Hepatic Lipid Accumulation and Dysregulation Associate with Enhanced Reactive Oxygen Species and Pro-Inflammatory Cytokine in Low-Birth-Weight Goats
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Husbandry
2.2. Biochemical Parameters Assays
2.3. RNA Sequencing and Analysis
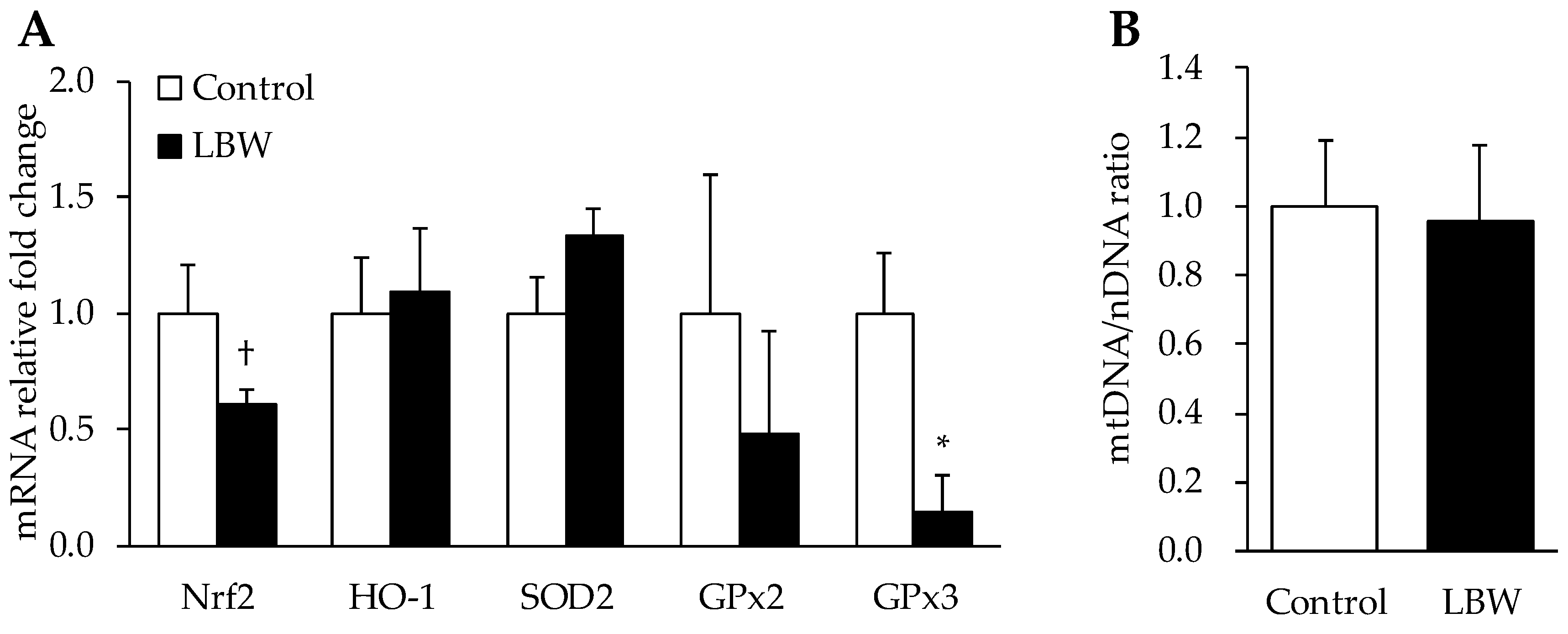
2.4. Quantitative Analysis of mRNA Expression and Mitochondrial DNA
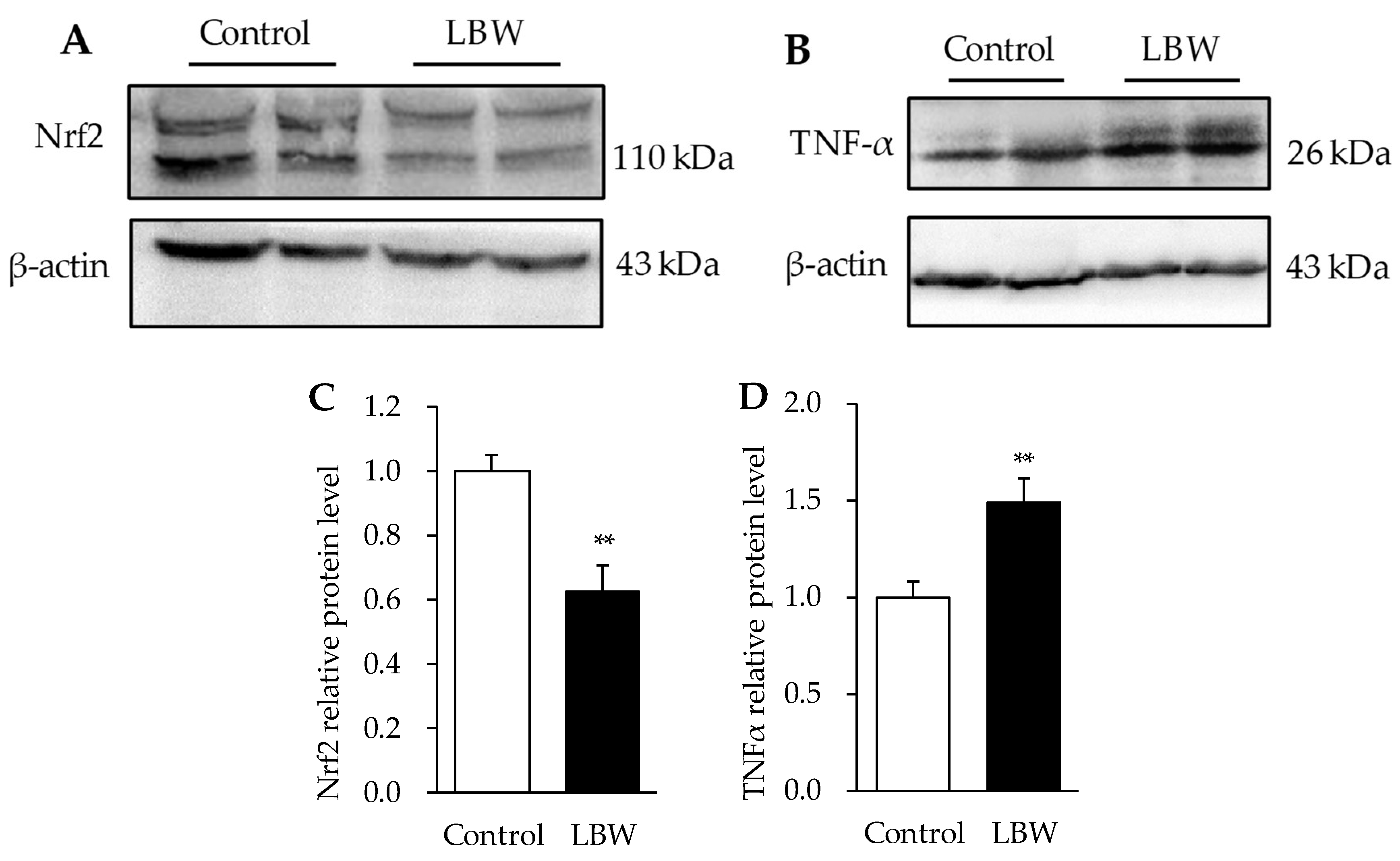
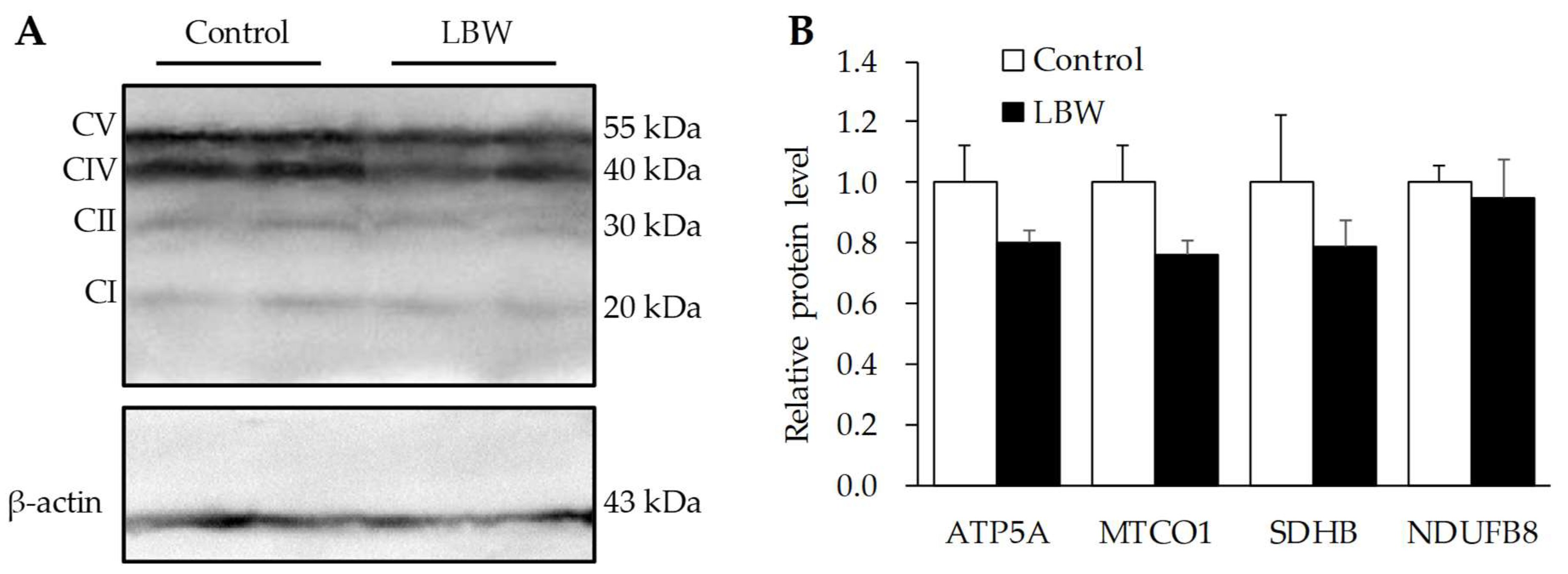
2.5. Western Blot Analysis
2.6. Histological Analyses
2.7. Statistical Analysis
3. Results
3.1. Weights
3.2. Morphological and Metabolic Features in Liver
3.3. Differential Gene Expression of RNAseq
3.4. Gene Expressions in Liver
3.5. Hepatic Protein Expressions
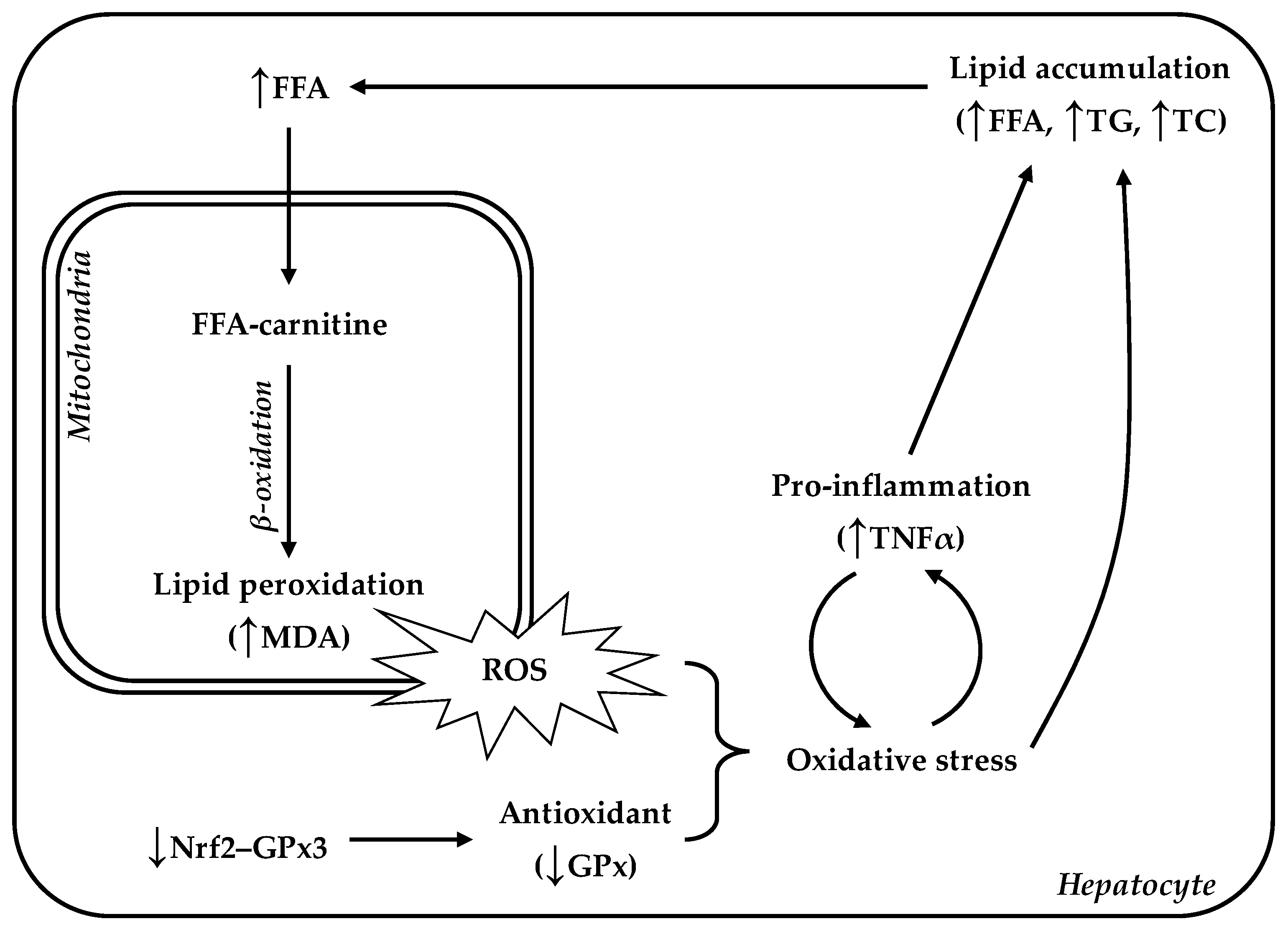
4. Discussion
4.1. Hepatic Lipid Accumulation and Oxidative Stress in LBW Goat Kids
4.2. Enhanced ROS Caused by Lower Antioxidant Capacity Damage Hepatic Lipid Regulation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wu, G.; Bazer, F.W.; Wallace, J.M.; Spencer, T.E. Board-Invited Review: Intrauterine Growth Retardation: Implications for the Animal Sciences. J. Anim. Sci. 2006, 84, 2316–2337. [Google Scholar] [CrossRef] [PubMed]
- Petry, C.J.; Dorling, M.W.; Pawlak, D.B.; Ozanne, S.E.; Hales, C.N. Diabetes in Old Male Offspring of Rat Dams Fed a Reduced Protein Diet. Int. J. Exp. Diabetes Res. 2001, 2, 139–143. [Google Scholar] [CrossRef] [PubMed]
- Simmons, R.A.; Templeton, L.J.; Gertz, S.J. Intrauterine Growth Retardation Leads to the Development of Type 2 Diabetes in the Rat. Diabetes 2001, 50, 2279–2286. [Google Scholar] [CrossRef]
