Ca 1 (mmol/L) | median | 2.42 | 2.41 | 2.40 | 2.42 | 2.32 | 2.26 | 2.36 | 2.40 | 2.45 | 2.45 |
| n | 148 | 336 | 332 | 322 | 99 | 1050 | 958 | 919 | 1430 | 1288 | |
| DIM 6 | −20.8 | −9.3 | −4.9 | −2.1 | 0 | 2.3 | 4.7 | 10.1 | 41.1 | 325.9 | |
| significance | * | * | * | * | * | ||||||
| PO4 2 (mmol/L) | median | 1.96 | 1.94 | 1.90 | 1.94 | 1.63 | 1.68 | 1.68 | 1.72 | 1.81 | 1.82 |
| n | 149 | 335 | 331 | 321 | 99 | 1052 | 977 | 1121 | 1620 | 1405 | |
| DIM | −20.8 | −9.3 | −4.9 | −2.1 | 0 | 2.3 | 4.8 | 10.3 | 40.3 | 316.3 | |
| significance | * | * | |||||||||
| In Urine | |||||||||||
| NABE 3 (mmol/L) | median | 105.5 | 88.0 | 88.0 | 72.0 | 35.5 | 95.0 | 88.0 | 90.0 | 126.0 | 121.0 |
| n | 112 | 284 | 238 | 233 | 32 | 431 | 503 | 667 | 1391 | 1375 | |
| DIM | −19.7 | −9.5 | −4.9 | −2.1 | 0 | 2.4 | 4.8 | 10.3 | 43.3 | 317.3 | |
| significance | * | * | * | ||||||||
| K 4 (mmol/L) | median | 254.7 | 263.9 | 257.0 | 247.4 | 164.3 | 221.7 | 217.4 | 207.6 | 214.8 | 217.7 |
| n | 117 | 285 | 237 | 235 | 32 | 437 | 507 | 667 | 1381 | 1324 | |
| DIM | −19.7 | −9.5 | −4.9 | −2.1 | 0 | 2.4 | 4.8 | 10.3 | 43.3 | 321.9 | |
| significance | * | * | |||||||||
| Na 5 (mmol/L) | median | 50.3 | 44.8 | 50.7 | 51.0 | 24.9 | 42.3 | 37.9 | 52.0 | 77.7 | 63.9 |
| n | 117 | 285 | 237 | 235 | 32 | 437 | 507 | 667 | 1381 | 1324 | |
| DIM | −19.7 | −9.5 | −4.9 | −2.1 | 0 | 2.4 | 4.8 | 10.3 | 43.3 | 321.9 | |
| significance | * | * | |||||||||
| Parameter | Days in Milk Classes | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| From −30 | From −14 | From −6 | From −3 | 0 | From 1 | From 4 | From 7 | From 16 | From 76 | ||
| to −15 | to −7 | to −4 | to −1 | to 3 | to 6 | to 15 | to 75 | to 300 | |||
| In Serum | |||||||||||
| Ca 1 (mmol/L) | median | 2.41 | 2.41 | 2.41 | 2.41 | 2.15 | 2.24 | 2.38 | 2.37 | 2.42 | 2.41 |
| n | 1248 | 2878 | 1729 | 1844 | 670 | 2758 | 2443 | 2651 | 6005 | 3218 | |
| DIM 6 | −19.3 | −10.0 | −5.0 | −2.0 | 0 | 2.2 | 4.9 | 9.9 | 44.9 | 252.4 | |
| significance | * | * | * | * | * | ||||||
| PO4 2 (mmol/L) | median | 1.92 | 1.91 | 1.93 | 1.92 | 1.52 | 1.68 | 1.76 | 1.65 | 1.68 | 1.76 |
| n | 1247 | 2867 | 1723 | 1836 | 671 | 2860 | 2477 | 3020 | 6631 | 3545 | |
| DIM | −19.3 | −10.0 | −5.0 | −2.0 | 0 | 2.2 | 4.9 | 10.1 | 44.2 | 245.4 | |
| significance | * | * | * | * | * | * | |||||
| In Urine | |||||||||||
| NABE 3 (mmol/L) | median | 103.0 | 92.0 | 80.0 | 70.0 | 78.0 | 86.0 | 89.0 | 98.0 | 134.0 | 124.0 |
| n | 1173 | 2590 | 1530 | 1615 | 239 | 1484 | 1597 | 2242 | 6161 | 3607 | |
| DIM | −19.4 | −10.0 | −5.0 | −2.0 | 0 | 2.3 | 4.9 | 10.2 | 45.3 | 239.7 | |
