A Case of XX Disorder of Sexual Development in a Female-Phenotype Roe Deer (Capreolus capreolus L.) Associated with Antlers Growth with Retained Velvet
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal
2.2. Computed Tomography Study
2.3. Molecular Study
2.4. Histopathological Study
3. Results
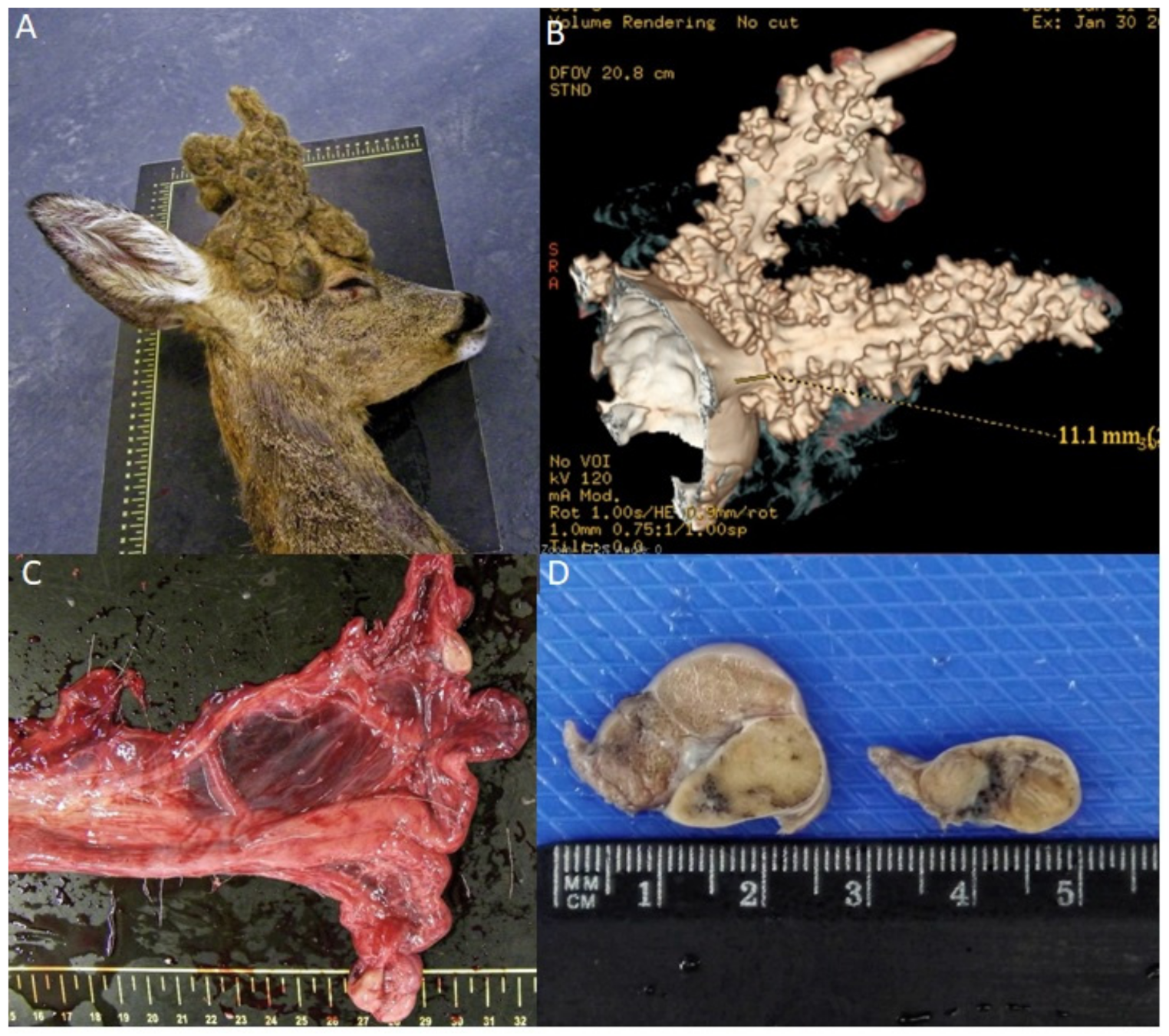
3.1. Age Determination and Clinical Evaluation
3.2. CT Study
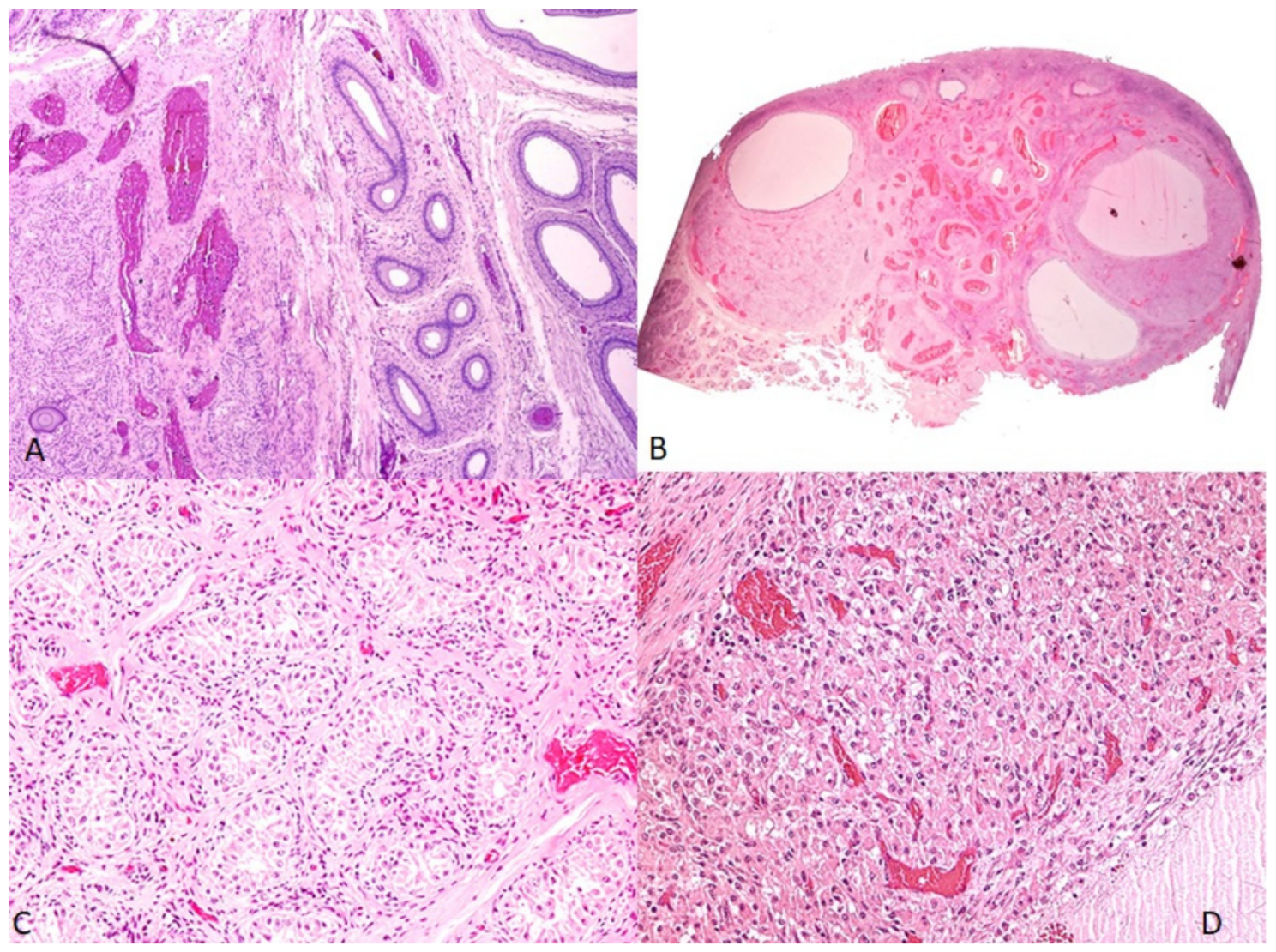
3.3. Histopathological Study
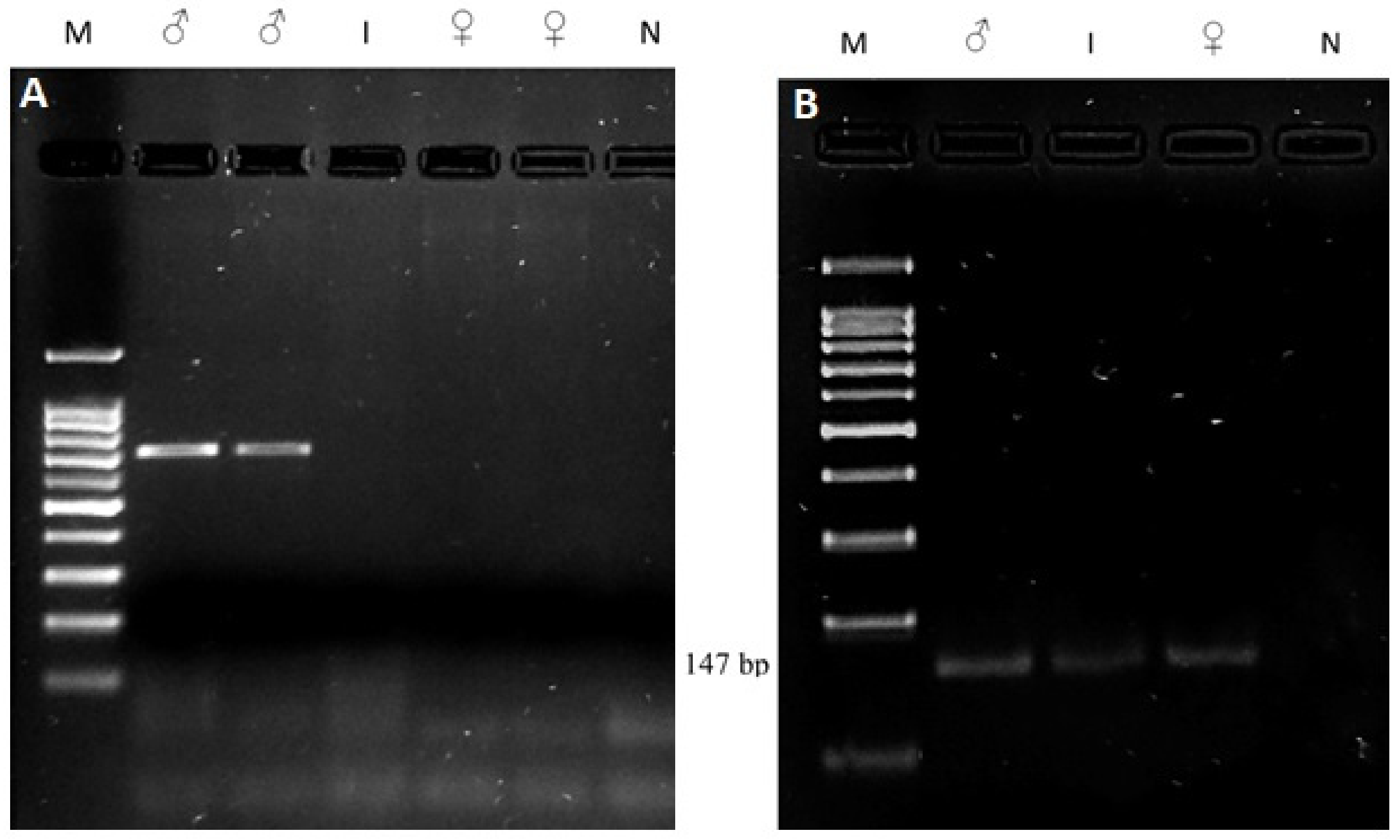
3.4. Molecular Study
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mysterud, A.; Østbye, E. The frequency of antlered female and antlerless male roe deer (Capreolus capreolus) in a population in south-east Norway. Z. Jagdwiss. 1999, 45, 208–211. [Google Scholar] [CrossRef]
- Flis, M. Does with antlers, ie intersex in roe deer (Capreolus capreolus L.)—Description of cases. Ann. UMCS Zootech. 2012, 30, 1–8. [Google Scholar] [CrossRef]
- Sempéré, A.J.; Boissin, J. Relationship between antler development and plasma androgen concentrations in adult roe deer (Capreolus capreolus). J. Reprod. Fertil. 1981, 62, 49–53. [Google Scholar] [CrossRef] [PubMed]