- Poore, K.R.; Fowden, A.L. The Effect of Birth Weight on Glucose Tolerance in Pigs at 3 and 12 Months of Age. Diabetologia 2002, 45, 1247–1254. [Google Scholar] [CrossRef] [PubMed][Green Version]
- De Blasio, M.J.; Gatford, K.L.; McMillen, I.C.; Robinson, J.S.; Owens, J.A. Placental Restriction of Fetal Growth Increases Insulin Action, Growth, and Adiposity in the Young Lamb. Endocrinology 2007, 148, 1350–1358. [Google Scholar] [CrossRef] [PubMed]
- Luo, N.; Wang, J.; Hu, Y.; Zhao, Z.; Zhao, Y.; Chen, X. Cold and Heat Climatic Variations Reduce Indigenous Goat Birth Weight and Enhance Pre-Weaning Mortality in Subtropical Monsoon Region of China. Trop. Anim. Health Prod. 2019, 52, 1385–1394. [Google Scholar] [CrossRef] [PubMed]
- Magee, T.R.; Han, G.; Cherian, B.; Khorram, O.; Ross, M.G.; Desai, M. Down-Regulation of Transcription Factor Peroxisome Proliferator-Activated Receptor in Programmed Hepatic Lipid Dysregulation and Inflammation in Intrauterine Growth-Restricted Offspring. Am. J. Obstet. Gynecol. 2008, 199, 271.e1–271.e5. [Google Scholar] [CrossRef]
- Desai, M.; Gayle, D.; Babu, J.; Ross, M.G. Programmed Obesity in Intrauterine Growth-Restricted Newborns: Modulation by Newborn Nutrition. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2005, 288, R91–R96. [Google Scholar] [CrossRef]
- He, J.; Dong, L.; Xu, W.; Bai, K.; Lu, C.; Wu, Y.; Huang, Q.; Zhang, L.; Wang, T. Dietary Tributyrin Supplementation Attenuates Insulin Resistance and Abnormal Lipid Metabolism in Suckling Piglets with Intrauterine Growth Retardation. PLoS ONE 2015, 10, e0136848. [Google Scholar] [CrossRef]