| significance | * | * | * | * | * | * | |||||
| K 4 (mmol/L) | median | 256.9 | 249.7 | 235.7 | 247.8 | 200.7 | 197.1 | 200.9 | 200.4 | 218.5 | 217.6 |
| n | 1175 | 2610 | 1541 | 1629 | 335 | 1490 | 1605 | 2240 | 6123 | 3469 | |
| DIM | −19.4 | −10.0 | −5.0 | −2.0 | 0 | 2.3 | 4.9 | 10.2 | 45.3 | 242.2 | |
| significance | * | * | * | * | |||||||
| Na 5 (mmol/L) | median | 46.2 | 47.6 | 49.1 | 43.5 | 21.6 | 45.2 | 44.3 | 57.1 | 73.5 | 58.7 |
| n | 1175 | 2611 | 1541 | 1629 | 335 | 1490 | 1605 | 2240 | 6123 | 3469 | |
| DIM | −19.4 | −10.0 | −5.0 | −2.0 | 0 | 2.3 | 4.9 | 10.2 | 45.3 | 242.2 | |
| significance | * | * | * | * | * | ||||||
References
- Barkema, H.W.; von Keyserlingk, M.A.; Kastelic, J.P.; Lam, T.J.; Luby, C.; Roy, J.P.; LeBlanc, S.J.; Keefe, G.P.; Kelton, D.F. Invited review: Changes in the dairy industry affecting dairy cattle health and welfare. J. Dairy Sci. 2015, 98, 7426–7445. [Google Scholar] [CrossRef]
- Van Saun, R.J. Indicators of dairy cow transition risks: Metabolic profiling revisited. Tierarztl. Prax. Ausg. G Grosstiere Nutztiere 2016, 44, 118–126. [Google Scholar] [CrossRef]
- LeBlanc, S. Monitoring metabolic health of dairy cattle in the transition period. J. Reprod. Dev. 2010, 56, S29–S35. [Google Scholar] [CrossRef]
- Knob, D.A.; Thaler Neto, A.; Schweizer, H.; Weigand, A.C.; Kappes, R.; Scholz, A.M. Energy Balance Indicators during the Transition Period and Early Lactation of Purebred Holstein and Simmental Cows and Their Crosses. Animals 2021, 11, 309. [Google Scholar] [CrossRef] [PubMed]
- Wathes, D.C.; Cheng, Z.; Bourne, N.; Taylor, V.J.; Coffey, M.P.; Brotherstone, S. Differences between primiparous and multiparous dairy cows in the inter-relationships between metabolic traits, milk yield and body condition score in the periparturient period. Domest. Anim. Endocrinol. 2007, 33, 203–225. [Google Scholar] [CrossRef]
- Cozzi, G.; Ravarotto, L.; Gottardo, F.; Stefani, A.L.; Contiero, B.; Moro, L.; Brscic, M.; Dalvit, P. Short communication: Reference values for blood parameters in Holstein dairy cows: Effects of parity, stage of lactation, and season of production. J. Dairy Sci. 2011, 94, 3895–3901. [Google Scholar] [CrossRef]
- Brscic, M.; Cozzi, G.; Lora, I.; Stefani, A.L.; Contiero, B.; Ravarotto, L.; Gottardo, F. Short communication: Reference limits for blood analytes in Holstein late-pregnant heifers and dry cows: Effects of parity, days relative to calving, and season. J. Dairy Sci. 2015, 98, 7886–7892. [Google Scholar] [CrossRef]
