The Role of Nutraceuticals and Phytonutrients in Chickens’ Gastrointestinal Diseases
Abstract
Simple Summary
Abstract
1. Introduction
2. Different Classes of Alternative Compounds
3. Salmonella spp. Infection
3.1. Probiotics and Prebiotics
3.2. Organic Acids
3.3. Vitamins
3.4. Phytogenic Feed Additives (PFAs)
4. Campylobacter jejuni Infection
4.1. Probiotics and Prebiotics
4.2. Organic Acids
4.3. Phytogenic Feed Additives (PFAs)
5. Clostridium perfringens Infection
5.1. Probiotics and Prebiotics
5.2. Organic Acids
5.3. Phytogenic Feed Additives (PFAs)
5.4. Vitamins
6. Coccidiosis
6.1. Probiotics
6.2. Organic Acids
6.3. Phytogenic Feed Additives (PFAs)
6.4. Antioxidants
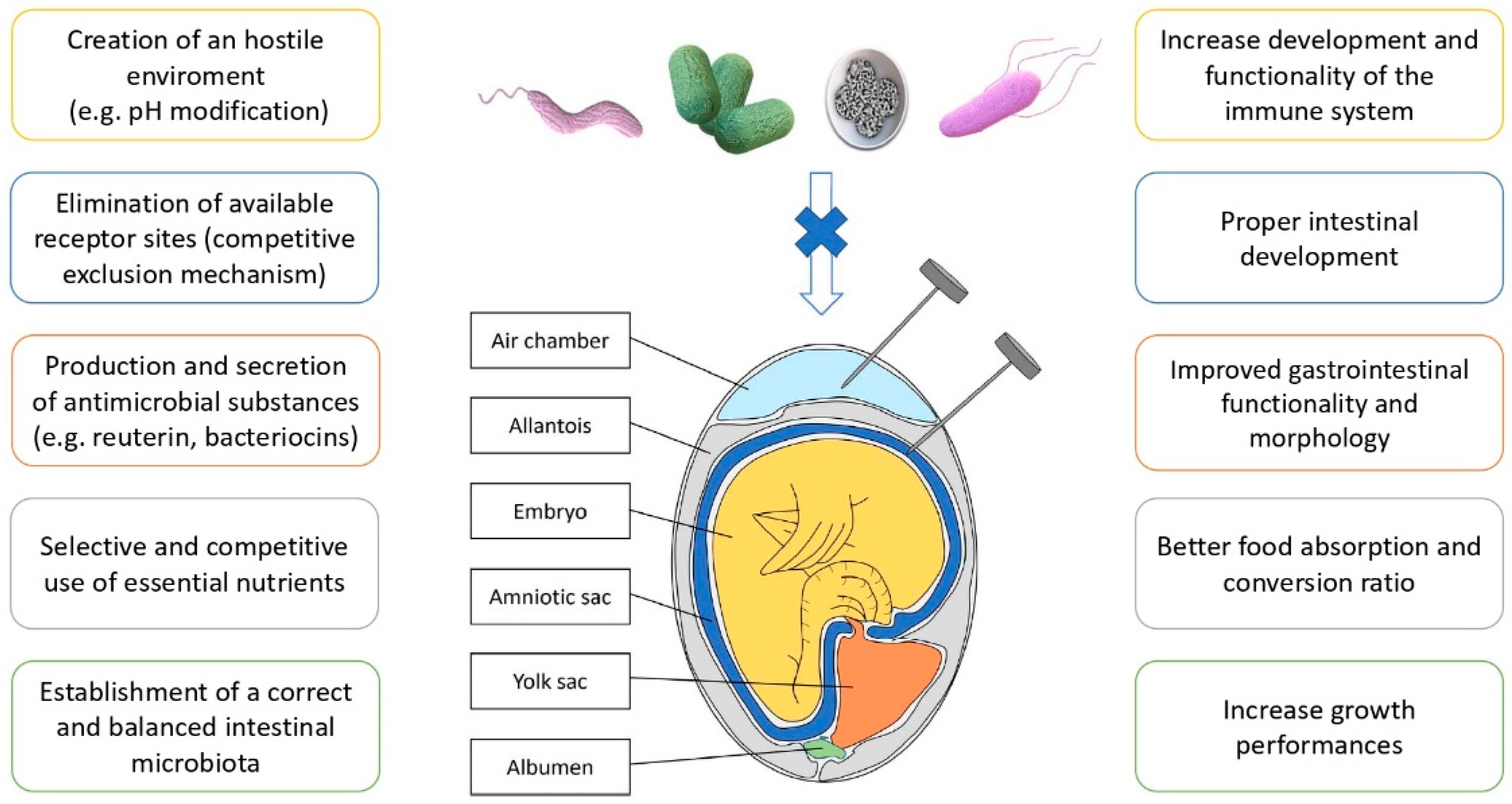
7. In Ovo Technique
Using in Ovo Inoculation Technique against GI Pathogens
8. Discussion and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Roth, N.; Käsbohrer, A.; Mayrhofer, S.; Zitz, U.; Hofacre, C.; Domig, K.J. The Application of Antibiotics in Broiler Production and the Resulting Antibiotic Resistance in Escherichia Coli: A Global Overview. Poult. Sci. 2019, 98, 1791–1804. [Google Scholar] [CrossRef]
- Antibiotic Resistance. Available online: https://www.who.int/news-room/fact-sheets/detail/antibiotic-resistance (accessed on 17 December 2021).
- Cosby, D.E.; Cox, N.A.; Harrison, M.A.; Wilson, J.L.; Buhr, R.J.; Fedorka-Cray, P.J. Salmonella and Antimicrobial Resistance in Broilers: A Review. J. Appl. Poult. Res. 2015, 24, 408–426. [Google Scholar] [CrossRef]
- World Health Organization. Critically Important Antimicrobials for Human Medicine; World Health Organization: Geneva, Switzerland, 2019; ISBN 978-92-4-151552-8. [Google Scholar]
- Castanon, J.I.R. History of the Use of Antibiotic as Growth Promoters in European Poultry Feeds. Poult. Sci. 2007, 86, 2466–2471. [Google Scholar] [CrossRef] [PubMed]
- EU, R. Ban on Antibiotics as Growth Promoters in Animal Feed Enters into Effect. Bruss. Eur. 2005. Available online: https://agenceurope.eu/aewebsite_dev/en/bulletin/article/9099/22 (accessed on 17 December 2021).
- Editors, A. US Bans Antibiotics Use for Enhancing Growth in Livestock. 2017. Available online: https://doi.org/10.1036/1097-8542.BR0125171 (accessed on 17 December 2021).
- Agricultural Production—Livestock and Meat. Available online: https://ec.europa.eu/eurostat/statistics-explained/index.php?title=Agricultural_production_-_livestock_and_meat (accessed on 17 December 2021).
- Shehata, A.A.; Yalçın, S.; Latorre, J.D.; Basiouni, S.; Attia, Y.A.; Abd El-Wahab, A.; Visscher, C.; El-Seedi, H.R.; Huber, C.; Hafez, H.M.; et al. Probiotics, Prebiotics, and Phytogenic Substances for Optimizing Gut Health in Poultry. Microorganisms 2022, 10, 395. [Google Scholar] [CrossRef] [PubMed]
- Huyghebaert, G.; Ducatelle, R.; Immerseel, F.V. An Update on Alternatives to Antimicrobial Growth Promoters for Broilers. Vet. J. 2011, 187, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Alagawany, M.; Elnesr, S.S.; Farag, M.R.; Abd El-Hack, M.E.; Barkat, R.A.; Gabr, A.A.; Foda, M.A.; Noreldin, A.E.; Khafaga, A.F.; El-Sabrout, K. Potential Role of Important Nutraceuticals in Poultry Performance and Health-A Comprehensive Review. Res. Vet. Sci. 2021, 137, 9–29. [Google Scholar] [CrossRef] [PubMed]
- Brower, V. Nutraceuticals: Poised for a Healthy Slice of the Healthcare Market? Nat. Biotechnol. 1998, 16, 728–731. [Google Scholar] [CrossRef] [PubMed]
- Das, L.; Bhaumik, E.; Raychaudhuri, U.; Chakraborty, R. Role of Nutraceuticals in Human Health. J. Food Sci. Technol. 2012, 49, 173–183. [Google Scholar] [CrossRef]
- Frank, J.; Fukagawa, N.K.; Bilia, A.R.; Johnson, E.J.; Kwon, O.; Prakash, V.; Miyazawa, T.; Clifford, M.N.; Kay, C.D.; Crozier, A. Terms and Nomenclature Used for Plant-Derived Components in Nutrition and Related Research: Efforts toward Harmonization. Nutr. Rev. 2020, 78, 451–458. [Google Scholar] [CrossRef]
- Sugiharto, S. Role of Nutraceuticals in Gut Health and Growth Performance of Poultry. J. Saudi Soc. Agric. Sci. 2016, 15, 99–111. [Google Scholar] [CrossRef]
- Swaggerty, C.L.; Bortoluzzi, C.; Lee, A.; Eyng, C.; Pont, G.D.; Kogut, M.H. Potential Replacements for Antibiotic Growth Promoters in Poultry: Interactions at the Gut Level and Their Impact on Host Immunity. In Recent Advances in Animal Nutrition and Metabolism; Wu, G., Ed.; Advances in Experimental Medicine and Biology; Springer International Publishing: Cham, Switzerland, 2022; pp. 145–159. ISBN 978-3-030-85686-1. [Google Scholar]
- Chauhan, B.; Kumar, G.; Kalam, N.; Ansari, S.H. Current Concepts and Prospects of Herbal Nutraceutical: A Review. J. Adv. Pharm. Technol. Res. 2013, 4, 4–8. [Google Scholar]
- Yan, L.; An, S.; Lv, Z.; Wang, Z.; Wu, Y.; Zhu, Y.; Zhao, M.; Sun, C.; Lv, M.; Zhu, Z.; et al. Effects of Phytonutrients on Growth Performance, Antioxidative Status, and Energy Utilization of Broilers Fed Low Energy Diets. Anim. Nutr. 2019, 5, 270–277. [Google Scholar] [CrossRef] [PubMed]
- El-Sherbiny, I.M.; El-Baz, N.M.; Hefnawy, A. Potential of Nanotechnology in Nutraceuticals Delivery for the Prevention and Treatment of Cancer. In Nutraceuticals; Grumezescu, A.M., Ed.; Nanotechnology in the Agri-Food Industry; Academic Press: Cambridge, MA, USA, 2016; pp. 117–152. ISBN 978-0-12-804305-9. [Google Scholar]
- Solicitation of Written Comments on Proposed Definition of Bioactive Food Components. Available online: https://www.federalregister.gov/documents/2004/09/16/04-20892/solicitation-of-written-comments-on-proposed-definition-of-bioactive-food-components (accessed on 3 January 2022).