- Peterside, I.E.; Selak, M.A.; Simmons, R.A. Impaired Oxidative Phosphorylation in Hepatic Mitochondria in Growth-Retarded Rats. Am. J. Physiol. Endocrinol. Metab. 2003, 285, E1258–E1266. [Google Scholar] [CrossRef] [PubMed]
- Niu, Y.; He, J.; Ahmad, H.; Shen, M.; Zhao, Y.; Gan, Z.; Zhang, L.; Zhong, X.; Wang, C.; Wang, T. Dietary Curcumin Supplementation Increases Antioxidant Capacity, Upregulates Nrf2 and Hmox1 Levels in the Liver of Piglet Model with Intrauterine Growth Retardation. Nutrients 2019, 11, 2978. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Valle, V.; Chávez-Tapia, N.C.; Uribe, M.; Méndez-Sánchez, N. Role of Oxidative Stress and Molecular Changes in Liver Fibrosis: A Review. Curr. Med. Chem. 2012, 19, 4850–4860. [Google Scholar] [CrossRef] [PubMed]
- Singal, A.K.; Jampana, S.C.; Weinman, S.A. Antioxidants as Therapeutic Agents for Liver Disease. Liver Int. 2011, 31, 1432–1448. [Google Scholar] [CrossRef] [PubMed]
- Gu, C.; Li, P.; Liu, W.; Zhou, Y.; Tan, W.-S. The Role of Insulin in Transdifferentiated Hepatocyte Proliferation and Function in Serum-Free Medium. J. Cell. Mol. Med. 2019, 23, 4165–4178. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Langmead, B.; Salzberg, S.L. HISAT: A Fast Spliced Aligner with Low Memory Requirements. Nat. Methods 2015, 12, 357–360. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Paggi, J.M.; Park, C.; Bennett, C.; Salzberg, S.L. Graph-Based Genome Alignment and Genotyping with HISAT2 and HISAT-Genotype. Nat. Biotechnol. 2019, 37, 907–915. [Google Scholar] [CrossRef] [PubMed]