- Gartner, T.; Gernand, E.; Gottschalk, J.; Donat, K. Relationships between body condition, body condition loss, and serum metabolites during the transition period in primiparous and multiparous cows. J. Dairy Sci. 2019, 102, 9187–9199. [Google Scholar] [CrossRef]
- Neave, H.W.; Lomb, J.; von Keyserlingk, M.A.G.; Behnam-Shabahang, A.; Weary, D.M. Parity differences in the behavior of transition dairy cows. J. Dairy Sci. 2017, 100, 548–561. [Google Scholar] [CrossRef]
- Soonberg, M.; Kass, M.; Kaart, T.; Barraclough, R.; Haskell, M.J.; Arney, D.R. Effect of grouping on behaviour of dairy heifers and cows in the transition period. J. Dairy Res. 2021, 88, 45–51. [Google Scholar] [CrossRef]
- Quiroz-Rocha, G.F.; LeBlanc, S.J.; Duffield, T.F.; Wood, D.; Leslie, K.E.; Jacobs, R.M. Reference limits for biochemical and hematological analytes of dairy cows one week before and one week after parturition. Can. Vet. J. 2009, 50, 383. [Google Scholar] [PubMed]
- Piccione, G.; Messina, V.; Marafioti, S.; Casella, S.; Giannetto, C.; Fazio, F. Changes of some haematochemical parameters in dairy cows during late gestation, post partum, lactation and dry periods. Vet. Med. Zoot. 2012, 58, 59–64. [Google Scholar]
- Bender, S.; Gelfert, C.C.; Staufenbiel, R. Einsatz der Harnuntersuchung zur Beurteilung des Säure-Basen-Haushalts in der Bestandsbetreuung von Milchkuhherden. Tierarztl. Prax. Ausg. G Grosstiere Nutztiere 2003, 31, 132–142. [Google Scholar] [CrossRef]
- Rerat, M.; Schlegel, P. Effect of dietary potassium and anionic salts on acid-base and mineral status in periparturient cows. J. Anim. Physiol. Anim. Nutr. 2014, 98, 458–466. [Google Scholar] [CrossRef] [PubMed]
- Enemark, J.M.D.; Jørgensen, R.J.; Kristensen, N.B. An evaluation of parameters for the detection of subclinical rumen acidosis in dairy herds. Vet. Res. Commun. 2004, 28, 687–709. [Google Scholar] [CrossRef]
- Fürll, M. Development and basics of metabolic monitoring in dairy cows. Focus on research in Eastern Germany and at the University of Leipzig, Germany. Tierarztl. Prax. Ausg. G Grosstiere Nutztiere 2016, 44, 107–117. [Google Scholar] [CrossRef]
- Donat, K.; Siebert, W.; Menzer, E.; Söllner-Donat, S. Long-term trends in the metabolic profile test results in German Holstein dairy herds in Thuringia, Germany. Tierärztl. Prax. Ausg. G Großtiere Nutztiere 2016, 44, 73–82. [Google Scholar]
- Trends of Animal Breeding in Thuringia. Reporting Year 2019. Report of the Thuringian State Office for Agriculture and Rural Area. Available online: https://tlllr.thueringen.de/fileadmin/TLLLR/Service/Publikationen/Schriftenreihe/2020_1_Tierzuchtbericht_2019.pdf (accessed on 14 February 2022).