- Nutrition Division. Probiotics in Food: Health and Nutritional Properties and Guidelines for Evaluation—Report of a Joint FAO/WHO Expert Consultation on Evaluation of Health and Nutritional Properties of Probiotics in Food Including Powder Milk with Live Lactic Acid Bacteria; FAO Food and Nutrition Paper; FAO/WHO: Rome, Italy, 2006; ISBN 978-92-5-105513-7. [Google Scholar]
- Bratz, K.; Gölz, G.; Janczyk, P.; Nöckler, K.; Alter, T. Analysis of in Vitro and in Vivo Effects of Probiotics against Campylobacter spp. Berl. Munch. Tierarztl. Wochenschr. 2015, 128, 155–162. [Google Scholar] [PubMed]
- Cox, C.M.; Dalloul, R.A. Immunomodulatory Role of Probiotics in Poultry and Potential in Ovo Application. Benef. Microbes 2015, 6, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Kemgang, T.S.; Kapila, S.; Shanmugam, V.P.; Kapila, R. Cross-talk between Probiotic Lactobacilli and Host Immune System. J. Appl. Microbiol. 2014, 117, 303–319. [Google Scholar] [CrossRef] [PubMed]
- Travers, M.-A.; Florent, I.; Kohl, L.; Grellier, P. Probiotics for the Control of Parasites: An Overview. J. Parasitol. Res. 2011, 2011, 610769. [Google Scholar] [CrossRef] [PubMed]
- Bai, S.P.; Wu, A.M.; Ding, X.M.; Lei, Y.; Bai, J.; Zhang, K.Y.; Chio, J.S. Effects of Probiotic-Supplemented Diets on Growth Performance and Intestinal Immune Characteristics of Broiler Chickens. Poult. Sci. 2013, 92, 663–670. [Google Scholar] [CrossRef] [PubMed]
- Sen, S.; Ingale, S.L.; Kim, Y.W.; Kim, J.S.; Kim, K.H.; Lohakare, J.D.; Kim, E.K.; Kim, H.S.; Ryu, M.H.; Kwon, I.K. Effect of Supplementation of Bacillus subtilis LS 1-2 to Broiler Diets on Growth Performance, Nutrient Retention, Caecal Microbiology and Small Intestinal Morphology. Res. Vet. Sci. 2012, 93, 264–268. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Xiao, K.; Ke, Y.L.; Jiao, L.F.; Hu, C.H.; Diao, Q.Y.; Shi, B.; Zou, X.T. Effect of a Probiotic Mixture on Intestinal Microflora, Morphology, and Barrier Integrity of Broilers Subjected to Heat Stress. Poult. Sci. 2014, 93, 581–588. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Sun, J.; Zhong, H.; Li, N.; Xu, H.; Zhu, Q.; Liu, Y. Effect of Probiotics on the Meat Flavour and Gut Microbiota of Chicken. Sci. Rep. 2017, 7, 6400. [Google Scholar] [CrossRef]
- Yadav, S.; Jha, R. Strategies to Modulate the Intestinal Microbiota and Their Effects on Nutrient Utilization, Performance, and Health of Poultry. J. Anim. Sci. Biotechnol. 2019, 10, 2. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.F.; Kim, I.H. Effects of Multistrain Probiotics on Growth Performance, Apparent Ileal Nutrient Digestibility, Blood Characteristics, Cecal Microbial Shedding, and Excreta Odor Contents in Broilers. Poult. Sci. 2014, 93, 364–370. [Google Scholar] [CrossRef] [PubMed]
- Pourabedin, M.; Zhao, X. Prebiotics and Gut Microbiota in Chickens. FEMS Microbiol. Lett. 2015, 362, fnv122. [Google Scholar] [CrossRef] [PubMed]
- Alloui, N.; Szczurek, W.; Swiatkiewicz, S. The Usefulness of Prebiotics and Probiotics in Modern Poultry Nutrition: A Review. Ann. Anim. Sci. 2013, 13, 17–32. [Google Scholar] [CrossRef]
- Micciche, A.C.; Foley, S.L.; Pavlidis, H.O.; McIntyre, D.R.; Ricke, S.C. A Review of Prebiotics against Salmonella in Poultry: Current and Future Potential for Microbiome Research Applications. Front. Vet. Sci. 2018, 5, 191. [Google Scholar] [CrossRef] [PubMed]
- Ricke, S.C. Potential of Fructooligosaccharide Prebiotics in Alternative and Nonconventional Poultry Production Systems. Poult. Sci. 2015, 94, 1411–1418. [Google Scholar] [CrossRef]
- Ricke, S.C. Focus: Nutrition and Food Science: Impact of Prebiotics on Poultry Production and Food Safety. Yale J. Biol. Med. 2018, 91, 151. [Google Scholar] [PubMed]
- Shojadoost, B.; Yitbarek, A.; Alizadeh, M.; Kulkarni, R.R.; Astill, J.; Boodhoo, N.; Sharif, S. Centennial Review: Effects of Vitamins A, D, E, and C on the Chicken Immune System. Poult. Sci. 2021, 100, 100930. [Google Scholar] [CrossRef] [PubMed]
- Elwinger, K.; Fisher, C.; Jeroch, H.; Sauveur, B.; Tiller, H.; Whitehead, C.C. A Brief History of Poultry Nutrition over the Last Hundred Years. Worlds Poult. Sci. J. 2016, 72, 701–720. [Google Scholar] [CrossRef]
- El-Senousey, H.K.; Chen, B.; Wang, J.Y.; Atta, A.M.; Mohamed, F.R.; Nie, Q.H. Effects of Dietary Vitamin C, Vitamin E, and Alpha-Lipoic Acid Supplementation on the Antioxidant Defense System and Immune-Related Gene Expression in Broilers Exposed to Oxidative Stress by Dexamethasone. Poult. Sci. 2018, 97, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Khan, R.U.; Rahman, Z.U.; Nikousefat, Z.; Javdani, M.; Tufarelli, V.; Dario, C.; Selvaggi, M.; Laudadio, V. Immunomodulating Effects of Vitamin E in Broilers. Worlds Poult. Sci. J. 2012, 68, 31–40. [Google Scholar] [CrossRef]
- Lucas, A.; Morales, J.; Velando, A. Differential Effects of Specific Carotenoids on Oxidative Damage and Immune Response of Gull Chicks. J. Exp. Biol. 2014, 217, 1253–1262. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Roshdy, A.R.; Guo, Y.; Wang, Y.; Guo, S. Effect of Dietary Vitamin A on Reproductive Performance and Immune Response of Broiler Breeders. PLoS ONE 2014, 9, e105677. [Google Scholar] [CrossRef]
- Kholif, A.E.; Olafadehan, O.A. Essential Oils and Phytogenic Feed Additives in Ruminant Diet: Chemistry, Ruminal Microbiota and Fermentation, Feed Utilization and Productive Performance. Phytochem. Rev. 2021, 20, 1087–1108. [Google Scholar] [CrossRef]
- Abdelli, N.; Solà-Oriol, D.; Pérez, J.F. Phytogenic Feed Additives in Poultry: Achievements, Prospective and Challenges. Animals 2021, 11, 3471. [Google Scholar] [CrossRef] [PubMed]
- Brenes, A.; Roura, E. Essential Oils in Poultry Nutrition: Main Effects and Modes of Action. Anim. Feed Sci. Technol. 2010, 158, 1–14. [Google Scholar] [CrossRef]
- Cherian, G.; Orr, A.; Burke, I.C.; Pan, W. Feeding Artemisia Annua Alters Digesta PH and Muscle Lipid Oxidation Products in Broiler Chickens. Poult. Sci. 2013, 92, 1085–1090. [Google Scholar] [CrossRef]
- Mohammadi Gheisar, M.; Kim, I.H. Phytobiotics in Poultry and Swine Nutrition—A Review. Ital. J. Anim. Sci. 2018, 17, 92–99. [Google Scholar] [CrossRef]
- Yakhkeshi, S.; Rahimi, S.; Gharib Naseri, K. The Effects of Comparison of Herbal Extracts, Antibiotic, Probiotic and Organic Acid on Serum Lipids, Immune Response, GIT Microbial Population, Intestinal Morphology and Performance of Broilers. J. Med. Plants 2011, 10, 80–95. [Google Scholar]
- Yang, C.; Chowdhury, M.A.K.; Huo, Y.; Gong, J. Phytogenic Compounds as Alternatives to In-Feed Antibiotics: Potentials and Challenges in Application. Pathogens 2015, 4, 137–156. [Google Scholar] [CrossRef] [PubMed]
- Dittoe, D.K.; Ricke, S.C.; Kiess, A.S. Organic Acids and Potential for Modifying the Avian Gastrointestinal Tract and Reducing Pathogens and Disease. Front. Vet. Sci. 2018, 5, 216. [Google Scholar] [CrossRef] [PubMed]
- Hassan, H.M.A.; Mohamed, M.A.; Youssef, A.W.; Hassan, E.R. Effect of Using Organic Acids to Substitute Antibiotic Growth Promoters on Performance and Intestinal Microflora of Broilers. Asian-Australas. J. Anim. Sci. 2010, 23, 1348–1353. [Google Scholar] [CrossRef]