- Pertea, M.; Pertea, G.M.; Antonescu, C.M.; Chang, T.-C.; Mendell, J.T.; Salzberg, S.L. StringTie Enables Improved Reconstruction of a Transcriptome from RNA-Seq Reads. Nat. Biotechnol. 2015, 33, 290–295. [Google Scholar] [CrossRef] [PubMed]
- Trapnell, C.; Williams, B.A.; Pertea, G.; Mortazavi, A.; Kwan, G.; van Baren, M.J.; Salzberg, S.L.; Wold, B.J.; Pachter, L. Transcript Assembly and Quantification by RNA-Seq Reveals Unannotated Transcripts and Isoform Switching during Cell Differentiation. Nat. Biotechnol. 2010, 28, 511–515. [Google Scholar] [CrossRef] [PubMed]
- Robinson, M.D.; McCarthy, D.J.; Smyth, G.K. EdgeR: A Bioconductor Package for Differential Expression Analysis of Digital Gene Expression Data. Bioinformatics 2010, 26, 139–140. [Google Scholar] [CrossRef] [PubMed]
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. Ser. B Methodol. 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Young, M.D.; Wakefield, M.J.; Smyth, G.K.; Oshlack, A. Gene Ontology Analysis for RNA-Seq: Accounting for Selection Bias. Genome Biol. 2010, 11, R14. [Google Scholar] [CrossRef] [PubMed]
- Luo, N.; Cheng, W.; Zhou, Y.; Gu, B.; Zhao, Z.; Zhao, Y. Screening Candidate Genes Regulating Placental Development from Trophoblast Transcriptome at Early Pregnancy in Dazu Black Goats (Capra hircus). Animals 2021, 11, 2132. [Google Scholar] [CrossRef] [PubMed]
- Pfaffl, M.W. A New Mathematical Model for Relative Quantification in Real-Time RT-PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-C.; Lee, W.-C.; Liao, S.-C.; Lee, L.-C.; Su, Y.-J.; Lee, C.-T.; Chen, J.-B. Mitochondrial DNA Copy Number Correlates with Oxidative Stress and Predicts Mortality in Nondiabetic Hemodialysis Patients. J. Nephrol. 2011, 24, 351–358. [Google Scholar] [CrossRef]
