Thermal and Circulatory Changes in Diverse Body Regions in Dogs and Cats Evaluated by Infrared Thermography
Abstract
:Simple Summary
Abstract
1. Introduction
2. Clinical Usefulness of IRT with Dogs and Cats
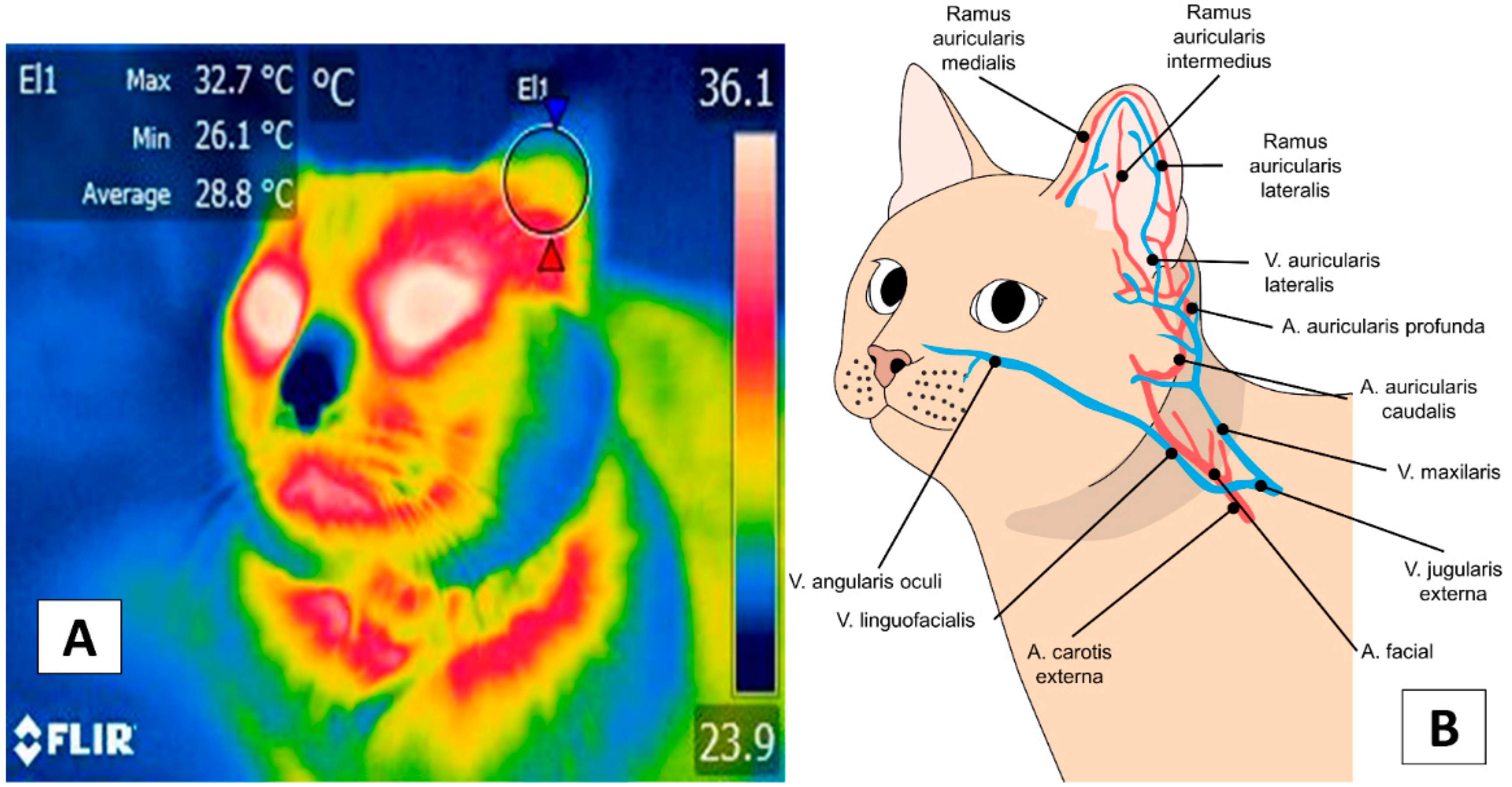
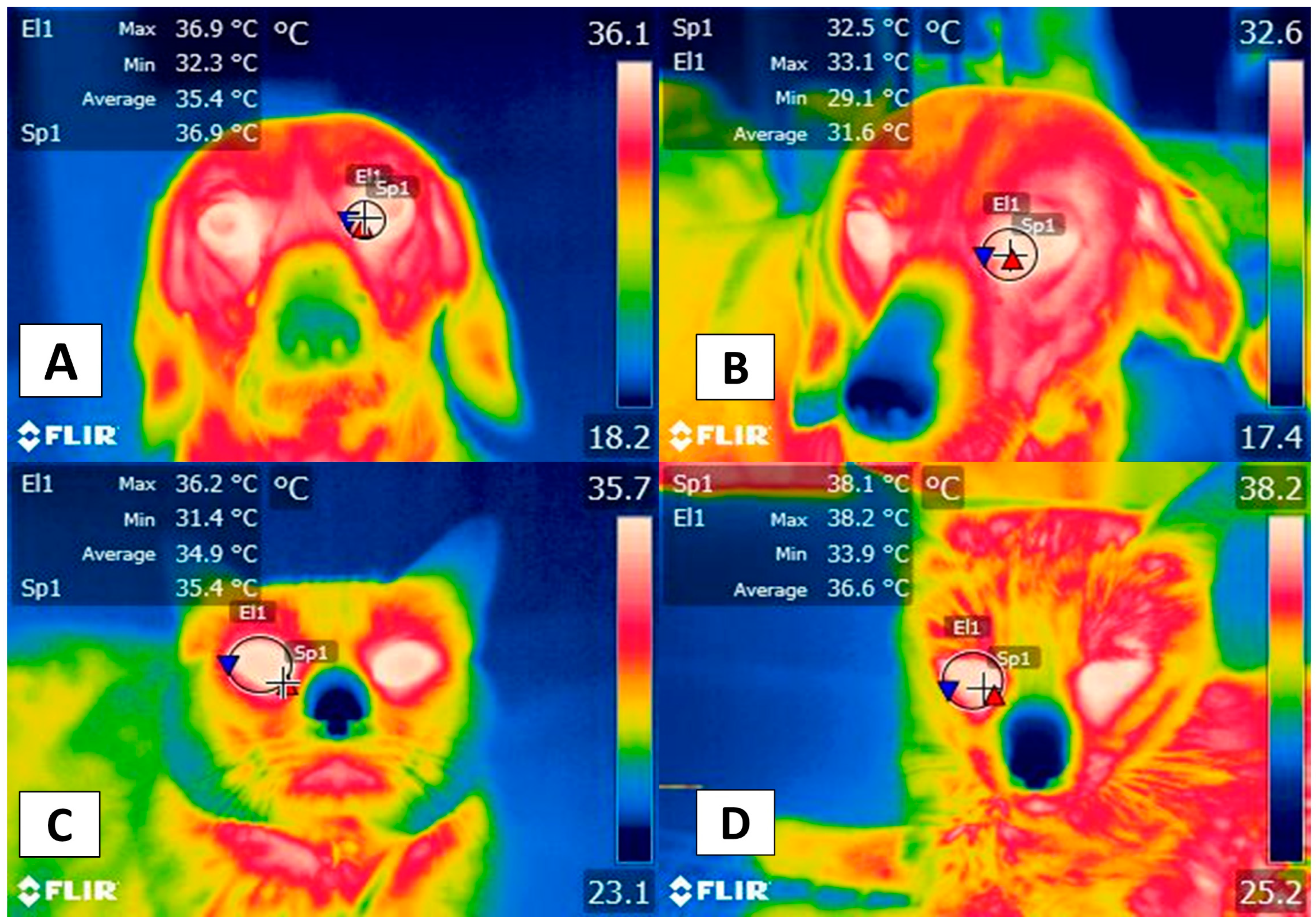
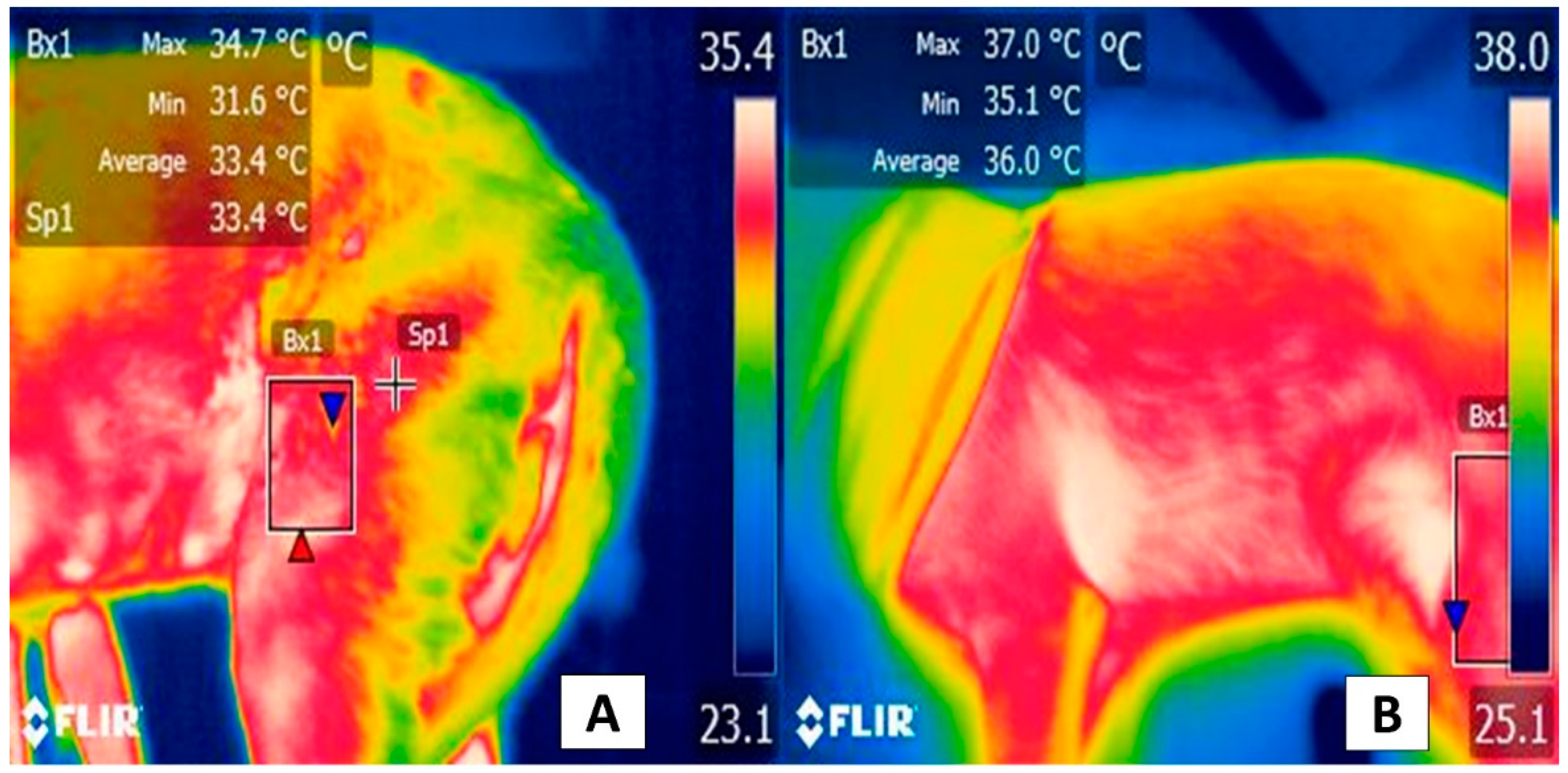
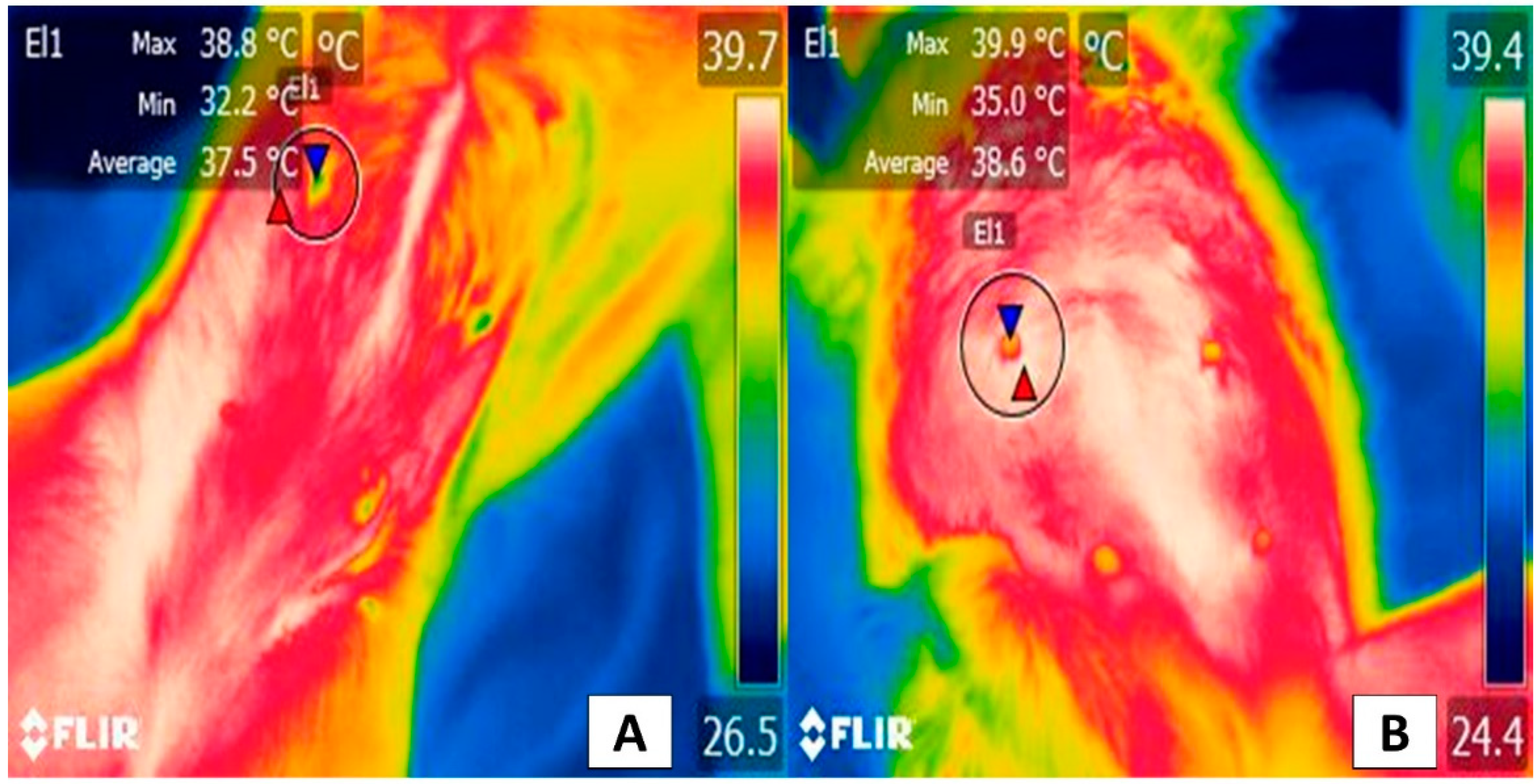
3. Facial Thermal Windows
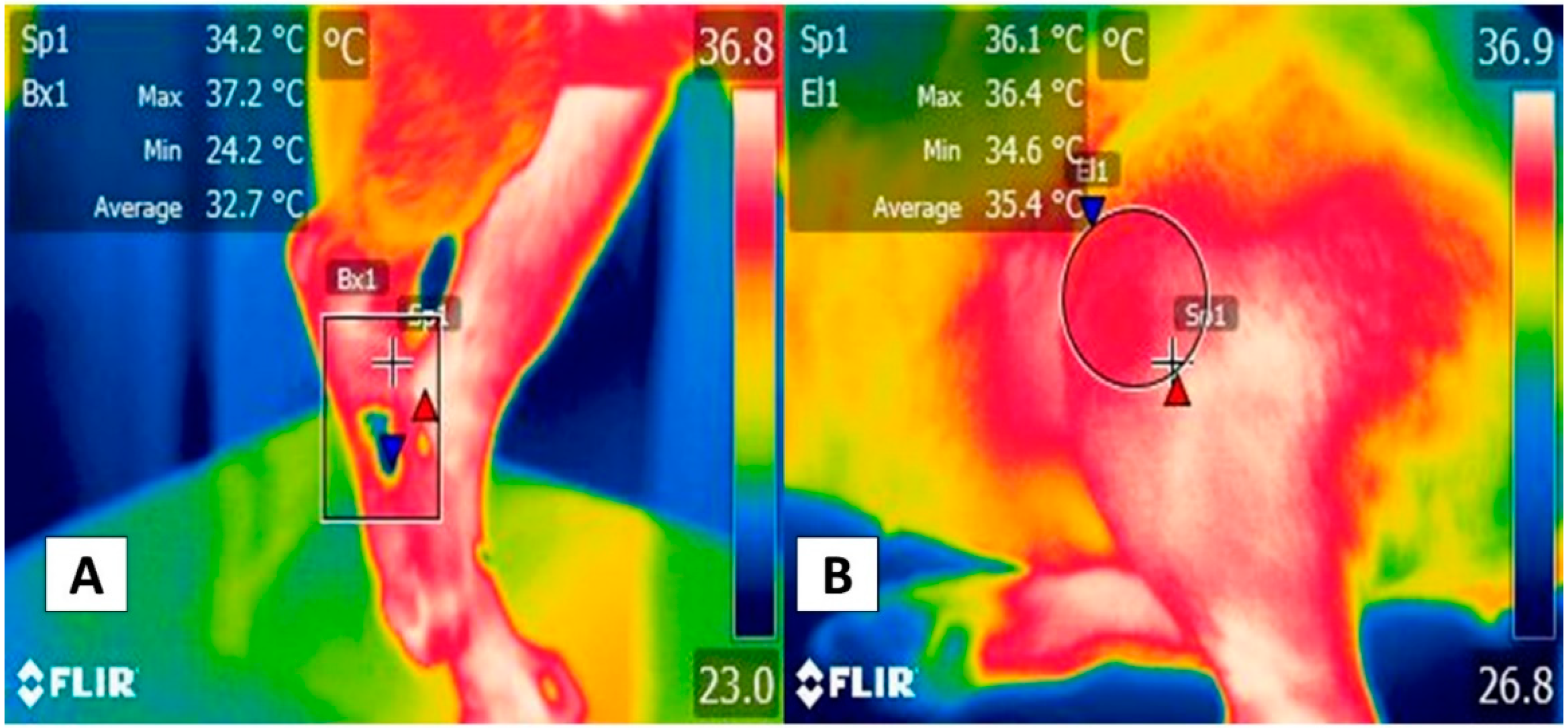
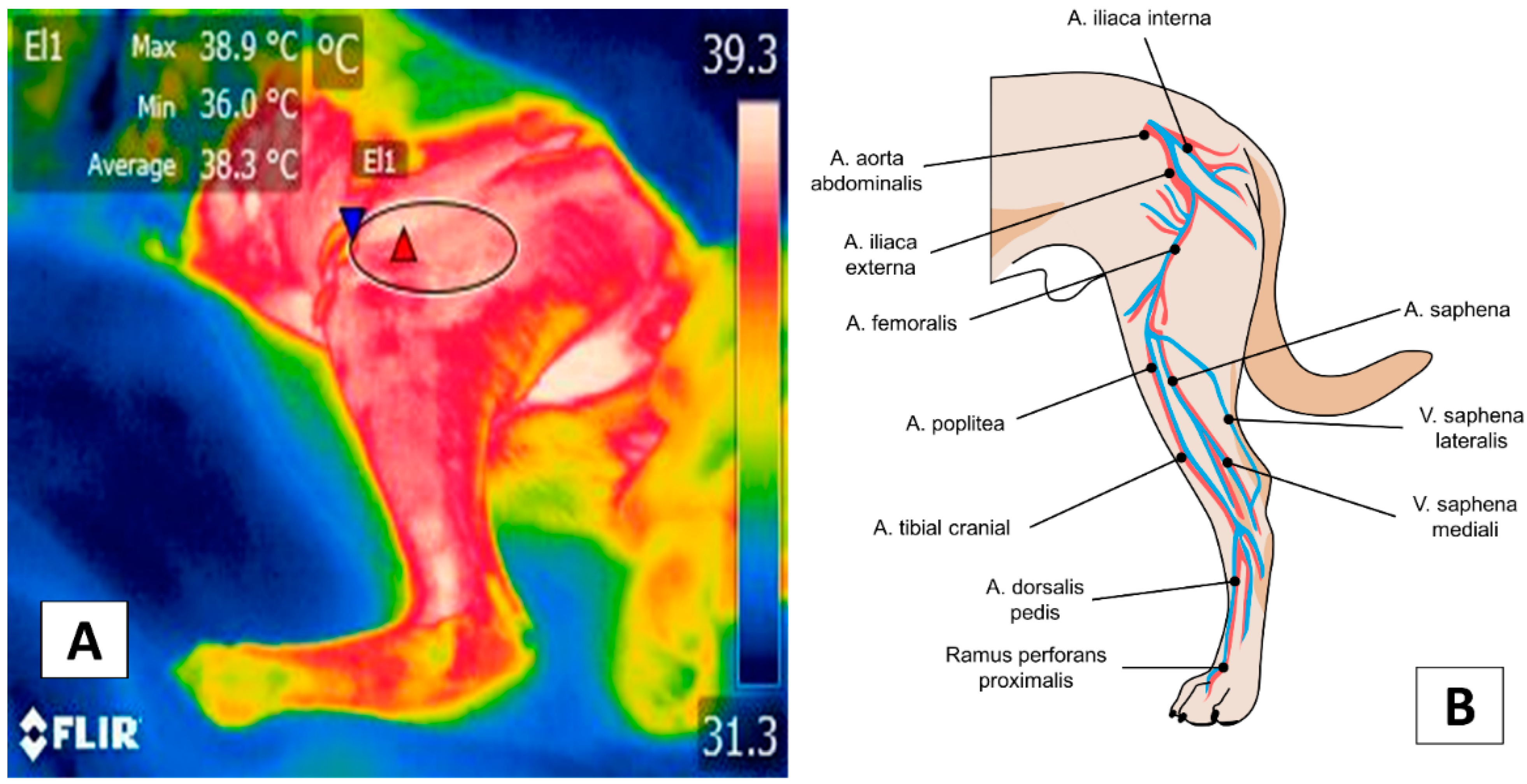
4. Appendicular Windows
5. Body Windows
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Aquino Rocha, J.H.; Vieira Póvoas Tavares, Y. Infrared thermography as a non-destructive test for the inspection of reinforced concrete bridges: A review of the state of the art. Rev. ALCONPAT 2017, 7, 200–214. [Google Scholar] [CrossRef] [Green Version]
- Casas-Alvarado, A.; Mota-Rojas, D.; Hernández-Ávalos, I.; Mora-Medina, P.; Olmos-Hernández, A.; Verduzco-Mendoza, A.; Reyes-Sotelo, B.; Martínez-Burnes, J. Advances in infrared thermography: Surgical aspects, vascular changes, and pain monitoring in veterinary medicine. J. Therm. Biol. 2020, 92, 102664. [Google Scholar] [CrossRef] [PubMed]
- Childs, C. Body temperature and clinical thermometry. In Thermoregulation: From Basic Neuroscience to Clinical Neurology, Part II; Romanovsky, A.A., Ed.; Elsevier: Edinburg, Scotland, 2018; pp. 467–482. [Google Scholar]
- Lezama-García, K.; Mota-Rojas, D.; Pereira, A.M.F.; Martínez-Burnes, J.; Ghezzi, M.; Domínguez, A.; Gómez, J.; de Mira Geraldo, A.; Lendez, P.; Hernández-Ávalos, I.; et al. Transient Receptor Potential (TRP) and thermoregulation in animals: Structural biology and neurophysiological aspects. Animals 2022, 12, 106. [Google Scholar] [CrossRef] [PubMed]
- Lush, J.; Ijichi, C. A Preliminary investigation into personality and pain in dogs. J. Vet. Behav. 2018, 24, 62–68. [Google Scholar] [CrossRef] [Green Version]
- Travain, T.; Colombo, E.S.; Grandi, L.C.; Heinzl, E.; Pelosi, A.; Prato Previde, E.; Valsecchi, P. How good is this food? A study on dogs’ emotional responses to a potentially pleasant event using infrared thermography. Physiol. Behav. 2016, 159, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Sotelo, B.; Mota-Rojas, D.; Martínez-Burnes, J.; Olmos-Hernández, A.; Hernández-Ávalos, I.; José, N.; Casas-Alvarado, A.; Gómez, J.; Mora-Medina, P. Thermal homeostasis in the newborn puppy: Behavioral and physiological responses. J. Anim. Behav. Biometeorol. 2021, 9, 1–25. [Google Scholar] [CrossRef]
- Travain, T.; Colombo, E.S.; Heinzl, E.; Bellucci, D.; Prato Previde, E.; Valsecchi, P. Hot dogs: Thermography in the assessment of stress in dogs (Canis familiaris)—A pilot study. J. Vet. Behav. 2015, 10, 17–23. [Google Scholar] [CrossRef]
- Huggins, J.; Rakobowchuk, M. Utility of lacrimal caruncle infrared thermography when monitoring alterations in autonomic activity in healthy humans. Eur. J. Appl. Physiol. 2019, 119, 531–538. [Google Scholar] [CrossRef]
- Stewart, M.; Stookey, J.M.; Stafford, K.J.; Tucker, C.B.; Rogers, A.R.; Dowling, S.K.; Verkerk, G.A.; Schaefer, A.L.; Webster, J.R. Effects of local anesthetic and a nonsteroidal antiinflammatory drug on pain responses of dairy calves to hot-iron dehorning. J. Dairy Sci. 2009, 92, 1512–1519. [Google Scholar] [CrossRef]