- Khan, S.H.; Iqbal, J. Recent Advances in the Role of Organic Acids in Poultry Nutrition. J. Appl. Anim. Res. 2016, 44, 359–369. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, X.; Xin, H.; Chen, S.; Yang, C.; Duan, Y.; Yang, X. Effects of a Protected Inclusion of Organic Acids and Essential Oils as Antibiotic Growth Promoter Alternative on Growth Performance, Intestinal Morphology and Gut Microflora in Broilers. Anim. Sci. J. 2017, 88, 1414–1424. [Google Scholar] [CrossRef] [PubMed]
- Saki, A.A.; Harcini, R.N.; Rahmatnejad, E.; Salary, J. Herbal Additives and Organic Acids as Antibiotic Alternatives in Broiler Chickens Diet for Organic Production. Afr. J. Biotechnol. 2012, 11, 2139–2145. [Google Scholar]
- Gast, R.K.; Porter, R.E., Jr. Salmonella Infections. In Diseases of Poultry; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2020; pp. 717–753. [Google Scholar]
- Kowalska, J.D.; Nowak, A.; Śliżewska, K.; Stańczyk, M.; Łukasiak, M.; Dastych, J. Anti-Salmonella Potential of New Lactobacillus Strains with the Application in the Poultry Industry. Pol. J. Microbiol. 2020, 69, 5. [Google Scholar] [CrossRef]
- Antunes, P.; Mourão, J.; Campos, J.; Peixe, L. Salmonellosis: The Role of Poultry Meat. Clin. Microbiol. Infect. 2016, 22, 110–121. [Google Scholar] [CrossRef]
- European Food Safety Authority and European Centre for Disease Prevention and Control. The European Union One Health 2019 Zoonoses Report. EFSA J. 2021, 19, e06406. [Google Scholar]
- Foley, S.L.; Nayak, R.; Hanning, I.B.; Johnson, T.J.; Han, J.; Ricke, S.C. Population Dynamics of Salmonella enterica Serotypes in Commercial Egg and Poultry Production. Appl. Environ. Microbiol. 2011, 77, 4273–4279. [Google Scholar] [CrossRef]
- Barrow, P.A.; Jones, M.A.; Smith, A.L.; Wigley, P. The Long View: Salmonella–the Last Forty Years. Avian Pathol. 2012, 41, 413–420. [Google Scholar] [CrossRef]
- Velasquez, C.G.; Macklin, K.S.; Kumar, S.; Bailey, M.; Ebner, P.E.; Oliver, H.F.; Martin-Gonzalez, F.S.; Singh, M. Prevalence and Antimicrobial Resistance Patterns of Salmonella Isolated from Poultry Farms in Southeastern United States. Poult. Sci. 2018, 97, 2144–2152. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, J.; Khan, S.; Su, J.Q.; Hesham, A.E.-L.; Ditta, A.; Nawab, J.; Ali, A. Antibiotics in Poultry Manure and Their Associated Health Issues: A Systematic Review. J. Soils Sediments 2020, 20, 486–497. [Google Scholar] [CrossRef]
- El-Shall, N.A.; Awad, A.M.; El-Hack, M.E.A.; Naiel, M.A.; Othman, S.I.; Allam, A.A.; Sedeik, M.E. The Simultaneous Administration of a Probiotic or Prebiotic with Live Salmonella Vaccine Improves Growth Performance and Reduces Fecal Shedding of the Bacterium in Salmonella-Challenged Broilers. Animals 2020, 10, 70. [Google Scholar] [CrossRef] [PubMed]
- El-Sharkawy, H.; Tahoun, A.; Rizk, A.M.; Suzuki, T.; Elmonir, W.; Nassef, E.; Shukry, M.; Germoush, M.O.; Farrag, F.; Bin-Jumah, M. Evaluation of Bifidobacteria and Lactobacillus Probiotics as Alternative Therapy for Salmonella Typhimurium Infection in Broiler Chickens. Animals 2020, 10, 1023. [Google Scholar] [CrossRef]
- Fuller, R. Probiotics in Man and Animals. J. Appl. Bacteriol. 1989, 66, 365–378. [Google Scholar]
- Nakphaichit, M.; Sobanbua, S.; Siemuang, S.; Vongsangnak, W.; Nakayama, J.; Nitisinprasert, S. Protective Effect of Lactobacillus Reuteri KUB-AC5 against Salmonella Enteritidis Challenge in Chickens. Benef. Microbes 2019, 10, 43–54. [Google Scholar] [CrossRef]
- Prado-Rebolledo, O.F.; de Jesus Delgado-Machuca, J.; Macedo-Barragan, R.J.; Garcia-Márquez, L.J.; Morales-Barrera, J.E.; Latorre, J.D.; Hernandez-Velasco, X.; Tellez, G. Evaluation of a Selected Lactic Acid Bacteria-Based Probiotic on Salmonella enterica Serovar Enteritidis Colonization and Intestinal Permeability in Broiler Chickens. Avian Pathol. 2017, 46, 90–94. [Google Scholar] [CrossRef]
- Carter, A.; Adams, M.; La Ragione, R.M.; Woodward, M.J. Colonisation of Poultry by Salmonella Enteritidis S1400 Is Reduced by Combined Administration of Lactobacillus Salivarius 59 and Enterococcus faecium PXN-33. Vet. Microbiol. 2017, 199, 100–107. [Google Scholar] [CrossRef]
- Bae, D.; Kim, D.-H.; Chon, J.-W.; Song, K.-Y.; Seo, K.-H. Synergistic Effects of the Early Administration of Lactobacillus Kefiranofaciens DN1 and Kluyveromyces Marxianus KU140723-05 on the Inhibition of Salmonella Enteritidis Colonization in Young Chickens. Poult. Sci. 2020, 99, 5999–6006. [Google Scholar] [CrossRef]
- Khochamit, N.; Siripornadulsil, S.; Sukon, P.; Siripornadulsil, W. Bacillus subtilis and Lactic Acid Bacteria Improve the Growth Performance and Blood Parameters and Reduce Salmonella Infection in Broilers. Vet. World 2020, 13, 2663–2672. [Google Scholar] [CrossRef]
- Neveling, D.P.; van Emmenes, L.; Ahire, J.J.; Pieterse, E.; Smith, C.; Dicks, L.M.T. Effect of a Multi-Species Probiotic on the Colonisation of Salmonella in Broilers. Probiotics Antimicrob. Proteins 2020, 12, 896–905. [Google Scholar] [CrossRef] [PubMed]
- Delgado, R.; Latorre, J.D.; Vicuña, E.; Hernandez-Velasco, X.; Vicente, J.L.; Menconi, A.; Kallapura, G.; Layton, S.; Hargis, B.M.; Tellez, G. Glycerol Supplementation Enhances the Protective Effect of Dietary FloraMax-B11 against Salmonella Enteritidis Colonization in Neonate Broiler Chickens. Poult. Sci. 2014, 93, 2363–2369. [Google Scholar] [CrossRef] [PubMed]
- Biloni, A.; Quintana, C.F.; Menconi, A.; Kallapura, G.; Latorre, J.; Pixley, C.; Layton, S.; Dalmagro, M.; Hernandez-Velasco, X.; Wolfenden, A. Evaluation of Effects of EarlyBird Associated with FloraMax-B11 on Salmonella Enteritidis, Intestinal Morphology, and Performance of Broiler Chickens. Poult. Sci. 2013, 92, 2337–2346. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Chousalkar, K.K. Short-Term Feeding of Probiotics and Synbiotics Modulates Caecal Microbiota during Salmonella Typhimurium Infection but Does Not Reduce Shedding and Invasion in Chickens. Appl. Microbiol. Biotechnol. 2020, 104, 319–334. [Google Scholar] [CrossRef]
- Lourenço, M.C.; Kuritza, L.N.; Hayashi, R.M.; Miglino, L.B.; Durau, J.F.; Pickler, L.; Santin, E. Effect of a Mannanoligosaccharide-Supplemented Diet on Intestinal Mucosa T Lymphocyte Populations in Chickens Challenged WithSalmonella Enteritidis. J. Appl. Poult. Res. 2015, 24, 15–22. [Google Scholar] [CrossRef]
- Park, S.H.; Kim, S.A.; Lee, S.I.; Rubinelli, P.M.; Roto, S.M.; Pavlidis, H.O.; McIntyre, D.R.; Ricke, S.C. Original XPCTM Effect on Salmonella Typhimurium and Cecal Microbiota from Three Different Ages of Broiler Chickens When Incubated in an Anaerobic In Vitro Culture System. Front. Microbiol. 2017, 8, 1070. [Google Scholar] [CrossRef]
- Feye, K.M.; Anderson, K.L.; Scott, M.F.; McIntyre, D.R.; Carlson, S.A. Inhibition of the Virulence, Antibiotic Resistance, and Fecal Shedding of Multiple Antibiotic-Resistant Salmonella Typhimurium in Broilers Fed Original XPCTM. Poult. Sci. 2016, 95, 2902–2910. [Google Scholar] [CrossRef]
- Lee, S.I.; Park, S.H.; Ricke, S.C. Assessment of Cecal Microbiota, Integron Occurrence, Fermentation Responses, and Salmonella Frequency in Conventionally Raised Broilers Fed a Commercial Yeast-Based Prebiotic Compound. Poult. Sci. 2016, 95, 144–153. [Google Scholar] [CrossRef]
- Hughes, R.-A.; Ali, R.A.; Mendoza, M.A.; Hassan, H.M.; Koci, M.D. Impact of Dietary Galacto-Oligosaccharide (GOS) on Chicken’s Gut Microbiota, Mucosal Gene Expression, and Salmonella Colonization. Front. Vet. Sci. 2017, 4, 192. [Google Scholar] [CrossRef]