- Fazzini, F.; Lamina, C.; Fendt, L.; Schultheiss, U.T.; Kotsis, F.; Hicks, A.A.; Meiselbach, H.; Weissensteiner, H.; Forer, L.; Krane, V.; et al. Mitochondrial DNA Copy Number Is Associated with Mortality and Infections in a Large Cohort of Patients with Chronic Kidney Disease. Kidney Int. 2019, 96, 480–488. [Google Scholar] [CrossRef]
- Luo, N.; Yue, F.; Jia, Z.; Chen, J.; Deng, Q.; Zhao, Y.; Kuang, S. Reduced Electron Transport Chain Complex I Protein Abundance and Function in Mfn2-Deficient Myogenic Progenitors Lead to Oxidative Stress and Mitochondria Swelling. FASEB J. 2021, 35, e21426. [Google Scholar] [CrossRef]
- Brown, L.D.; Hay, W.W. Impact of Placental Insufficiency on Fetal Skeletal Muscle Growth. Mol. Cell. Endocrinol. 2016, 435, 69–77. [Google Scholar] [CrossRef]
- Nobili, V.; Marcellini, M.; Marchesini, G.; Vanni, E.; Manco, M.; Villani, A.; Bugianesi, E. Intrauterine Growth Retardation, Insulin Resistance, and Nonalcoholic Fatty Liver Disease in Children. Diabetes Care 2007, 30, 2638–2640. [Google Scholar] [CrossRef]
- Newton, K.P.; Feldman, H.S.; Chambers, C.D.; Wilson, L.; Behling, C.; Clark, J.M.; Molleston, J.P.; Chalasani, N.; Sanyal, A.J.; Fishbein, M.H.; et al. Low and High Birth Weights Are Risk Factors for Nonalcoholic Fatty Liver Disease in Children. J. Pediatr. 2017, 187, 141–146.e1. [Google Scholar] [CrossRef]
- Camacho, L.E.; Chen, X.; Hay, W.W.; Limesand, S.W. Enhanced Insulin Secretion and Insulin Sensitivity in Young Lambs with Placental Insufficiency-Induced Intrauterine Growth Restriction. Am. J. Physiol. Integr. Comp. Physiol. 2017, 313, R101–R109. [Google Scholar] [CrossRef]