- Alves, J.C.A.; dos Santos, A.M.M.P.; Jorge, P.I.F.; Branco Lavrador, C.F.T.V.; Carreira, L.M. Thermographic imaging of police working dogs with bilateral naturally occurring hip osteoarthritis. Acta Vet. Scand. 2020, 62, 60. [Google Scholar] [CrossRef]
- Wang, F.-K.; Shih, J.-Y.; Juan, P.-H.; Su, Y.-C.; Wang, Y.-C. Non-invasive cattle body temperature measurement using infrared thermography and auxiliary sensor. Sensors 2021, 21, 2425. [Google Scholar] [CrossRef] [PubMed]
- Kwon, C.J.; Brundage, C.M. Quantifying body surface temperature differences in canine coat types using infrared thermography. J. Therm. Biol. 2019, 82, 18–22. [Google Scholar] [CrossRef]
- Tattersall, G.J. Infrared thermography: A non-invasive window into thermal physiology. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2016, 202, 78–98. [Google Scholar] [CrossRef] [PubMed]
- Loughin, C.A.; Marino, D.J. Evaluation of thermographic imaging of the limbs of healthy dogs. Am. J. Vet. Res. 2007, 68, 1064–1069. [Google Scholar] [CrossRef] [PubMed]
- Wan-Tae, K.; Min-Su, K.; Sun Young, K.; Kang-Moon, S.; Tchi-Chou, N. Use of digital infrared thermography on experimental spinal cord compression in dogs. J. Vet. Clin. 2005, 22, 302–308. [Google Scholar]
- Rekant, S.I.; Lyons, M.A.; Pacheco, J.M.; Arzt, J.; Rodriguez, L.L. Veterinary applications of infrared thermography. Am. J. Vet. Res. 2016, 77, 98–107. [Google Scholar] [CrossRef]
- Mota-Rojas, D.; Miranda- Córtes, A.; Casas-Alvarado, A.; Mora-Medina, P.; Boscato, L.; Hernández-Ávalos, I. Neurobiology and modulation of stress- induced hyperthermia and fever in animals. Abanico Vet. 2021, 11, 1–17. [Google Scholar]
- Schaefer, A.L.; Cook, N.; Tessaro, S.V.; Deregt, D.; Desroches, G.; Dubeski, P.L.; Tong, A.K.W.; Godson, D.L. Early detection and prediction of infection using infrared thermography. Can. J. Anim. Sci. 2004, 84, 73–80. [Google Scholar] [CrossRef]
- Sung, J.; Loughin, C.; Marino, D.; Leyva, F.; Dewey, C.; Umbaugh, S.; Lesser, M. Medical infrared thermal imaging of canine appendicular bone neoplasia. BMC Vet. Res. 2019, 15, 430. [Google Scholar] [CrossRef]
- Pavelski, M.; Silva, D.M.; Leite, N.C.; Junior, D.A.; de Sousa, R.; Guérios, S.D.; Dornbusch, P.T. Infrared thermography in dogs with mammary tumors and healthy dogs. J. Vet. Intern. Med. 2015, 29, 1578–1583. [Google Scholar] [CrossRef]
- Zanghi, B.M. Eye and ear temperature using infrared thermography are related to rectal temperature in dogs at rest or with exercise. Front. Vet. Sci. 2016, 3, 111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nääs, I.A.; Garcia, R.G.; Caldara, F.R. Infrared thermal image for assessing animal health and welfare. J. Anim. Behav. Biometeorol. 2014, 2, 66–72. [Google Scholar] [CrossRef] [Green Version]
- Quimby, J.M.; Smith, M.L.; Lunn, K.F. Evaluation of the effects of hospital visit stress on physiologic parameters in the cat. J. Feline Med. Surg. 2011, 13, 733–737. [Google Scholar] [CrossRef] [PubMed]