- Guilloteau, P.; Martin, L.; Eeckhaut, V.; Ducatelle, R.; Zabielski, R.; Van Immerseel, F. From the Gut to the Peripheral Tissues: The Multiple Effects of Butyrate. Nutr. Res. Rev. 2010, 23, 366–384. [Google Scholar] [CrossRef]
- Onrust, L.; Baeyen, S.; Haesebrouck, F.; Ducatelle, R.; Van Immerseel, F. Effect of in Feed Administration of Different Butyrate Formulations on Salmonella Enteritidis Colonization and Cecal Microbiota in Broilers. Vet. Res. 2020, 51, 56. [Google Scholar] [CrossRef] [PubMed]
- Bedford, A.; Gong, J. Implications of Butyrate and Its Derivatives for Gut Health and Animal Production. Anim. Nutr. 2018, 4, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Moquet, P.C.A.; Salami, S.A.; Onrust, L.; Hendriks, W.H.; Kwakkel, R.P. Butyrate Presence in Distinct Gastrointestinal Tract Segments Modifies Differentially Digestive Processes and Amino Acid Bioavailability in Young Broiler Chickens. Poult. Sci. 2018, 97, 167–176. [Google Scholar] [CrossRef] [PubMed]
- van den Borne, J.J.G.C.; Heetkamp, M.J.W.; Buyse, J.; Niewold, T.A. Fat Coating of Ca Butyrate Results in Extended Butyrate Release in the Gastrointestinal Tract of Broilers. Livest. Sci. 2015, 175, 96–100. [Google Scholar] [CrossRef]
- Adhikari, P.; Kiess, A.; Adhikari, R.; Jha, R. An Approach to Alternative Strategies to Control Avian Coccidiosis and Necrotic Enteritis. J. Appl. Poult. Res. 2020, 29, 515–534. [Google Scholar] [CrossRef]
- van Kuijk, S.J.A.; Han, Y. Efficacy of a Synergistic Blend of Organic Acids and SS-1,4 Mannobiose on Cecal Salmonella Counts and Growth Performance in Salmonella Challenged Broiler Chickens: A Meta-Analysis. Animals 2021, 11, 2988. [Google Scholar] [CrossRef] [PubMed]
- Bourassa, D.V.; Wilson, K.M.; Ritz, C.R.; Kiepper, B.K.; Buhr, R.J. Evaluation of the Addition of Organic Acids in the Feed and/or Water for Broilers and the Subsequent Recovery of Salmonella Typhimurium from Litter and Ceca. Poult. Sci. 2018, 97, 64–73. [Google Scholar] [CrossRef]
- Sobotik, E.B.; Ramirez, S.; Roth, N.; Tacconi, A.; Pender, C.; Murugesan, R.; Archer, G.S. Evaluating the Effects of a Dietary Synbiotic or Synbiotic plus Enhanced Organic Acid on Broiler Performance and Cecal and Carcass Salmonella Load. Poult. Sci. 2021, 100, 101508. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, T.; Srivastava, S.K.; Gaurav, A.; Kumar, A.; Kumar, P.; Yadav, A.S.; Pathania, R.; Navani, N.K. A Combination of Linalool, Vitamin C, and Copper Synergistically Triggers Reactive Oxygen Species and DNA Damage and Inhibits Salmonella enterica subsp. enterica Serovar Typhi and Vibrio fluvialis. Appl. Environ. Microbiol. 2019, 85, e02487-18. [Google Scholar] [CrossRef]
- Hernandez-Patlan, D.; Solis-Cruz, B.; Méndez-Albores, A.; Latorre, J.D.; Hernandez-Velasco, X.; Tellez, G.; López-Arellano, R. Comparison of PrestoBlue® and Plating Method to Evaluate Antimicrobial Activity of Ascorbic Acid, Boric Acid and Curcumin in an in Vitro Gastrointestinal Model. J. Appl. Microbiol. 2018, 124, 423–430. [Google Scholar] [CrossRef]
- Hernandez-Patlan, D.; Solis-Cruz, B.; Pontin, K.P.; Latorre, J.D.; Hernandez-Velasco, X.; Merino-Guzman, R.; Mendez-Albores, A.; Hargis, B.M.; Lopez-Arellano, R.; Tellez-Isaias, G. Evaluation of Ascorbic Acid or Curcumin Formulated in a Solid Dispersion on Salmonella Enteritidis Infection and Intestinal Integrity in Broiler Chickens. Pathogens 2019, 8, 229. [Google Scholar] [CrossRef] [PubMed]
- Gan, L.; Fan, H.; Mahmood, T.; Guo, Y. Dietary Supplementation with Vitamin C Ameliorates the Adverse Effects of Salmonella Enteritidis-Challenge in Broilers by Shaping Intestinal Microbiota. Poult. Sci. 2020, 99, 3663–3674. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.J.; Zhao, L.H.; Mosenthin, R.; Zhang, J.Y.; Ji, C.; Ma, Q.G. Protective Effect of Vitamin E on Laying Performance, Antioxidant Capacity, and Immunity in Laying Hens Challenged with Salmonella Enteritidis. Poult. Sci. 2019, 98, 5847–5854. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Byrd, J.A.; Farnell, M.; Ruiz-Feria, C.A. Arginine and Vitamin E Improve the Immune Response after a Salmonella Challenge in Broiler Chicks. Poult. Sci. 2014, 93, 882–890. [Google Scholar] [CrossRef]
- Abudabos, A.M.; Alyemni, A.H.; Dafalla, Y.M.; Khan, R.U. The Effect of Phytogenic Feed Additives to Substitute In-Feed Antibiotics on Growth Traits and Blood Biochemical Parameters in Broiler Chicks Challenged with Salmonella Typhimurium. Environ. Sci. Pollut. Res. 2016, 23, 24151–24157. [Google Scholar] [CrossRef]
- Murugesan, G.R.; Syed, B.; Haldar, S.; Pender, C. Phytogenic Feed Additives as an Alternative to Antibiotic Growth Promoters in Broiler Chickens. Front. Vet. Sci. 2015, 2, 21. [Google Scholar] [PubMed]
- Wati, T.; Ghosh, T.K.; Syed, B.; Haldar, S. Comparative Efficacy of a Phytogenic Feed Additive and an Antibiotic Growth Promoter on Production Performance, Caecal Microbial Population and Humoral Immune Response of Broiler Chickens Inoculated with Enteric Pathogens. Anim. Nutr. 2015, 1, 213–219. [Google Scholar] [CrossRef]
- Casanova, N.A.; Redondo, L.M.; Redondo, E.A.; Joaquim, P.E.; Dominguez, J.E.; Fernández-Miyakawa, M.E.; Chacana, P.A. Efficacy of Chestnut and Quebracho Wood Extracts to Control Salmonella in Poultry. J. Appl. Microbiol. 2021, 131, 135–145. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Sahin, O. Campylobacteriosis. In Diseases of Poultry; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2020; pp. 754–769. [Google Scholar]
- Humphrey, S.; Chaloner, G.; Kemmett, K.; Davidson, N.; Williams, N.; Kipar, A.; Humphrey, T.; Wigley, P. Campylobacter jejuni Is Not Merely a Commensal in Commercial Broiler Chickens and Affects Bird Welfare. mBio 2014, 5, e01364-14. [Google Scholar] [CrossRef]
- Awad, W.A.; Hess, C.; Hess, M. Re-Thinking the Chicken-Campylobacter Jejuni Interaction: A Review. Avian Pathol. J. WVPA 2018, 47, 352–363. [Google Scholar] [CrossRef] [PubMed]
- García-Sánchez, L.; Melero, B.; Jaime, I.; Hänninen, M.-L.; Rossi, M.; Rovira, J. Campylobacter jejuni Survival in a Poultry Processing Plant Environment. Food Microbiol. 2017, 65, 185–192. [Google Scholar] [CrossRef]
- Hue, O.; Allain, V.; Laisney, M.-J.; Le Bouquin, S.; Lalande, F.; Petetin, I.; Rouxel, S.; Quesne, S.; Gloaguen, P.-Y.; Picherot, M.; et al. Campylobacter Contamination of Broiler Caeca and Carcasses at the Slaughterhouse and Correlation with Salmonella Contamination. Food Microbiol. 2011, 28, 862–868. [Google Scholar] [CrossRef] [PubMed]
- Agunos, A.; Waddell, L.; Léger, D.; Taboada, E. A Systematic Review Characterizing On-Farm Sources of Campylobacter Spp. for Broiler Chickens. PLoS ONE 2014, 9, e104905. [Google Scholar] [CrossRef] [PubMed]
- Śmiałek, M.; Kowalczyk, J.; Koncicki, A. The Use of Probiotics in the Reduction of Campylobacter Spp. Prevalence in Poultry. Animals 2021, 11, 1355. [Google Scholar] [CrossRef] [PubMed]
- Kemmett, K. Probiotics and Enzymes: A Good Combination. AFMA Matrix 2015, 24, 35–37. [Google Scholar]
- Wang, X.; Zhang, X.; Dong, X.; Samie, M.; Li, X.; Cheng, X.; Goschka, A.; Shen, D.; Zhou, Y.; Harlow, J.; et al. TPC proteins are phosphoinositide-activated sodium-selective ion channels in endosomes and lysosomes. Cell 2012, 151, 372–383. [Google Scholar] [CrossRef] [PubMed]