- Martin-Gronert, M.S.; Ozanne, S.E. Experimental IUGR and Later Diabetes. J. Intern. Med. 2007, 261, 437–452. [Google Scholar] [CrossRef] [PubMed]
- Sarr, O.; Mathers, K.E.; Vanderboor, C.; Wiggers, K.; Devgan, A.; Hardy, D.B.; Zhao, L.; Regnault, T.R.H. Sex-Specific Alterations in Hepatic Cholesterol Metabolism in Low Birth Weight Adult Guinea Pigs. Pediatr. Res. 2021, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Zinkhan, E.K.; Zalla, J.M.; Carpenter, J.R.; Yu, B.; Yu, X.; Chan, G.; Joss-Moore, L.; Lane, R.H. Intrauterine Growth Restriction Combined with a Maternal High-Fat Diet Increases Hepatic Cholesterol and Low-Density Lipoprotein Receptor Activity in Rats. Physiol. Rep. 2016, 4, e12862. [Google Scholar] [CrossRef]
- Lane, R.H.; Kelley, D.E.; Gruetzmacher, E.M.; Devaskar, S.U. Uteroplacental Insufficiency Alters Hepatic Fatty Acid-Metabolizing Enzymes in Juvenile and Adult Rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2001, 280, R183–R190. [Google Scholar] [CrossRef]
- Chen, Z.; Tian, R.; She, Z.; Cai, J.; Li, H. Role of Oxidative Stress in the Pathogenesis of Nonalcoholic Fatty Liver Disease. Free Radic. Biol. Med. 2020, 152, 116–141. [Google Scholar] [CrossRef] [PubMed]
- Zelber-Sagi, S.; Ivancovsky-Wajcman, D.; Fliss-Isakov, N.; Hahn, M.; Webb, M.; Shibolet, O.; Kariv, R.; Tirosh, O. Serum Malondialdehyde Is Associated with Non-Alcoholic Fatty Liver and Related Liver Damage Differentially in Men and Women. Antioxidants 2020, 9, 578. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.-Z.; Yang, S.; Wu, G. Free Radicals, Antioxidants, and Nutrition. Nutrition 2002, 18, 872–879. [Google Scholar] [CrossRef]