- Kutas, F. Determination of net acid-base excretion in the urine of cattle. A method for the estimation of acid-base equilibrium. Acta Vet. Acad. Sci. Hung. 1965, 15, 147–153. [Google Scholar]
- Kruskal, W.H.; Wallis, W.A. Use of ranks in one-criterion variance analysis. J. Am. Stat. Assoc. 1952, 47, 583–621. [Google Scholar] [CrossRef]
- Abdi, H. Bonferroni and Šidák corrections for multiple comparisons. Encycl. Meas. Stat. 2007, 3, 103–107. [Google Scholar]
- Tukey, J.W. Exploratory Data Analysis (1970–1971: Preliminary Edition); Addison-Wesley: Boston, MA, USA, 1977. [Google Scholar]
- Fürll, M. Specific diagnosis in ruminants [Spezielle Untersuchungen beim Wiederkäuer]. In Klinische Labordiagnostik in der Tiermedizin, 7th ed.; Moritz, A., Ed.; Schattauer: Stuttgart, Germany, 2014; pp. 726–777. [Google Scholar]
- Ruprechter, G.; Adrien, M.L.; Larriestra, A.; Meotti, O.; Batista, C.; Meikle, A.; Noro, M. Metabolic predictors of peri-partum diseases and their association with parity in dairy cows. Res. Vet. Sci. 2018, 118, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Cabezas-Garcia, E.H.; Gordon, A.W.; Mulligan, F.J.; Ferris, C.P. Revisiting the Relationships between Fat-to-Protein Ratio in Milk and Energy Balance in Dairy Cows of Different Parities, and at Different Stages of Lactation. Animals 2021, 11, 3256. [Google Scholar] [CrossRef]
- Janovick, N.A.; Boisclair, Y.R.; Drackley, J.K. Prepartum dietary energy intake affects metabolism and health during the periparturient period in primiparous and multiparous Holstein cows. J. Dairy Sci. 2011, 94, 1385–1400. [Google Scholar] [CrossRef] [PubMed]
- Kaufmann, T.B.; Drillich, M.; Tenhagen, B.-A.; Heuwieser, W. Correlations between periparturient serum concentrations of non-esterified fatty acids, beta-hydroxybutyric acid, bilirubin, and urea and the occurrence of clinical and subclinical postpartum bovine endometritis. BMC Vet. Res. 2010, 6, 47. [Google Scholar] [CrossRef] [PubMed]
- Guretzky, N.J.; Carlson, D.; Garrett, J.; Drackley, J. Lipid metabolite profiles and milk production for Holstein and Jersey cows fed rumen-protected choline during the periparturient period. J. Dairy Sci. 2006, 89, 188–200. [Google Scholar] [CrossRef]
- Drackley, J.K. Biology of dairy cows during the transition period: The final frontier? J. Dairy Sci. 1999, 82, 2259–2273. [Google Scholar] [CrossRef]
- Ferreira, M.F.L.; Renno, L.N.; Rodrigues, I.I.; Detmann, E.; Paulino, M.F.; de Campos Valadares Filho, S.; Martins, H.C.; Moreira, S.S.; de Lana, D.S. Effects of parity order on performance, metabolic, and hormonal parameters of grazing beef cows during pre-calving and lactation periods. BMC Vet. Res. 2021, 17, 311. [Google Scholar] [CrossRef] [PubMed]
- Meikle, A.; Kulcsar, M.; Chilliard, Y.; Febel, H.; Delavaud, C.; Cavestany, D.; Chilibroste, P. Effects of parity and body condition at parturition on endocrine and reproductive parameters of the cow. Reproduction 2004, 127, 727–737. [Google Scholar] [CrossRef]
- Duffield, T.F.; Kelton, D.F.; Leslie, K.E.; Lissemore, K.D.; Lumsden, J.H. Use of test day milk fat and milk protein to detect subclinical ketosis in dairy cattle in Ontario. Can. Vet. J. 1997, 38, 713. [Google Scholar]
- Vanholder, T.; Papen, J.; Bemers, R.; Vertenten, G.; Berge, A.C. Risk factors for subclinical and clinical ketosis and association with production parameters in dairy cows in the Netherlands. J. Dairy Sci. 2015, 98, 880–888. [Google Scholar] [CrossRef]
- McArt, J.A.; Nydam, D.V.; Oetzel, G.R.; Overton, T.R.; Ospina, P.A. Elevated non-esterified fatty acids and beta-hydroxybutyrate and their association with transition dairy cow performance. Vet. J. 2013, 198, 560–570. [Google Scholar] [CrossRef] [PubMed]