- Bartoš, L.; Bubenik, G.A.; Tománek, M. The role of adrenal androgens in antler growth of castrated fallow deer (Dama dama): Possible stimulation of adrenal secretion of testosterone by stress. In Antler Science and Product Technology; Suttie, J.M., Haines, S.R., Li, C., Eds.; AgResearch, Invermay Agricultural Centre: Mosgiel, New Zealand, 2004; pp. 45–53. [Google Scholar]
- Quinn, A.; Koopman, P. The molecular genetics of sex determination and sex reversal in mammals. Semin. Reprod. Med. 2012, 30, 351–363. [Google Scholar] [CrossRef] [PubMed]
- Vaiman, D.; Pailhoux, E. Mammalian sex reversal and intersexuality: Deciphering the sex-determination cascade. Trends Genet. 2000, 16, 488–494. [Google Scholar] [CrossRef]
- Christensen, B.W. Disorders of sexual development in dogs and cats. Veter.-Clin. N. Am. Small Anim. Pract. 2012, 42, 515–526. [Google Scholar] [CrossRef] [PubMed]
- Mastromonaco, G.F.; Houck, M.L.; Bergfelt, D.R. Disorders of sexual development in wild and captive exotic animals. Sex. Dev. 2012, 6, 84–95. [Google Scholar] [CrossRef]
- Pajares, G.; Balseiro, A.; Pérez-Pardal, L.; Gamarra, J.A.; Monteagudo, L.V.; Goyache, F.; Royo, L.J. SRY-negative XX true hermaphroditism in a roe deer. Anim. Reprod. Sci. 2009, 112, 190–197. [Google Scholar] [CrossRef]