- Polit, M.; Rząsa, A.; Rafajłowicz, W.; Niżański, W. Infrared technology for estrous detection in Chinchilla lanigera. Anim. Reprod. Sci. 2018, 197, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Elias, B.; Starling, M.; Wilson, B.; McGreevy, P. Influences on infrared thermography of the canine eye in relation to the stress and arousal of racing greyhounds. Animals 2021, 11, 103. [Google Scholar] [CrossRef] [PubMed]
- Mota-Rojas, D.; Pereira, A.M.F.; Wang, D.; Martínez-Burnes, J.; Ghezzi, M.; Hernández-Avalos, I.; Lendez, P.; Mora-Medina, P.; Casas, A.; Olmos-Hernández, A.; et al. Clinical applications and factors involved in validating thermal windows used in infrared thermography in cattle and river buffalo to assess health and productivity. Animals 2021, 11, 2247. [Google Scholar] [CrossRef] [PubMed]
- Hildebrandt, C.; Raschner, C.; Ammer, K. An overview of recent application of medical infrared thermography in sports medicine in Austria. Sensors 2010, 10, 4700–4715. [Google Scholar] [CrossRef] [Green Version]
- Van Hoogmoed, L.M.; Snyder, J.R. Use of infrared thermography to detect injections and palmar digital neurectomy in horses. Vet. J. 2002, 164, 129–141. [Google Scholar] [CrossRef]
- Tunley, B.V.; Henson, F.M.D. Reliability and repeatability of thermographic examination and the normal thermographic image of the thoracolumbar region in the horse. Equine Vet. J. 2010, 36, 306–312. [Google Scholar] [CrossRef]
- Mota-Rojas, D.; Olmos-Hernández, A.; Verduzco-Mendoza, A.; Lecona-Butrón, H.; Martínez-Burnes, J.; Mora-Medina, P.; Gómez-Prado, J.; Orihuela, A. Infrared thermal imaging associated with pain in laboratory animals. Exp. Anim. 2021, 70, 1–12. [Google Scholar] [CrossRef]
- Ng, W.K.; Ng, Y.K.; Tan, Y.K. Qualitative study of sexual functioning in couples with erectile dysfunction: Prospective evaluation of the thermography diagnostic system. J. Reprod. Med. 2009, 54, 698–705. [Google Scholar] [PubMed]
- Pouzot-Nevoret, C.; Barthélemy, A.; Goy-Thollot, I.; Boselli, E.; Cambournac, M.; Guillaumin, J.; Bonnet-Garin, J.-M.; Allaouchiche, B. Infrared thermography: A rapid and accurate technique to detect feline aortic thromboembolism. J. Feline Med. Surg. 2018, 20, 780–785. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Avalos, I.; Mota-Rojas, D.; Mendoza-Flores, J.E.; Casas-Alvarado, A.; Flores-Padilla, K.; Miranda-Cortes, A.E.; Torres-Bernal, F.; Gómez-Prado, J.; Mora-Medina, P. Nociceptive pain and anxiety in equines: Physiological and behavioral alterations. Vet. World 2021, in press. [Google Scholar] [CrossRef] [PubMed]
- Bertoni, A.; Mota-Rojas, D.; Álvarez-Macias, A.; Mora-Medina, P.; Guerrero-Legarreta, I.; Morales-Canela, A.; Gómez-Prado, J.; José-Pérez, N.; Martínez-Burnes, J. Scientific findings related to changes in vascular microcirculation using infrared thermography in the river buffalo. J. Anim. Behav. Biometeorol. 2020, 8, 288–297. [Google Scholar] [CrossRef]
- Hankenson, F.C.; Marx, J.O.; Gordon, C.J.; David, J.M. Effects of rodent thermoregulation on animal models in the research environment. Comp. Med. 2018, 68, 425–438. [Google Scholar] [CrossRef]