- Bionaz, M.; Trevisi, E.; Calamari, L.; Librandi, F.; Ferrari, A.; Bertoni, G. Plasma paraoxonase, health, inflammatory conditions, and liver function in transition dairy cows. J. Dairy Sci. 2007, 90, 1740–1750. [Google Scholar] [CrossRef] [PubMed]
- Moretti, P.; Paltrinieri, S.; Trevisi, E.; Probo, M.; Ferrari, A.; Minuti, A.; Giordano, A. Reference intervals for hematological and biochemical parameters, acute phase proteins and markers of oxidation in Holstein dairy cows around 3 and 30 days after calving. Res. Vet. Sci. 2017, 114, 322–331. [Google Scholar] [CrossRef]
- Reid, I.; Harrison, R.; Collins, R. Fasting and refeeding in the lactating dairy cow: 2. The recovery of liver cell structure and function following a six-day fast. J. Comp. Pathol. 1977, 87, 253–265. [Google Scholar] [CrossRef]
- Naylor, J.M.; Kronfeld, D.S.; Johnson, K. Fasting Hyperbilirubinemia and Its Relationship to Free Fatty Acids and Triglycerides in the Horse. Proc. Soc. Exp. Biol. Med. 1980, 165, 86–90. [Google Scholar] [CrossRef] [PubMed]
- Cowan, R.E.; Thompson, R.P. Fatty acids and the control of bilirubin levels in blood. Med. Hypotheses 1983, 11, 343–351. [Google Scholar] [CrossRef]
- McSherry, B.; Lumsden, J.; Valli, V.; Baird, J. Hyperbilirubinemia in sick cattle. Can. J. Comp. Med. 1984, 48, 237. [Google Scholar]
- Kinoshita, A.; Wolf, C.; Zeyner, A. Studies on the incidence of hyperketonemia with and without hyperbilirubinaemia in cows in Mecklenburg-Vorpommern (in Germany) in the course of the year. Tierärztl. Praxis. Ausg. G Grosstiere Nutztiere 2010, 38, 7–15. [Google Scholar] [CrossRef]
- West, H. Effect on liver function of acetonaemia and the fat cow syndrome in cattle. Res. Vet. Sci. 1990, 48, 221–227. [Google Scholar] [CrossRef]
- Bertoni, G.; Trevisi, E.; Han, X.; Bionaz, M. Effects of inflammatory conditions on liver activity in puerperium period and consequences for performance in dairy cows. J. Dairy Sci. 2008, 91, 3300–3310. [Google Scholar] [CrossRef]
- Zhou, Z.; Trevisi, E.; Luchini, D.; Loor, J. Differences in liver functionality indexes in peripartal dairy cows fed rumen-protected methionine or choline are associated with performance, oxidative stress status, and plasma amino acid profiles. J. Dairy Sci. 2017, 100, 6720–6732. [Google Scholar] [CrossRef]
- Weber, J.; Zenker, M.; Koller, G.; Furll, M.; Freick, M. Clinical Chemistry Investigations in Recumbent and Healthy German Holstein Cows After the Fifth Day in Milk. J. Vet. Res. 2019, 63, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Peterson, R.; Waldern, D. Repeatabilities of serum constituents in Holstein-Friesians affected by feeding, age, lactation, and pregnancy. J. Dairy Sci. 1981, 64, 822–831. [Google Scholar] [CrossRef]
- Kessler, E.; Gross, J.J.; Bruckmaier, R.; Albrecht, C. Cholesterol metabolism, transport, and hepatic regulation in dairy cows during transition and early lactation. J. Dairy Sci. 2014, 97, 5481–5490. [Google Scholar] [CrossRef]
- Bertoni, G.; Trevisi, E. Use of the liver activity index and other metabolic variables in the assessment of metabolic health in dairy herds. Vet. Clin. Food Anim. Pract. 2013, 29, 413–431. [Google Scholar] [CrossRef] [PubMed]
- Roubies, N.; Panousis, N.; Fytianou, A.; Katsoulos, P.-D.; Giadinis, N.; Karatzias, H. Effects of age and reproductive stage on certain serum biochemical parameters of Chios sheep under Greek rearing conditions. J. Vet. Med. Ser. A 2006, 53, 277–281. [Google Scholar] [CrossRef] [PubMed]