- Wooten, J.; Liu, X.; Miller, M.J. Draft Genome Sequence of Lactobacillus crispatus JCM5810, Which Can Reduce Campylobacter jejuni Colonization in Chicken Intestine. Genome Announc. 2016, 4, e00255-16. [Google Scholar] [CrossRef]
- Arsi, K.; Donoghue, A.M.; Woo-Ming, A.; Blore, P.J.; Donoghue, D.J. The Efficacy of Selected Probiotic and Prebiotic Combinations in Reducing Campylobacter Colonization in Broiler Chickens. J. Appl. Poult. Res. 2015, 24, 327–334. [Google Scholar] [CrossRef]
- Ghareeb, K.; Awad, W.A.; Mohnl, M.; Porta, R.; Biarnés, M.; Böhm, J.; Schatzmayr, G. Evaluating the Efficacy of an Avian-Specific Probiotic to Reduce the Colonization of Campylobacter jejuni in Broiler Chickens. Poult. Sci. 2012, 91, 1825–1832. [Google Scholar] [CrossRef]
- Guyard-Nicodème, M.; Keita, A.; Quesne, S.; Amelot, M.; Poezevara, T.; Le Berre, B.; Sánchez, J.; Vesseur, P.; Martín, Á.; Medel, P.; et al. Efficacy of Feed Additives against Campylobacter in Live Broilers during the Entire Rearing Period. Poult. Sci. 2016, 95, 298–305. [Google Scholar] [CrossRef]
- Saint-Cyr, M.J.; Haddad, N.; Taminiau, B.; Poezevara, T.; Quesne, S.; Amelot, M.; Daube, G.; Chemaly, M.; Dousset, X.; Guyard-Nicodème, M. Use of the Potential Probiotic Strain Lactobacillus Salivarius SMXD51 to Control Campylobacter jejuni in Broilers. Int. J. Food Microbiol. 2017, 247, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Šikić Pogačar, M.; Langerholc, T.; Mičetić-Turk, D.; Možina, S.S.; Klančnik, A. Effect of Lactobacillus Spp. on Adhesion, Invasion, and Translocation of Campylobacter jejuni in Chicken and Pig Small-Intestinal Epithelial Cell Lines. BMC Vet. Res. 2020, 16, 34. [Google Scholar] [CrossRef] [PubMed]
- Eeckhaut, V.; Wang, J.; Van Parys, A.; Haesebrouck, F.; Joossens, M.; Falony, G.; Raes, J.; Ducatelle, R.; Van Immerseel, F. The Probiotic Butyricicoccus Pullicaecorum Reduces Feed Conversion and Protects from Potentially Harmful Intestinal Microorganisms and Necrotic Enteritis in Broilers. Front. Microbiol. 2016, 7, 1416. [Google Scholar] [CrossRef] [PubMed]
- Thomrongsuwannakij, T.; Chuanchuen, R.; Chansiripornchai, N. Identification of Competitive Exclusion and Its Ability to Protect Against Campylobacter jejuni in Broilers. Thai Vet. Med. 2016, 46, 279–286. [Google Scholar]
- Baffoni, L.; Gaggìa, F.; Garofolo, G.; Di Serafino, G.; Buglione, E.; Di Giannatale, E.; Di Gioia, D. Evidence of Campylobacter jejuni Reduction in Broilers with Early Synbiotic Administration. Int. J. Food Microbiol. 2017, 251, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Peng, M.; Reichmann, G.; Biswas, D. Lactobacillus Casei and Its Byproducts Alter the Virulence Factors of Foodborne Bacterial Pathogens. J. Funct. Foods 2015, 15, 418–428. [Google Scholar] [CrossRef]
- Tabashsum, Z.; Peng, M.; Kahan, E.; Rahaman, S.O.; Biswas, D. Effect of Conjugated Linoleic Acid Overproducing Lactobacillus with Berry Pomace Phenolic Extracts on Campylobacter jejuni Pathogenesis. Food Funct. 2019, 10, 296–303. [Google Scholar] [CrossRef] [PubMed]
- Nothaft, H.; Perez-Muñoz, M.E.; Gouveia, G.J.; Duar, R.M.; Wanford, J.J.; Lango-Scholey, L.; Panagos, C.G.; Srithayakumar, V.; Plastow, G.S.; Coros, C. Coadministration of the Campylobacter Jejuni N-Glycan-Based Vaccine with Probiotics Improves Vaccine Performance in Broiler Chickens. Appl. Environ. Microbiol. 2017, 83, e01523-17. [Google Scholar] [CrossRef] [PubMed]
- Beier, R.C.; Byrd, J.A.; Caldwell, D.; Andrews, K.; Crippen, T.L.; Anderson, R.C.; Nisbet, D.J. Inhibition and Interactions of Campylobacter Jejuni from Broiler Chicken Houses with Organic Acids. Microorganisms 2019, 7, 223. [Google Scholar] [CrossRef] [PubMed]
- Skånseng, B.; Kaldhusdal, M.; Moen, B.; Gjevre, A.-G.; Johannessen, G.S.; Sekelja, M.; Trosvik, P.; Rudi, K. Prevention of Intestinal Campylobacter Jejuni Colonization in Broilers by Combinations of In-Feed Organic Acids. J. Appl. Microbiol. 2010, 109, 1265–1273. [Google Scholar] [CrossRef]
- Hankel, J.; Popp, J.; Meemken, D.; Zeiger, K.; Beyerbach, M.; Taube, V.; Klein, G.; Visscher, C. Influence of Lauric Acid on the Susceptibility of Chickens to an Experimental Campylobacter jejuni Colonisation. PLoS ONE 2018, 13, e0204483. [Google Scholar] [CrossRef] [PubMed]
- Peh, E.; Kittler, S.; Reich, F.; Kehrenberg, C. Antimicrobial Activity of Organic Acids against Campylobacter Spp. and Development of Combinations—A Synergistic Effect? PLoS ONE 2020, 15, e0239312. [Google Scholar] [CrossRef]
- Gracia, M.I.; Millán, C.; Sánchez, J.; Guyard-Nicodème, M.; Mayot, J.; Carre, Y.; Csorbai, A.; Chemaly, M.; Medel, P. Efficacy of Feed Additives against Campylobacter in Live Broilers during the Entire Rearing Period: Part B. Poult. Sci. 2016, 95, 886–892. [Google Scholar] [CrossRef] [PubMed]
- Hermans, D.; Martel, A.; Garmyn, A.; Verlinden, M.; Heyndrickx, M.; Gantois, I.; Haesebrouck, F.; Pasmans, F. Application of Medium-Chain Fatty Acids in Drinking Water Increases Campylobacter jejuni Colonization Threshold in Broiler Chicks. Poult. Sci. 2012, 91, 1733–1738. [Google Scholar] [CrossRef] [PubMed]
- Greene, G.; Koolman, L.; Whyte, P.; Lynch, H.; Coffey, A.; Lucey, B.; Egan, J.; O’Connor, L.; Bolton, D. The Efficacy of Organic Acid, Medium Chain Fatty Acid and Essential Oil Based Broiler Treatments; in Vitro Anti-Campylobacter jejuni Activity and the Effect of These Chemical-Based Treatments on Broiler Performance. J. Appl. Microbiol. 2021, 132, 687–695. [Google Scholar] [CrossRef] [PubMed]
- Haughton, P.N.; Lyng, J.; Fanning, S.; Whyte, P. Potential of a Commercially Available Water Acidification Product for Reducing Campylobacter in Broilers Prior to Slaughter. Br. Poult. Sci. 2013, 54, 319–324. [Google Scholar] [PubMed]
- Hermans, D.; Martel, A.; van Deun, K.; van Immerseel, F.; Heyndrickx, M.; Haesebrouck, F.; Pasmans, F. The Cinnamon-Oil Ingredient Trans-Cinnamaldehyde Fails to Target Campylobacter jejuni Strain KC 40 in the Broiler Chicken Cecum despite Marked in Vitro Activity. J. Food Prot. 2011, 74, 1729–1734. [Google Scholar] [CrossRef] [PubMed]
- Robyn, J.; Rasschaert, G.; Hermans, D.; Pasmans, F.; Heyndrickx, M. Is Allicin Able to Reduce Campylobacter jejuni Colonization in Broilers When Added to Drinking Water? Poult. Sci. 2013, 92, 1408–1418. [Google Scholar] [CrossRef]
- Anderson, R.C.; Vodovnik, M.; Min, B.R.; Pinchak, W.E.; Krueger, N.A.; Harvey, R.B.; Nisbet, D.J. Bactericidal Effect of Hydrolysable and Condensed Tannin Extracts on Campylobacter jejuni in Vitro. Folia Microbiol. 2012, 57, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Epps, S.V.R.; Harvey, R.B.; Byrd, J.A.; Petrujkić, B.T.; Sedej, I.; Beier, R.C.; Phillips, T.D.; Hume, M.E.; Anderson, R.C.; Nisbet, D.J. Comparative Effect of Thymol or Its Glucose Conjugate, Thymol-β-D-Glucopyranoside, on Campylobacter in Avian Gut Contents. J. Environ. Sci. Health B 2015, 50, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Kurekci, C.; Al Jassim, R.; Hassan, E.; Bishop-Hurley, S.L.; Padmanabha, J.; McSweeney, C.S. Effects of Feeding Plant-Derived Agents on the Colonization of Campylobacter jejuni in Broiler Chickens. Poult. Sci. 2014, 93, 2337–2346. [Google Scholar] [CrossRef] [PubMed]
- Arsi, K.; Donoghue, A.M.; Venkitanarayanan, K.; Kollanoor-Johny, A.; Fanatico, A.C.; Blore, P.J.; Donoghue, D.J. The Efficacy of the Natural Plant Extracts, Thymol and Carvacrol against Campylobacter Colonization in Broiler Chickens. J. Food Saf. 2014, 34, 321–325. [Google Scholar] [CrossRef]