- Zhang, H.; Li, Y.; Wang, T. Antioxidant Capacity and Concentration of Redox-Active Trace Mineral in Fully Weaned Intra-Uterine Growth Retardation Piglets. J. Anim. Sci. Biotechnol. 2015, 6, 48. [Google Scholar] [CrossRef][Green Version]
- Ferenc, K.; Pietrzak, P.; Wierzbicka, M.; Matyba, P.; Grzesiuk, E.; Gajewski, Z.; Zabielski, R. Alterations in the Liver of Intrauterine Growth Retarded Piglets May Predispose to Development of Insulin Resistance and Obesity in Later Life. J. Physiol. Pharmacol. 2018, 69, 1–8. [Google Scholar] [CrossRef]
- Cheng, K.; Ji, S.; Jia, P.; Zhang, H.; Wang, T.; Song, Z.; Zhang, L.; Wang, T. Resveratrol Improves Hepatic Redox Status and Lipid Balance of Neonates with Intrauterine Growth Retardation in a Piglet Model. BioMed Res. Int. 2020, 2020, 7402645. [Google Scholar] [CrossRef]
- Gveric-Ahmetasevic, S.; Sunjic, S.B.; Skala, H.; Andrisic, L.; Stroser, M.; Zarkovic, K.; Skrablin, S.; Tatzber, F.; Cipak, A.; Jaganjac, M.; et al. Oxidative Stress in Small-for-Gestational Age (SGA) Term Newborns and Their Mothers. Free Radic. Res. 2009, 43, 376–384. [Google Scholar] [CrossRef] [PubMed]
- Pendleton, A.L.; Wesolowski, S.R.; Regnault, T.R.H.; Lynch, R.M.; Limesand, S.W. Dimming the Powerhouse: Mitochondrial Dysfunction in the Liver and Skeletal Muscle of Intrauterine Growth Restricted Fetuses. Front. Endocrinol. 2021, 12, 612888. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Niu, Y.; He, J.; Zhang, L.; Wang, C.; Wang, T. Dietary Dihydroartemisinin Supplementation Attenuates Hepatic Oxidative Damage of Weaned Piglets with Intrauterine Growth Retardation through the Nrf2/ARE Signaling Pathway. Animals 2019, 9, 1144. [Google Scholar] [CrossRef] [PubMed]