- Romanovsky, A.A. Skin temperature: Its role in thermoregulation. Acta Physiol. 2014, 210, 498–507. [Google Scholar] [CrossRef]
- Tattersall, G.J.; Cadena, V. Insights into animal temperature adaptations revealed through thermal imaging. Imaging Sci. J. 2010, 58, 261–268. [Google Scholar] [CrossRef]
- Seixas, A.; Ammer, K. Utility of infrared thermography when monitoring autonomic activity. Eur. J. Appl. Physiol. 2019, 119, 1455–1457. [Google Scholar] [CrossRef]
- Sutherland, M.A.; Worth, G.M.; Dowling, S.K.; Lowe, G.L.; Cave, V.M.; Stewart, M. Evaluation of infrared thermography as a non-invasive method of measuring the autonomic nervous response in sheep. PLoS ONE 2020, 15, e0233558. [Google Scholar] [CrossRef]
- Foster, S.; Ijichi, C. The association between infrared thermal imagery of core eye temperature, personality, age and housing in cats. Appl. Anim. Behav. Sci. 2017, 189, 79–84. [Google Scholar] [CrossRef] [Green Version]
- Riemer, S.; Assis, L.; Pike, T.W.; Mills, D.S. Dynamic changes in ear temperature in relation to separation distress in dogs. Physiol. Behav. 2016, 167, 86–91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, D.; Menassa, C.C.; Kamat, V.R. Non-intrusive interpretation of human thermal comfort through analysis of facial infrared thermography. Energy Build. 2018, 176, 246–261. [Google Scholar] [CrossRef]
- Evans, H.E.; de Lahunta, A. Miller’s Anatomy of the Dog, 4th ed.; Elsevier Saunders: St. Louis, MO, USA, 2012; 849p. [Google Scholar]
- International Committee on Veterinary Gross Anatomical Nomenclature. Nomina Anatomica Veterinaria; World Association of Veterinary Anatomists: Hanover, Germany, 2017; pp. 1–178. [Google Scholar]
- Cannas, S.; Palestrini, C.; Canali, E.; Cozzi, B.; Ferri, N.; Heinzl, E.; Minero, M.; Chincarini, M.; Vignola, G.; Dalla Costa, E. Thermography as a non-invasive measure of stress and fear of humans in sheep. Animals 2018, 8, 146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stewart, M.; Verkerk, G.A.; Stafford, K.J.; Schaefer, A.L.; Webster, J.R. Noninvasive assessment of autonomic activity for evaluation of pain in calves, using surgical castration as a model. J. Dairy Sci. 2010, 93, 3602–3609. [Google Scholar] [CrossRef]
- Lowe, G.; McCane, B.; Sutherland, M.; Waas, J.; Schaefer, A.; Cox, N.; Stewart, M. Automated collection and analysis of infrared thermograms for measuring eye and cheek temperatures in calves. Animals 2020, 10, 292. [Google Scholar] [CrossRef] [Green Version]
- Hudson, L.C.; Hamilton, W.P. Atlas of Feline Anatomy for Veterinarians, 2nd ed.; Teton NewMedia: Jackson, WY, USA, 2010; p. 275. [Google Scholar]
- Całkosiński, I.; Dobrzyński, M.; Rosińczuk, J.; Dudek, K.; Chrószcz, A.; Fita, K.; Dymarek, R. The use of infrared thermography as a rapid, quantitative, and noninvasive method for evaluation of inflammation response in different anatomical regions of rats. BioMed Res. Int. 2015, 2015, 972535. [Google Scholar] [CrossRef]
- Verduzco-Mendoza, A.; Bueno-Nava, A.; Wang, D.; Martínez-Burnes, J.; Olmos-Hernández, A.; Casas, A.; Domínguez, A.; Mota-Rojas, D. Experimental applications and factors involved in validating thermal windows using infrared thermography to assess the health and thermostability of laboratory animals. Animals 2021, 11, 3448. [Google Scholar] [CrossRef]