- Kurekci, C.; Padmanabha, J.; Bishop-Hurley, S.L.; Hassan, E.; Al Jassim, R.A.M.; McSweeney, C.S. Antimicrobial Activity of Essential Oils and Five Terpenoid Compounds against Campylobacter jejuni in Pure and Mixed Culture Experiments. Int. J. Food Microbiol. 2013, 166, 450–457. [Google Scholar] [CrossRef]
- Boulianne, M.; Uzal, F.A.; Opengart, K. Clostridial Diseases. In Diseases of Poultry; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2020; pp. 966–994. ISBN 978-1-119-37119-9. [Google Scholar]
- M’Sadeq, S.A.; Wu, S.; Swick, R.A.; Choct, M. Towards the Control of Necrotic Enteritis in Broiler Chickens with In-Feed Antibiotics Phasing-out Worldwide. Anim. Nutr. 2015, 1, 1–11. [Google Scholar] [CrossRef]
- Caly, D.L.; D’Inca, R.; Auclair, E.; Drider, D. Alternatives to Antibiotics to Prevent Necrotic Enteritis in Broiler Chickens: A Microbiologist’s Perspective. Front. Microbiol. 2015, 6, 1336. [Google Scholar] [CrossRef] [PubMed]
- Van Immerseel, F.; De Buck, J.; Pasmans, F.; Huyghebaert, G.; Haesebrouck, F.; Ducatelle, R. Clostridium perfringens in Poultry: An Emerging Threat for Animal and Public Health. Avian Pathol. J. WVPA 2004, 33, 537–549. [Google Scholar] [CrossRef]
- Jayaraman, S.; Thangavel, G.; Kurian, H.; Mani, R.; Mukkalil, R.; Chirakkal, H. Bacillus subtilis PB6 Improves Intestinal Health of Broiler Chickens Challenged with Clostridium perfringens-Induced Necrotic Enteritis. Poult. Sci. 2013, 92, 370–374. [Google Scholar] [CrossRef] [PubMed]
- Mongkolthanaruk, W. Classification of Bacillus Beneficial Substances Related to Plants, Humans and Animals. J. Microbiol. Biotechnol. 2012, 22, 1597–1604. [Google Scholar] [CrossRef]
- Abudabos, A. Bacillus subtilis PB6 Based-Probiotic (CloSTATTM) Improves Intestinal Morphological and Microbiological Status of Broiler Chickens under Clostridium perfringens Challenge. Int. J. Agric. Biol. 2013, 15, 978–982. [Google Scholar]
- Ramlucken, U.; Ramchuran, S.O.; Moonsamy, G.; Lalloo, R.; Thantsha, M.S.; Jansen van Rensburg, C. A Novel Bacillus Based Multi-Strain Probiotic Improves Growth Performance and Intestinal Properties of Clostridium perfringens Challenged Broilers. Poult. Sci. 2020, 99, 331–341. [Google Scholar] [CrossRef]
- Bortoluzzi, C.; Vieira, B.S.; Applegate, T.J. Influence of Dietary Zinc, Copper, and Manganese on the Intestinal Health of Broilers under Eimeria Challenge. Front. Vet. Sci. 2020, 7, 13. [Google Scholar] [CrossRef] [PubMed]
- Hussein, E.O.S.; Ahmed, S.H.; Abudabos, A.M.; Suliman, G.M.; Abd El-Hack, M.E.; Swelum, A.A.; Alowaimer, A.N. Ameliorative Effects of Antibiotic-, Probiotic- and Phytobiotic-Supplemented Diets on the Performance, Intestinal Health, Carcass Traits, and Meat Quality of Clostridium perfringens-Infected Broilers. Animals 2020, 10, 669. [Google Scholar] [CrossRef] [PubMed]
- Layton, S.L.; Hernandez-Velasco, X.; Chaitanya, S.; Xavier, J.; Menconi, A.; Latorre, J.D.; Kallapura, G.; Kuttappan, V.A.; Wolfenden, R.E.; Andreatti Filho, R.L.; et al. The Effect of a Lactobacillus-Based Probiotic for the Control of Necrotic Enteritis in Broilers. Food Nutr. Sci. 2013, 4, 1–7. [Google Scholar]
- Guo, S.; Liu, D.; Zhang, B.; Li, Z.; Li, Y.; Ding, B.; Guo, Y. Two Lactobacillus Species Inhibit the Growth and α-Toxin Production of Clostridium perfringens and Induced Proinflammatory Factors in Chicken Intestinal Epithelial Cells in Vitro. Front. Microbiol. 2017, 8, 2081. [Google Scholar] [CrossRef]
- Qing, X.; Zeng, D.; Wang, H.; Ni, X.; Liu, L.; Lai, J.; Khalique, A.; Pan, K.; Jing, B. Preventing Subclinical Necrotic Enteritis through Lactobacillus Johnsonii BS15 by Ameliorating Lipid Metabolism and Intestinal Microflora in Broiler Chickens. AMB Express 2017, 7, 139. [Google Scholar] [CrossRef]
- Wang, H.; Ni, X.; Liu, L.; Zeng, D.; Lai, J.; Qing, X.; Li, G.; Pan, K.; Jing, B. Controlling of Growth Performance, Lipid Deposits and Fatty Acid Composition of Chicken Meat through a Probiotic, Lactobacillus Johnsonii during Subclinical Clostridium perfringens Infection. Lipids Health Dis. 2017, 16, 38. [Google Scholar] [CrossRef]
- Abudabos, A.M.; Yehia, H.M. Effect of Dietary Mannan Oligosaccharide from Saccharomyces Cerevisiae on Live Performance of Broilers Under Clostridium perfringens Challenge. Ital. J. Anim. Sci. 2013, 12, e38. [Google Scholar] [CrossRef]
- M’Sadeq, S.A.; Wu, S.-B.; Choct, M.; Forder, R.; Swick, R.A. Use of Yeast Cell Wall Extract as a Tool to Reduce the Impact of Necrotic Enteritis in Broilers. Poult. Sci. 2015, 94, 898–905. [Google Scholar] [CrossRef]
- Abdelli, N.; Pérez, J.F.; Vilarrasa, E.; Cabeza Luna, I.; Melo-Duran, D.; D’Angelo, M.; Solà-Oriol, D. Targeted-Release Organic Acids and Essential Oils Improve Performance and Digestive Function in Broilers under a Necrotic Enteritis Challenge. Animals 2020, 10, 259. [Google Scholar] [CrossRef]
- Kumar, A.; Toghyani, M.; Kheravii, S.; Pineda, L.; Han, Y.; Swick, R.; Wu, S.-B. Potential of Blended Organic Acids to Improve Performance and Health of Broilers Infected with Necrotic Enteritis. Anim. Nutr. 2021, 7, 440–449. [Google Scholar] [CrossRef]
- Pham, V.H.; Kan, L.; Huang, J.; Geng, Y.; Zhen, W.; Guo, Y.; Abbas, W.; Wang, Z. Dietary Encapsulated Essential Oils and Organic Acids Mixture Improves Gut Health in Broiler Chickens Challenged with Necrotic Enteritis. J. Anim. Sci. Biotechnol. 2020, 11, 18. [Google Scholar] [CrossRef] [PubMed]
- Pham, V.H.; Abbas, W.; Huang, J.; He, Q.; Zhen, W.; Guo, Y.; Wang, Z. Effect of Blending Encapsulated Essential Oils and Organic Acids as an Antibiotic Growth Promoter Alternative on Growth Performance and Intestinal Health in Broilers with Necrotic Enteritis. Poult. Sci. 2022, 101, 101563. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Ni, A.; Jiang, Y.; Li, Y.; Huang, Z.; Shi, L.; Xu, H.; Chen, C.; Li, D.; Han, Y.; et al. Effects of Replacing In-Feed Antibiotics with Synergistic Organic Acids on Growth Performance, Health, Carcass, and Immune and Oxidative Statuses of Broiler Chickens Under Clostridium perfringens Type A Challenge. Avian Dis. 2020, 64, 393–400. [Google Scholar] [CrossRef] [PubMed]
- Diaz Carrasco, J.M.; Redondo, L.M.; Redondo, E.A.; Dominguez, J.E.; Chacana, A.P.; Fernandez Miyakawa, M.E. Use of Plant Extracts as an Effective Manner to Control Clostridium perfringens Induced Necrotic Enteritis in Poultry. BioMed Res. Int. 2016, 2016, 3278359. [Google Scholar] [CrossRef] [PubMed]
- Du, E.; Guo, Y. Dietary Supplementation of Essential Oils and Lysozyme Reduces Mortality and Improves Intestinal Integrity of Broiler Chickens with Necrotic Enteritis. Anim. Sci. J. Nihon Chikusan Gakkaiho 2021, 92, e13499. [Google Scholar] [CrossRef] [PubMed]
- Elizondo, A.M.; Mercado, E.C.; Rabinovitz, B.C.; Fernandez-Miyakawa, M.E. Effect of Tannins on the in Vitro Growth of Clostridium perfringens. Vet. Microbiol. 2010, 145, 308–314. [Google Scholar] [CrossRef]
- Viveros, A.; Chamorro, S.; Pizarro, M.; Arija, I.; Centeno, C.; Brenes, A. Effects of Dietary Polyphenol-Rich Grape Products on Intestinal Microflora and Gut Morphology in Broiler Chicks. Poult. Sci. 2011, 90, 566–578. [Google Scholar] [CrossRef]
- Tosi, G.; Massi, P.; Antongiovanni, M.; Buccioni, A.; Minieri, S.; Marenchino, L.; Mele, M. Efficacy Test of a Hydrolysable Tannin Extract Against Necrotic Enteritis in Challenged Broiler Chickens. Ital. J. Anim. Sci. 2013, 12, e62. [Google Scholar] [CrossRef]