- Kropatsch, R.; Dekomien, G.; Akkad, D.A.; Gerding, W.M.; Petrasch-Parwez, E.; Young, N.D.; Altmüller, J.; Nürnberg, P.; Gasser, R.B.; Epplen, J.T. SOX9 duplication linked to intersex in deer. PLoS ONE 2013, 8, e73734. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stévant, I.; Nef, S. Genetic control of gonadal sex determination and development. Trends Genet. 2019, 35, 346–358. [Google Scholar] [CrossRef]
- Miyawaki, S.; Tachibana, M. Role of epigenetic regulation in mammalian sex determination. Curr. Top. Dev. Biol. 2019, 134, 195–221. [Google Scholar] [CrossRef]
- Monteagudo, L.V.; Arruga, M.V.; Bonafonte, J.I.; Ordás, M.; Whyte, A.; Gallego, M.; Bascuas, J.A.; Sierra, I. Bilateral Leydig cell tumour in a six-year-old intersex goat affected by Polled Intersex Syndrome. Veter. Pathol. 2008, 45, 42–45. [Google Scholar] [CrossRef]
- Pailhoux, E.; Vigier, B.; Chaffaux, S.; Servel, N.; Taourit, S.; Furet, J.P.; Fellous, M.; Grosclaude, F.; Cribiu, E.P.; Cotinot, C.; et al. A 11.7-kb deletion triggers intersexuality and polledness in goats. Nat. Genet. 2001, 29, 453–458. [Google Scholar] [CrossRef] [PubMed]
- Simon, R.; Lischer, H.E.L.; Pieńkowska-Schelling, A.; Keller, I.; Häfliger, I.M.; Letko, A.; Schelling, C.; Lühken, G.; Drögemüller, C. New genomic features of the polled intersex syndrome variant in goats unraveled by long-read whole-genome sequencing. Anim. Genet. 2020, 51, 439–448. [Google Scholar] [CrossRef] [PubMed]