- Taylor, S.; Webb, L.; Montrose, V.T.; Williams, J. The behavioral and physiological effects of dog appeasing pheromone on canine behavior during separation from the owner. J. Vet. Behav. 2020, 40, 36–42. [Google Scholar] [CrossRef]
- Riemer, S.; Heritier, C.; Windschnurer, I.; Pratsch, L.; Arhant, C.; Affenzeller, N. A review on mitigating fear and aggression in dogs and cats in a veterinary setting. Animals 2021, 11, 158. [Google Scholar] [CrossRef]
- Travain, T.; Valsecchi, P. Infrared thermography in the study of animals’ emotional responses: A critical review. Animals 2021, 11, 2510. [Google Scholar] [CrossRef]
- Casas-Alvarado, A. Evaluación de la Analgesia Epidural de Lidocaína Sola y en Combinación con Opioides en Perras Bajo Ooforosalpingohisterectomia Electiva Mediante Termografía Infrarroja y Respuesta Fisiometabólica Sanguínea. Master’s Thesis, Universidad Autónoma Metropolitana, Ciudad de México, México, 2020. [Google Scholar]
- Czaplik, M.; Hochhausen, N.; Dohmeier, H.; Pereira, C.B.; Rossaint, R. Development of a “thermal-associated pain index” score using infrared-thermography for objective pain assessment. In Proceedings of the 2017 39th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Jeju, Korea, 11–15 July 2017; pp. 3831–3834. [Google Scholar] [CrossRef]
- Antonaci, F.; Rossi, E.; Voiticovschi-Iosob, C.; Dalla Volta, G.; Marceglia, S. Frontal infrared thermography in healthy individuals and chronic migraine patients: Reliability of the method. Cephalalgia 2019, 39, 489–496. [Google Scholar] [CrossRef] [Green Version]
- Mazzotti, G.A.; Boere, V. The right ear but not the left ear temperature is related to stress-induced cortisolaemia in the domestic cat (Felis catus). Laterality 2009, 14, 196–204. [Google Scholar] [CrossRef] [PubMed]
- Polgár, Z.; Blackwell, E.J.; Rooney, N.J. Assessing the welfare of kennelled dogs—A review of animal-based measures. Appl. Anim. Behav. Sci. 2019, 213, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Hall, E.J.; Carter, A.J. Comparison of rectal and tympanic membrane temperature in healthy exercising dogs. Comp. Exerc. Physiol. 2017, 13, 37–44. [Google Scholar] [CrossRef] [Green Version]
- Sousa, M.G.; Carareto, R.; Pereira-Junior, V.A.; Aquino, M.C. Agreement between auricular and rectal measurements of body temperature in healthy cats. J. Feline Med. Surg. 2013, 15, 275–279. [Google Scholar] [CrossRef] [PubMed]
- Kunkle, G.A.; Nicklin, C.F.; Sullivan-Tamboe, D.L. Comparison of body temperature in cats using a veterinary infrared thermometer and a digital rectal thermometer. J. Am. Anim. Hosp. Assoc. 2004, 40, 42–46. [Google Scholar] [CrossRef]
- Nutt, K.R.; Levy, J.K.; Tucker, S.J. A Comparison of non-contact infrared thermometry and rectal thermometry in cats. 2015, 18, 798–803. J. Feline Med. Surg. [CrossRef]
- Smith, V.A.; Lamb, V.; McBrearty, A.R. Comparison of axillary, tympanic membrane and rectal temperature measurement in cats. J. Feline Med. Surg. 2015, 17, 1028–1034. [Google Scholar] [CrossRef]