- Kang, M.; Oh, J.-Y.; Cha, S.-Y.; Kim, W.-I.; Cho, H.-S.; Jang, H.-K. Efficacy of Polymers from Spontaneous Carotenoid Oxidation in Reducing Necrotic Enteritis in Broilers. Poult. Sci. 2018, 97, 3058–3062. [Google Scholar] [CrossRef]
- McDougald, L.R.; Cervantes, H.M.; Jenkins, M.C.; Hess, M.; Beckstead, R. Protozoal Infections. In Diseases of Poultry; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2020; pp. 1192–1254. [Google Scholar]
- Williams, R.B. Intercurrent Coccidiosis and Necrotic Enteritis of Chickens: Rational, Integrated Disease Management by Maintenance of Gut Integrity. Avian Pathol. 2005, 34, 159–180. [Google Scholar] [CrossRef]
- Madlala, T.; Okpeku, M.; Adeleke, M.A. Understanding the Interactions between Eimeria Infection and Gut Microbiota, towards the Control of Chicken Coccidiosis: A Review. Parasite 2021, 28, 48. [Google Scholar] [CrossRef] [PubMed]
- Mohsin, M.; Abbas, R.Z.; Yin, G.; Sindhu, Z.-U.-D.; Abbas, A.; Huang, Z.; Aleem, M.T.; Saeed, Z.; Afzal, M.Z.; Ejaz, A. Probiotics as Therapeutic, Antioxidant and Immunomodulatory Agents against Poultry Coccidiosis. Worlds Poult. Sci. J. 2021, 77, 331–345. [Google Scholar] [CrossRef]
- Abdelrahman, W.; Mohnl, M.; Teichmann, K.; Doupovec, B.; Schatzmayr, G.; Lumpkins, B.; Mathis, G. Comparative Evaluation of Probiotic and Salinomycin Effects on Performance and Coccidiosis Control in Broiler Chickens. Poult. Sci. 2014, 93, 3002–3008. [Google Scholar] [CrossRef] [PubMed]
- Ritzi, M.M.; Abdelrahman, W.; van-Heerden, K.; Mohnl, M.; Barrett, N.W.; Dalloul, R.A. Combination of Probiotics and Coccidiosis Vaccine Enhances Protection against an Eimeria Challenge. Vet. Res. 2016, 47, 111. [Google Scholar] [CrossRef]
- Erdoğmuş, S.Z.; Gülmez, N.; Findik, A.; Hüseyin, Ş.; Gülmez, M. Efficacy of Probiotics on Health Status and Growth Performance of Eimeria Tenella Infected Broiler Chickens. Kafkas Üniversitesi Vet. Fakültesi Derg. 2019, 25, 311–320. [Google Scholar]
- Pender, C.M.; Kim, S.; Potter, T.D.; Ritzi, M.M.; Young, M.; Dalloul, R.A. Effects of in Ovo Supplementation of Probiotics on Performance and Immunocompetence of Broiler Chicks to an Eimeria Challenge. Benef. Microbes 2016, 7, 699–705. [Google Scholar] [CrossRef]
- Stringfellow, K.; Caldwell, D.; Lee, J.; Mohnl, M.; Beltran, R.; Schatzmayr, G.; Fitz-Coy, S.; Broussard, C.; Farnell, M. Evaluation of Probiotic Administration on the Immune Response of Coccidiosis-Vaccinated Broilers. Poult. Sci. 2011, 90, 1652–1658. [Google Scholar] [CrossRef]
- Behnamifar, A.R.; Rahimi, S.; Kiaei, M.M.; Fayazi, H. Comparison of the Effect of Probiotic, Prebiotic, Salinomycin and Vaccine in Control of Coccidiosis in Broiler Chickens. Iran. J. Vet. Res. 2019, 20, 51. [Google Scholar]
- Wang, X.; Peebles, E.D.; Kiess, A.S.; Wamsley, K.G.S.; Zhai, W. Effects of Coccidial Vaccination and Dietary Antimicrobial Alternatives on the Growth Performance, Internal Organ Development, and Intestinal Morphology of Eimeria-Challenged Male Broilers. Poult. Sci. 2019, 98, 2054–2065. [Google Scholar] [CrossRef]
- Chand, N.; Faheem, H.; Khan, R.U.; Qureshi, M.S.; Alhidary, I.A.; Abudabos, A.M. Anticoccidial Effect of Mananoligosacharide against Experimentally Induced Coccidiosis in Broiler. Environ. Sci. Pollut. Res. Int. 2016, 23, 14414–14421. [Google Scholar] [CrossRef]
- Khater, H.F.; Ziam, H.; Abbas, A.; Abbas, R.Z.; Raza, M.A.; Hussain, K.; Younis, E.Z.; Radwan, I.T.; Selim, A. Avian Coccidiosis: Recent Advances in Alternative Control Strategies and Vaccine Development. Agrobiol. Rec. 2020, 1, 11–25. [Google Scholar] [CrossRef]
- Abbas, R.Z.; Munawar, S.H.; Manzoor, Z.; Iqbal, Z.; Khan, M.N.; Saleemi, M.K.; Zia, M.A.; Yousaf, A. Anticoccidial Effects of Acetic Acid on Performance and Pathogenic Parameters in Broiler Chickens Challenged with Eimeria Tenella. Pesqui. Veterinária Bras. 2011, 31, 99–103. [Google Scholar] [CrossRef]
- Fortuoso, B.F.; Dos Reis, J.H.; Gebert, R.R.; Barreta, M.; Griss, L.G.; Casagrande, R.A.; de Cristo, T.G.; Santiani, F.; Campigotto, G.; Rampazzo, L. Glycerol Monolaurate in the Diet of Broiler Chickens Replacing Conventional Antimicrobials: Impact on Health, Performance and Meat Quality. Microb. Pathog. 2019, 129, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.M.; Seddiek, S.A.; Khater, H.F. Effect of Butyrate, Clopidol and Their Combination on the Performance of Broilers Infected with Eimeria maxima. Br. Poult. Sci. 2014, 55, 474–482. [Google Scholar] [CrossRef] [PubMed]
- Aristimunha, P.C.; Rosa, A.P.; Boemo, L.S.; Garcez, D.C.; Rosa, D.P.; Londero, A.; Scher, A.; Forgiarini, J. A Blend of Benzoic Acid and Essential Oil Compounds as an Alternative to Antibiotic Growth Promoters in Broiler Diets. J. Appl. Poult. Res. 2016, 25, 455–463. [Google Scholar] [CrossRef]
- El-Shall, N.A.; Abd El-Hack, M.E.; Albaqami, N.M.; Khafaga, A.F.; Taha, A.E.; Swelum, A.A.; El-Saadony, M.T.; Salem, H.M.; El-Tahan, A.M.; AbuQamar, S.F.; et al. Phytochemical Control of Poultry Coccidiosis: A Review. Poult. Sci. 2022, 101, 101542. [Google Scholar] [CrossRef] [PubMed]
- Georgieva, N.V.; Gabrashanska, M.; Koinarski, V.; Ermidou-Pollet, S. Antioxidant Status in Eimeria Acervulina Infected Chickens after Dietary Selenium Treatment. Trace Elem. Electrolytes 2011, 28, 42. [Google Scholar] [CrossRef]
- Georgieva, N.V.; Gabrashanska, M.; Koinarski, V.; Yaneva, Z. Zinc Supplementation against Eimeria Acervulina-Induced Oxidative Damage in Broiler Chickens. Vet. Med. Int. 2011, 2011, 647124. [Google Scholar] [CrossRef]
- Galli, G.M.; Gerbet, R.R.; Griss, L.G.; Fortuoso, B.F.; Petrolli, T.G.; Boiago, M.M.; Souza, C.F.; Baldissera, M.D.; Mesadri, J.; Wagner, R. Combination of Herbal Components (Curcumin, Carvacrol, Thymol, Cinnamaldehyde) in Broiler Chicken Feed: Impacts on Response Parameters, Performance, Fatty Acid Profiles, Meat Quality and Control of Coccidia and Bacteria. Microb. Pathog. 2020, 139, 103916. [Google Scholar] [CrossRef]
- Perez-Carbajal, C.; Caldwell, D.; Farnell, M.; Stringfellow, K.; Pohl, S.; Casco, G.; Pro-Martinez, A.; Ruiz-Feria, C.A. Immune Response of Broiler Chickens Fed Different Levels of Arginine and Vitamin E to a Coccidiosis Vaccine and Eimeria Challenge. Poult. Sci. 2010, 89, 1870–1877. [Google Scholar] [CrossRef]
- Khan, R.U.; Naz, S.; Javdani, M.; Nikousefat, Z.; Selvaggi, M.; Tufarelli, V.; Laudadio, V. The Use of Turmeric (Curcuma longa) in Poultry Feed. Worlds Poult. Sci. J. 2012, 68, 97–103. [Google Scholar] [CrossRef]
- Sharma, J.M.; Burmester, B.R. Disease Control in Avian Species by Embryonal Vaccination. U.S. Patent 4,458,630, 10 July 1984. [Google Scholar]
- Peebles, E.D. In Ovo Applications in Poultry: A Review. Poult. Sci. 2018, 97, 2322–2338. [Google Scholar] [CrossRef]
- Uni, Z.; Ferket, P.R. Enhancement of Development of Oviparous Species by in Ovo Feeding. U.S. Patent 6,592,878 B2, 15 July 2003. [Google Scholar]
- Jha, R.; Singh, A.K.; Yadav, S.; Berrocoso, J.F.D.; Mishra, B. Early Nutrition Programming (in Ovo and Post-Hatch Feeding) as a Strategy to Modulate Gut Health of Poultry. Front. Vet. Sci. 2019, 6, 82. [Google Scholar] [CrossRef] [PubMed]