- Fernández, M.C.; Markina, F.; Garnica, R. Un método simple para la determinación de las diferentes clases de edad del corzo. Bol. Asc. Corzo Español. 2003, 5, 36–42. (In Spanish) [Google Scholar]
- Meadows, J.R.S.; Hawken, R.J.; Kijas, J.W. Nucleotide diversity on the ovine Y chromosome. Anim. Genet. 2004, 35, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Meyers-Wallen, V.N.; Patterson, D.F. Sexual differentiation and inherited disorders of sexual development in the dog. J. Reprod. Fertil. Suppl. 1989, 39, 57–64. [Google Scholar]
- Aruna, N.; Purushottam, R.M.; Sayee, R. 46,XX/46, XY chimerism—A case report. J. Anat. Soc. 2006, 55, 24–26. [Google Scholar]
| Amplicon | Primer Sequence | Expected | Reference |
|---|---|---|---|
| SRY | F: 5′-ctgctatgttcagagtattg-3′ R: 5′-tcaatattgaacataagcgc-3′ | 528 bp fragment, if male | [17] |
| PIS | F: 5′-ttccactgcttttggtgtgt-3′ R: 5′-aacaagagaggtgccctgaa-3′ | 147 bp fragment: PIS-negative No amplification: PIS-positive | [13] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, F.; Pires, I.; Prada, J.; Queirós, M.; Monzón, A.; Almeida, J.; Sargo, R.; Loureiro, F.; Sousa, L.; Valente, J.; et al. A Case of XX Disorder of Sexual Development in a Female-Phenotype Roe Deer (Capreolus capreolus L.) Associated with Antlers Growth with Retained Velvet. Animals 2022, 12, 865. https://doi.org/10.3390/ani12070865