- Saeki, K.; Kutara, K.; Iwata, E.; Miyabe, M.; Shimizu, Y.; Wada, Y.; Ohnishi, A.; Matsuda, A.; Miyama, T.S.; Asanuma, T. Noninvasive thermographic photographing as an assessment of the state of discomfort in a dog receiving radiation therapy. Animals 2021, 11, 2496. [Google Scholar] [CrossRef]
- Mota-Rojas, D.; Marcet-Rius, M.; Ogi, A.; Hernández-Ávalos, I.; Mariti, C.; Martínez-Burnes, J.; Mora-Medina, P.; Casas, A.; Domínguez, A.; Reyes, B.; et al. Current advances in assessment of dog’s emotions, facial expressions, and their use for clinical recognition of pain. Animals 2021, 11, 3334. [Google Scholar] [CrossRef]
- Fernández-Cuevas, I.; Bouzas, J.C.; Arnáiz, J.; Gómez, P.M.; Piñonosa, S.; García-Concepción, M.Á.; Sillero-Quintana, M. Classification of factors influencing the use of infrared thermography in humans: A review. Infrared Phys. Technol. 2015, 71, 28–55. [Google Scholar] [CrossRef]
- Loughmiller, J.A.; Spire, M.E.; Dritz, S.S.; Fenwick, B.W.; Hosni, M.H.; Hogge, S.B. Relationship between mean body surface temperature measured by use of infrared thermography and ambient temperature in clinically normal pigs and pigs inoculated with Actinobacillus pleuropneumoniae. Am. J. Vet. Res. 2001, 62, 676–681. [Google Scholar] [CrossRef] [PubMed]
- Soroko, M.; Howell, K.; Dudek, K. The Effect of Ambient Temperature on Infrared Thermographic Images of Joints in the Distal Forelimbs of Healthy Racehorses. J. Therm. Biol. 2017, 66, 63–67. [Google Scholar] [CrossRef] [PubMed]
- Church, J.S.; Hegadoren, P.R.; Paetkau, M.J.; Miller, C.C.; Regev-Shoshani, G.; Schaefer, A.L.; Schwartzkopf-Genswein, K.S. Influence of Environmental Factors on Infrared Eye Temperature Measurements in Cattle. Res. Vet. Sci. 2014, 96, 220–226. [Google Scholar] [CrossRef]
- Malafaia, O.; Brioschi, M.L.; Aoki, S.M.S.; Dias, F.G.; Gugelmin, B.S.; Aoki, M.S.; Aoki, Y.S. Infrared imaging contribution for intestinal ischemia detection in wound healing. Acta Cir. Bras. 2008, 23, 511–519. [Google Scholar] [CrossRef] [Green Version]
- Vainionpää, M. Thermographic Imaging in Cats and Dogs: Usability as a Clinical Method. Ph.D. Thesis, University of Helsinki, Helsinki, Finland, 2014. [Google Scholar]
- Rizzo, M.; Arfuso, F.; Alberghina, D.; Giudice, E.; Gianesella, M.; Piccione, G. Monitoring changes in body surface temperature associated with treadmill exercise in dogs by use of infrared methodology. J. Therm. Biol. 2017, 69, 64–68. [Google Scholar] [CrossRef]
- Repac, J.; Alvarez, L.X.; Lamb, K.; Gillette, R.L. Evaluation of thermographic imaging in canine hindlimb muscles after 6 min of walking—A pilot study. Front. Vet. Sci. 2020, 7, 224. [Google Scholar] [CrossRef]
- Sturion, M.A.T. Termografia de Infravermelho na Avaliação de Cães-Guia em Treinamento. Ph.D. Thesis, Facultade de Medicina Veterinária e Zootecnia, Botucatu, Brazil, 2019. (in Portuguese). [Google Scholar]
- Nitrini, A.G.C.; Cogliati, B.; Matera, J.M. Thermographic assessment of skin and soft tissue tumors in cats. J. Feline Med. Surg. 2020, 23, 513–518. [Google Scholar] [CrossRef]