- Van Saun, R.J. Metabolic profiles for evaluation of the transition period. In Proceedings of the American Association of Bovine Practitioners Proceedings of the Annual Conference, Saint Paul, MN, USA, 21–23 September 2006; pp. 130–138. [Google Scholar]
- Stojević, Z.; Piršljin, J.; Milinković-Tur, S.; Zdelar-Tuk, M.; Ljubić, B.B. Activities of AST, ALT and GGT in clinically healthy dairy cows during lactation and in the dry period. Vet. Arh. 2005, 75, 67–73. [Google Scholar]
- Sattler, T.; Fürll, M. Creatine kinase and aspartate aminotransferase in cows as indicators for endometritis. J. Vet. Med. Ser. A 2004, 51, 132–137. [Google Scholar] [CrossRef]
- Van der Drift, S.; Houweling, M.; Schonewille, J.; Tielens, A.; Jorritsma, R. Protein and fat mobilization and associations with serum β-hydroxybutyrate concentrations in dairy cows. J. Dairy Sci. 2012, 95, 4911–4920. [Google Scholar] [CrossRef]
- Zurek, E.; Foxcroft, G.; Kennelly, J. Metabolic status and interval to first ovulation in postpartum dairy cows. J. Dairy Sci. 1995, 78, 1909–1920. [Google Scholar] [CrossRef]
- Hørder, M.; Elser, R.C.; Gerhardt, W.; Mathieu, M.; Sampson, E.J. International Federation of Clinical Chemistry (IFCC): Scientific Division, Committee on Enzymes. IFCC methods for the measurement of catalytic concentration of enzymes. Part 7. IFCC method for creatine kinase (ATP: Creatine (N-phosphotransferase, EC 2.7.3.2). IFCC Recommendation. J. Autom. Chem. 1990, 12, 628560. [Google Scholar] [CrossRef]
- Hoedemaker, M.; Prange, D.; Zerbe, H.; Frank, J.; Daxenberger, A.; Meyer, H. Peripartal propylene glycol supplementation and metabolism, animal health, fertility, and production in dairy cows. J. Dairy Sci. 2004, 87, 2136–2145. [Google Scholar] [CrossRef]
- Hachenberg, S.; Weinkauf, C.; Hiss, S.; Sauerwein, H. Evaluation of classification modes potentially suitable to identify metabolic stress in healthy dairy cows during the peripartal period. J. Anim. Sci. 2007, 85, 1923–1932. [Google Scholar] [CrossRef] [PubMed][Green Version]
- McGill, M.R.; Jaeschke, H. Biomarkers of mitotoxicity after acute liver injury: Further insights into the interpretation of glutamate dehydrogenase. J. Clin. Transl. Res. 2021, 7, 61. [Google Scholar]
- Venjakob, P.; Staufenbiel, R.; Heuwieser, W.; Borchardt, S. Serum calcium dynamics within the first 3 days in milk and the associated risk of acute puerperal metritis. J. Dairy Sci. 2019, 102, 11428–11438. [Google Scholar] [CrossRef]
- Goff, J.; Horst, R. Effects of the addition of potassium or sodium, but not calcium, to prepartum rations on milk fever in dairy cows. J. Dairy Sci. 1997, 80, 176–186. [Google Scholar] [CrossRef]
- Kimura, K.; Reinhardt, T.; Goff, J. Parturition and hypocalcemia blunts calcium signals in immune cells of dairy cattle. J. Dairy Sci. 2006, 89, 2588–2595. [Google Scholar] [CrossRef]
- Marquardt, J.; Horst, R.; Jorgensen, N. Effect of parity on dry matter intake at parturition in dairy cattle. J. Dairy Sci. 1977, 60, 929–934. [Google Scholar] [CrossRef]
- Horst, R.L.; Goff, J.P.; Reinhardt, T.A. Adapting to the transition between gestation and lactation: Differences between rat, human and dairy cow. J. Mammary Gland Biol. Neoplasia 2005, 10, 141–156. [Google Scholar] [CrossRef]
- Reinhardt, T.A.; Lippolis, J.D.; McCluskey, B.J.; Goff, J.P.; Horst, R.L. Prevalence of subclinical hypocalcemia in dairy herds. Vet. J. 2011, 188, 122–124. [Google Scholar] [CrossRef]
- Kincaid, R.; Hillers, J.; Cronrath, J. Calcium and phosphorus supplementation of rations for lactating cows. J. Dairy Sci. 1981, 64, 754–758. [Google Scholar] [CrossRef]
- Goff, J.P. Macromineral physiology and application to the feeding of the dairy cow for prevention of milk fever and other periparturient mineral disorders. Anim. Feed. Sci. Technol. 2006, 126, 237–257. [Google Scholar] [CrossRef]