- Tako, E.; Glahn, R.P.; Knez, M.; Stangoulis, J.C. The Effect of Wheat Prebiotics on the Gut Bacterial Population and Iron Status of Iron Deficient Broiler Chickens. Nutr. J. 2014, 13, 1–10. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, J.E.; Van der Hoeven-Hangoor, E.; Van de Linde, I.B.; Montijn, R.C.; Van der Vossen, J. In Ovo Inoculation of Chicken Embryos with Probiotic Bacteria and Its Effect on Posthatch Salmonella Susceptibility. Poult. Sci. 2014, 93, 818–829. [Google Scholar] [CrossRef] [PubMed]
- Madej, J.P.; Stefaniak, T.; Bednarczyk, M. Effect of in Ovo-Delivered Prebiotics and Synbiotics on Lymphoid-Organs’ Morphology in Chickens. Poult. Sci. 2015, 94, 1209–1219. [Google Scholar] [CrossRef] [PubMed]
- Nurmi, E.; Rantala, M. New Aspects of Salmonella Infection in Broiler Production. Nature 1973, 241, 210–211. [Google Scholar] [CrossRef] [PubMed]
- Hosseini-Mansoub, N.; Vahdatpour, T.; Arjomandi, M.; Vahdatpour, S. Comparison of Different Methods of Probiotic Prescription against Salmonella Infection in Hatchery Broiler Chickens. Adv. Envrion. Biol 2011, 5, 1857–1860. [Google Scholar]
- Teague, K.D.; Graham, L.E.; Dunn, J.R.; Cheng, H.H.; Anthony, N.; Latorre, J.D.; Menconi, A.; Wolfenden, R.E.; Wolfenden, A.D.; Mahaffey, B.D. In Ovo Evaluation of FloraMax®-B11 on Marek’s Disease HVT Vaccine Protective Efficacy, Hatchability, Microbiota Composition, Morphometric Analysis, and Salmonella Enteritidis Infection in Broiler Chickens. Poult. Sci. 2017, 96, 2074–2082. [Google Scholar] [CrossRef]
- Silva, I.G.O.; Vellano, I.H.B.; Moraes, A.C.; Lee, I.M.; Alvarenga, B.; Milbradt, E.L.; Hataka, A.; Okamoto, A.S.; Andreatti, R.L. Evaluation of a Probiotic and a Competitive Exclusion Product Inoculated in Ovo on Broiler Chickens Challenged with Salmonella Heidelberg. Braz. J. Poult. Sci. 2017, 19, 19–26. [Google Scholar] [CrossRef][Green Version]
- Badri, F.B.A.; Amin, S.A. Influence of in ovo inoculation of bifidobacteria on productive performance, antioxidant and immune status and gut microfloraof broiler chickens. Egypt. J. Nutr. Feed. 2017, 20, 267–276. [Google Scholar] [CrossRef][Green Version]
- Lee, S.H.; Lillehoj, H.S.; Jang, S.I.; Jeong, M.S.; Xu, S.Z.; Kim, J.B.; Park, H.J.; Kim, H.R.; Lillehoj, E.P.; Bravo, D.M. Effects of in Ovo Injection with Selenium on Immune and Antioxidant Responses during Experimental Necrotic Enteritis in Broiler Chickens. Poult. Sci. 2014, 93, 1113–1121. [Google Scholar] [CrossRef] [PubMed]
- Stadnicka, K.; Bogucka, J.; Stanek, M.; Graczyk, R.; Krajewski, K.; Maiorano, G.; Bednarczyk, M. Injection of Raffinose Family Oligosaccharides at 12 Days of Egg Incubation Modulates the Gut Development and Resistance to Opportunistic Pathogens in Broiler Chickens. Animals 2020, 10, 592. [Google Scholar] [CrossRef] [PubMed]
- Angwech, H.; Tavaniello, S.; Ongwech, A.; Kaaya, A.N.; Maiorano, G. Efficacy of In Ovo Delivered Prebiotics on Growth Performance, Meat Quality and Gut Health of Kuroiler Chickens in the Face of a Natural Coccidiosis Challenge. Animals 2019, 9, 876. [Google Scholar] [CrossRef] [PubMed]
- Fatemi, S.A.; Elliott, K.E.C.; Bello, A.; Peebles, E.D. Effects of the in Ovo Injection of Vitamin D3 and 25-Hydroxyvitamin D3 in Ross 708 Broilers Subsequently Challenged with Coccidiosis. I. Performance, Meat Yield and Intestinal Lesion Incidence1,2,3. Poult. Sci. 2021, 100, 101382. [Google Scholar] [CrossRef] [PubMed]
| Compounds | Definition | Origin |
|---|---|---|
| Nutraceutical | A food (or its part) that provides medical or health benefits, including the prevention and/or treatment of a disease [17] | Plant or animal |
| Phytonutrient | Plant derived compound [18] | Plant |
| Phytochemical | A variety of plant-derived compounds with therapeutic activities such as anticarcinogenic, antimutagenic, anti-inflammatory, and antioxidant [19] | Plant |
| Bioactive Compound | Components in foods or dietary supplements, other than those necessary to the basic nutritional needs, which are responsible for changes in health status [20] | Plant or animal |
| Items | Definition | Mechanism of Action |
|---|---|---|
| Probiotics | Live microorganisms which, when administered in adequate amounts, confer a health benefit on the host [21] | Competitive exclusion Production of antimicrobial substances Stimulation of immune system Increased intestinal absorption surface Increased growth performance and feed intake Modulation of respiratory and GI microbiota [22,23,24,25,26,27,28,29,30,31] |
| Prebiotics | A nondigestible compound that, through its metabolization by microorganisms in the gut, modulates composition and/or activity of the gut microbiota, thus conferring a beneficial physiological effect on the host [32] | Nutrient source for the selective growth of beneficial bacteria of the intestinal microbiota Stimulation of short-chain fatty acids production Inhibition of bacterial adhesion to gut lining Change in mucin production Immunity boost Improvement in intestinal health and functionality. [15,33,34,35,36] |
| Vitamins | Vitamins are nutritional elements which are necessary for essential activities such as development, growth, and metabolism of cells [37] | Antioxidant effect Reduction in free radicals Increase in mucosal immunity Anti-inflammatory effect Immunostimulatory effects Increase in cellular immunity [37,38,39,40,41,42] |
| Phytogenic feed additives (or Phytobiotics) | Compounds of plant origin incorporated into animal feed to enhance livestock productivity through the improvement of digestibility, nutrient absorption, and elimination of intestinal pathogens [43] | Increase in growth performance, nutrient digestibility and gut health Introduction into the cell membrane of pathogens and consequent destruction with consequent ions leakage Antioxidant activity Modulation of intestinal microbiota composition [44,45,46,47,48,49] |
| Organic acids | Primarily composed of short-chain fatty acids (SCFA), also commonly referred to as volatile short-chain fatty acids (VSCFA), such as fumaric, propionic, acetic, lactic, butyric, and others. Other organic acids consist of medium-chain fatty acids (MCFA), and long-chain fatty acids (LCFA) [50] | Lowering pH of GI tract (reduction in acid sensitive bacteria) Potential for incorporation into cell membranes of target cells and promoting the loss of protons or cell ions (such as in Gram-positive bacteria) Promotion of gut health and performance [10,51,52,53,54] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Biagini, L.; Galosi, L.; Roncarati, A.; Attili, A.-R.; Mangiaterra, S.; Rossi, G. The Role of Nutraceuticals and Phytonutrients in Chickens’ Gastrointestinal Diseases. Animals 2022, 12, 892. https://doi.org/10.3390/ani12070892
Biagini L, Galosi L, Roncarati A, Attili A-R, Mangiaterra S, Rossi G. The Role of Nutraceuticals and Phytonutrients in Chickens’ Gastrointestinal Diseases. Animals. 2022; 12(7):892. https://doi.org/10.3390/ani12070892
Chicago/Turabian StyleBiagini, Lucia, Livio Galosi, Alessandra Roncarati, Anna-Rita Attili, Sara Mangiaterra, and Giacomo Rossi. 2022. "The Role of Nutraceuticals and Phytonutrients in Chickens’ Gastrointestinal Diseases" Animals 12, no. 7: 892. https://doi.org/10.3390/ani12070892
APA StyleBiagini, L., Galosi, L., Roncarati, A., Attili, A.-R., Mangiaterra, S., & Rossi, G. (2022). The Role of Nutraceuticals and Phytonutrients in Chickens’ Gastrointestinal Diseases. Animals, 12(7), 892. https://doi.org/10.3390/ani12070892