- Mota-Rojas, D.; Titto, C.G.; Orihuela, A.; Martínez-Burnes, J.; Gómez-Prado, J.; Torres-Bernal, F.; Flores-Padilla, K.; Carvajal-de la Fuente, V.; Wang, D. Physiological and behavioral mechanisms of thermoregulation in mammals. Animals 2021, 11, 1733. [Google Scholar] [CrossRef]
- Mota-Rojas, D.; Titto, C.G.; de Mira, A.; Martínez-Burnes, J.; Gómez, J.; Hernández-Ávalos, I.; Casas, A.; Domínguez, A.; José, N.; Bertoni, A.; et al. Efficacy and function of feathers, hair, and glabrous skin in the thermoregulation strategies of domestic animals. Animals 2021, 11, 3472. [Google Scholar] [CrossRef]
- Nomura, R.H.C.; de Freitas, I.B.; Guedes, R.L.; Araújo, F.F.; Mafra, A.C.D.N.; Ibañez, J.F.; Dornbusch, P.T. Thermographic images from healthy knees between dogs with long and short hair. Ciência Rural 2018, 48, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Meisfjord Jørgensen, G.H.; Mejdell, C.M.; Bøe, K.E. Effects of hair coat characteristics on radiant surface temperature in horses. J. Therm. Biol. 2020, 87, 102474. [Google Scholar] [CrossRef] [PubMed]
- Magnin, M.; Junot, S.; Cardinali, M.; Ayoub, J.Y.; Paquet, C.; Louzier, V.; Garin, J.M.B.; Allaouchiche, B. Use of infrared thermography to detect early alterations of peripheral perfusion: Evaluation in a porcine model. Biomed. Opt. Express 2020, 11, 2431. [Google Scholar] [CrossRef] [PubMed]
- Vainionpää, M.H.; Raekallio, M.R.; Junnila, J.J.; Hielm-Björkman, A.K.; Snellman, M.P.; Vainio, O.M. A comparison of thermographic imaging, physical examination and modified questionnaire as an instrument to assess painful conditions in cats. J. Feline Med. Surg. 2013, 15, 124–131. [Google Scholar] [CrossRef] [Green Version]
- Reyes-Sotelo, B.; Mota-Rojas, D.; Mora-Medina, P.; Ogi, A.; Mariti, C.; Olmos-Hernandez, A.; Martínez, J.; Hernández, I.; Jose, S.; Gazzano, A. Blood biomarker profile alterations in newborn canines: Effect of the mother´s weight. Animals 2021, 11, 2307. [Google Scholar] [CrossRef]
- Topalidou, A.; Ali, N.; Sekulic, S.; Downe, S. Thermal imaging applications in neonatal care: A scoping review. BMC Pregnancy Childbirth 2019, 19, 381. [Google Scholar] [CrossRef]
- Soerensen, D.D.; Pedersen, L.J. Infrared skin temperature measurements for monitoring health in pigs: A review. Acta Vet. Scand. 2015, 57, 5. [Google Scholar] [CrossRef] [Green Version]
- Mota-Rojas, D.; Napolitano, F.; Braghieri, A.; Guerrero-Legarreta, I.; Bertoni, A.; Martínez-Burnes, J.; Cruz-Monterrosa, R.; Gómez, J.; Ramírez-Bribiesca, E.; Barrios-García, H.; et al. Thermal biology in river buffalo in the humid tropics: Neurophysiological and behavioral responses assessed by infrared thermography. J. Anim. Behav. Biometeorol. 2021, 9, 1–12. [Google Scholar] [CrossRef]
- Axiak-Bechtel, S.M.; Mathew, L.M.; Amorim, J.R.; DeClue, A.E. Dogs with osteosarcoma have altered pro- and anti-inflammatory cytokine profiles. Vet. Med. Sci. 2019, 5, 485–493. [Google Scholar] [CrossRef] [Green Version]
- Carvalho, M.I.; Silva-Carvalho, R.; Pires, I.; Prada, J.; Bianchini, R.; Jensen-Jarolim, E.; Queiroga, F.L. A comparative approach of tumor-associated inflammation in mammary cancer between humans and dogs. Biomed. Res. Int. 2016, 2016, 4917387. [Google Scholar] [CrossRef]