Silva F, Pires I, Prada J, Queirós M, Monzón A, Almeida J, Sargo R, Loureiro F, Sousa L, Valente J, et al. A Case of XX Disorder of Sexual Development in a Female-Phenotype Roe Deer (Capreolus capreolus L.) Associated with Antlers Growth with Retained Velvet. Animals. 2022; 12(7):865. https://doi.org/10.3390/ani12070865
Chicago/Turabian StyleSilva, Filipe, Isabel Pires, Justina Prada, Miguel Queirós, Aurora Monzón, José Almeida, Roberto Sargo, Filipa Loureiro, Luís Sousa, Joana Valente, and et al. 2022. "A Case of XX Disorder of Sexual Development in a Female-Phenotype Roe Deer (Capreolus capreolus L.) Associated with Antlers Growth with Retained Velvet" Animals 12, no. 7: 865. https://doi.org/10.3390/ani12070865
APA StyleSilva, F., Pires, I., Prada, J., Queirós, M., Monzón, A., Almeida, J., Sargo, R., Loureiro, F., Sousa, L., Valente, J., Viegas, C., Ginja, M., Bastos, E., Martins-Bessa, A., & Dias, I. (2022). A Case of XX Disorder of Sexual Development in a Female-Phenotype Roe Deer (Capreolus capreolus L.) Associated with Antlers Growth with Retained Velvet. Animals, 12(7), 865. https://doi.org/10.3390/ani12070865