- Grünberg, W. Treatment of phosphorus balance disorders. Vet. Clin. Food Anim. Pract. 2014, 30, 383–408. [Google Scholar] [CrossRef] [PubMed]
- Brzeziñska, M.; Krawczyk, M. Changes of the mineral profile of serum of goats in various physiological states. J. Elem. 2009, 14, 649–656. [Google Scholar] [CrossRef]
- Constable, P.D.; Gelfert, C.-C.; Fürll, M.; Staufenbiel, R.; Stämpfli, H.R. Application of strong ion difference theory to urine and the relationship between urine pH and net acid excretion in cattle. Am. J. Vet. Res. 2009, 70, 915–925. [Google Scholar] [CrossRef] [PubMed]
- Tucker, W.; Hogue, J. Influence of sodium chloride or potassium chloride on systemic acid-base status, milk yield, and mineral metabolism in lactating dairy cows. J. Dairy Sci. 1990, 73, 3485–3493. [Google Scholar] [CrossRef]
- Hu, W.; Murphy, M. Dietary cation-anion difference effects on performance and acid-base status of lactating dairy cows: A meta-analysis. J. Dairy Sci. 2004, 87, 2222–2229. [Google Scholar] [CrossRef]
- Hu, W.; Murphy, M.R.; Constable, P.D.; Block, E. Dietary cation-anion difference effects on performance and acid-base status of dairy cows postpartum. J. Dairy Sci. 2007, 90, 3367–3375. [Google Scholar] [CrossRef]
- Hu, W.; Murphy, M.R.; Constable, P.D.; Block, E. Dietary cation-anion difference and dietary protein effects on performance and acid-base status of dairy cows in early lactation. J. Dairy Sci. 2007, 90, 3355–3366. [Google Scholar] [CrossRef]
- Rerat, M.; Philipp, A.; Hess, H.D.; Liesegang, A. Effect of different potassium levels in hay on acid-base status and mineral balance in periparturient dairy cows. J. Dairy Sci. 2009, 92, 6123–6133. [Google Scholar] [CrossRef]
- Gelfert, C.C.; Leonie Loeffler, S.; Fromer, S.; Engel, M.; Hartmann, H.; Manner, K.; Baumgartner, W.; Staufenbiel, R. The impact of dietary cation anion difference (DCAD) on the acid-base balance and calcium metabolism of non-lactating, non-pregnant dairy cows fed equal amounts of different anionic salts. J. Dairy Res. 2007, 74, 311–322. [Google Scholar] [CrossRef] [PubMed]
- Berg, M.; Plontzke, J.; Leonhard-Marek, S.; Muller, K.E.; Roblitz, S. A dynamic model to simulate potassium balance in dairy cows. J. Dairy Sci. 2017, 100, 9799–9814. [Google Scholar] [CrossRef] [PubMed]
- Apper-Bossard, E.; Faverdin, P.; Meschy, F.; Peyraud, J.-L. Effects of dietary cation-anion difference on ruminal metabolism and blood acid-base regulation in dairy cows receiving 2 contrasting levels of concentrate in diets. J. Dairy Sci. 2010, 93, 4196–4210. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Walter, L.L.; Gärtner, T.; Gernand, E.; Wehrend, A.; Donat, K. Effects of Parity and Stage of Lactation on Trend and Variability of Metabolic Markers in Dairy Cows. Animals 2022, 12, 1008. https://doi.org/10.3390/ani12081008
Walter LL, Gärtner T, Gernand E, Wehrend A, Donat K. Effects of Parity and Stage of Lactation on Trend and Variability of Metabolic Markers in Dairy Cows. Animals. 2022; 12(8):1008. https://doi.org/10.3390/ani12081008
Chicago/Turabian StyleWalter, Linda L., Tanja Gärtner, Erhard Gernand, Axel Wehrend, and Karsten Donat. 2022. "Effects of Parity and Stage of Lactation on Trend and Variability of Metabolic Markers in Dairy Cows" Animals 12, no. 8: 1008. https://doi.org/10.3390/ani12081008
APA StyleWalter, L. L., Gärtner, T., Gernand, E., Wehrend, A., & Donat, K. (2022). Effects of Parity and Stage of Lactation on Trend and Variability of Metabolic Markers in Dairy Cows. Animals, 12(8), 1008. https://doi.org/10.3390/ani12081008