- Gurjarpadhye, A.A.; Parekh, M.B.; Dubnika, A.; Rajadas, J.; Inayathullah, M. Infrared imaging tools for diagnostic applications in dermatology. SM J. Clin. Med. Imaging 2015, 1, 1–5. [Google Scholar] [PubMed]
- Waddell, R.E.; Marino, D.J.; Loughin, C.A.; Tumulty, J.W.; Dewey, C.W.; Sackman, J. Medical infrared thermal imaging of cats with hyperthyroidism. Am. J. Vet. Res. 2015, 76, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Olğaç, K.T.; Akçay, E.; Çil, B.; Uçar, B.M.; Daşkın, A. The use of infrared thermography to detect the stages of estrus cycle and ovulation time in anatolian shepherd dogs. J. Anim. Sci. Technol. 2017, 59, 21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Talukder, S.; Thomson, P.C.; Kerrisk, K.L.; Clark, C.E.F.; Celi, P. Evaluation of infrared thermography body temperature and collar-mounted accelerometer and acoustic technology for predicting time of ovulation of cows in a pasture-based system. Theriogenology 2015, 83, 739–748. [Google Scholar] [CrossRef]
- Simões, V.G.; Lyazrhi, F.; Picard-Hagen, N.; Gayrard, V.; Martineau, G.-P.; Waret-Szkuta, A. Variations in the vulvar temperature of sows during proestrus and estrus as determined by infrared thermography and its relation to ovulation. Theriogenology 2014, 82, 1080–1085. [Google Scholar] [CrossRef]
- Cugmas, B.; Šušterič, P.; Gorenjec, N.R.; Plavec, T. Comparison between rectal and body surface temperature in dogs by the calibrated infrared thermometer. Vet. Anim. Sci. 2020, 9, 100120. [Google Scholar] [CrossRef]
- Aloweni, F.A.B.; Ang, S.Y.; Chang, Y.Y.; Ng, X.P.; Teo, K.Y.; Choh, A.C.L.; Goh, I.H.Q.; Lim, S.H. Evaluation of infrared technology to detect category I and suspected deep tissue injury in hospitalised patients. J. Wound Care 2019, 28, S9–S16. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Casas-Alvarado, A.; Martínez-Burnes, J.; Mora-Medina, P.; Hernández-Avalos, I.; Domínguez-Oliva, A.; Lezama-García, K.; Gómez-Prado, J.; Mota-Rojas, D. Thermal and Circulatory Changes in Diverse Body Regions in Dogs and Cats Evaluated by Infrared Thermography. Animals 2022, 12, 789. https://doi.org/10.3390/ani12060789
Casas-Alvarado A, Martínez-Burnes J, Mora-Medina P, Hernández-Avalos I, Domínguez-Oliva A, Lezama-García K, Gómez-Prado J, Mota-Rojas D. Thermal and Circulatory Changes in Diverse Body Regions in Dogs and Cats Evaluated by Infrared Thermography. Animals. 2022; 12(6):789. https://doi.org/10.3390/ani12060789
Chicago/Turabian StyleCasas-Alvarado, Alejandro, Julio Martínez-Burnes, Patricia Mora-Medina, Ismael Hernández-Avalos, Adriana Domínguez-Oliva, Karina Lezama-García, Jocelyn Gómez-Prado, and Daniel Mota-Rojas. 2022. "Thermal and Circulatory Changes in Diverse Body Regions in Dogs and Cats Evaluated by Infrared Thermography" Animals 12, no. 6: 789. https://doi.org/10.3390/ani12060789
APA StyleCasas-Alvarado, A., Martínez-Burnes, J., Mora-Medina, P., Hernández-Avalos, I., Domínguez-Oliva, A., Lezama-García, K., Gómez-Prado, J., & Mota-Rojas, D. (2022). Thermal and Circulatory Changes in Diverse Body Regions in Dogs and Cats Evaluated by Infrared Thermography. Animals, 12(6), 789. https://doi.org/10.3390/ani12060789