- Sarr, O.; Blake, A.; Thompson, J.A.; Zhao, L.; Rabicki, K.; Walsh, J.C.; Welch, I.; Regnault, T.R.H. The Differential Effects of Low Birth Weight and Western Diet Consumption upon Early Life Hepatic Fibrosis Development in Guinea Pig: Placental Insufficiency and LBW, Western Diet and Fibrosis. J. Physiol. 2016, 594, 1753–1772. [Google Scholar] [CrossRef]
- Tarry-Adkins, J.L.; Fernandez-Twinn, D.S.; Hargreaves, I.P.; Neergheen, V.; Aiken, C.E.; Martin-Gronert, M.S.; McConnell, J.M.; Ozanne, S.E. Coenzyme Q10 Prevents Hepatic Fibrosis, Inflammation, and Oxidative Stress in a Male Rat Model of Poor Maternal Nutrition and Accelerated Postnatal Growth. Am. J. Clin. Nutr. 2016, 103, 579–588. [Google Scholar] [CrossRef] [PubMed]
- Mun, J.; Kim, S.; Yoon, H.-G.; You, Y.; Kim, O.-K.; Choi, K.-C.; Lee, Y.-H.; Lee, J.; Park, J.; Jun, W. Water Extract of Curcuma Longa L. Ameliorates Non-Alcoholic Fatty Liver Disease. Nutrients 2019, 11, 2536. [Google Scholar] [CrossRef]
- Manuelian, C.L.; Pitino, R.; Simoni, M.; Mavrommatis, A.; De Marchi, M.; Righi, F.; Tsiplakou, E. Plant Feed Additives as Natural Alternatives to the Use of Synthetic Antioxidant Vitamins on Livestock Mammals’ Performances, Health, and Oxidative Status: A Review of the Literature in the Last 20 Years. Antioxidants 2021, 10, 1461. [Google Scholar] [CrossRef] [PubMed]
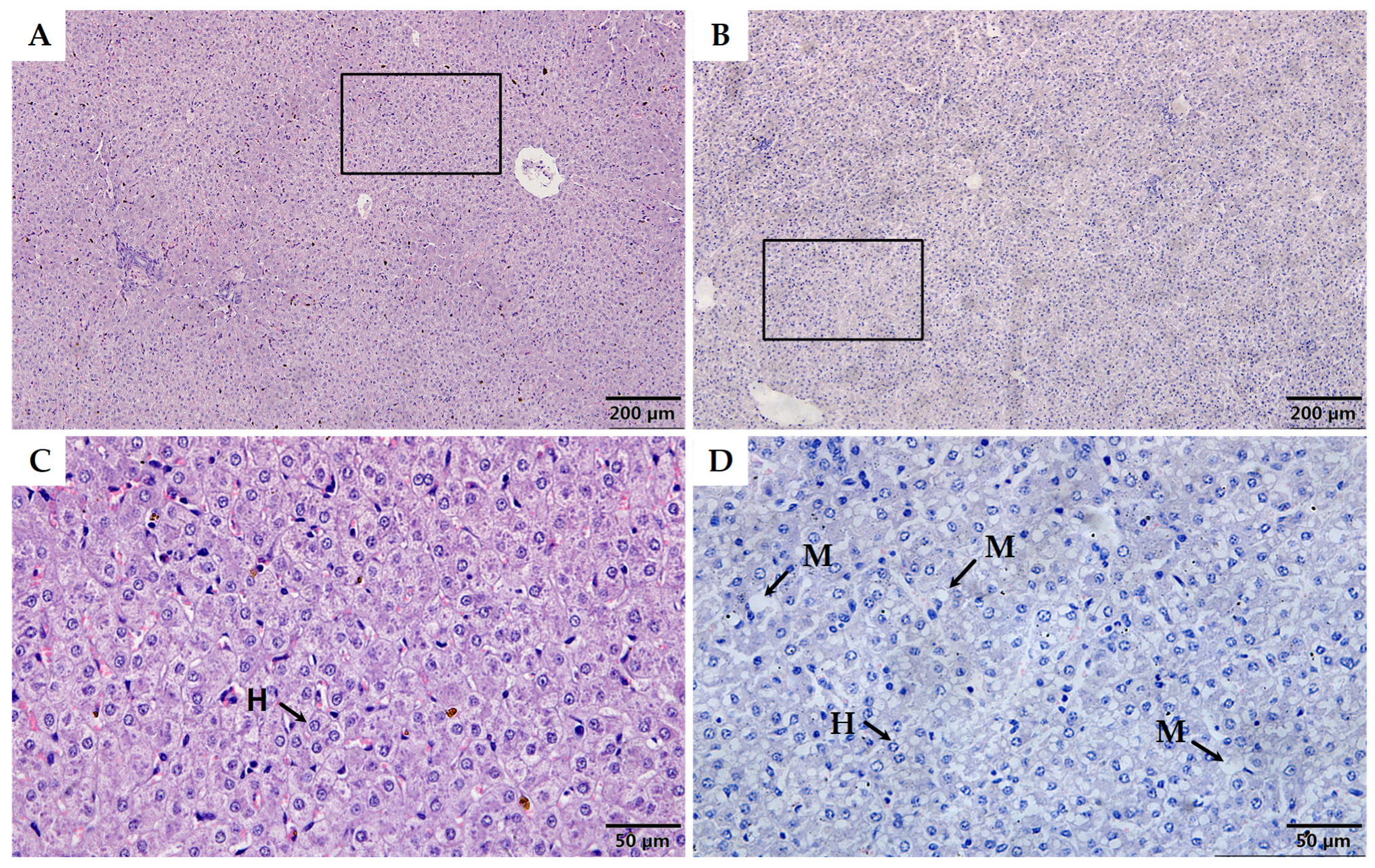

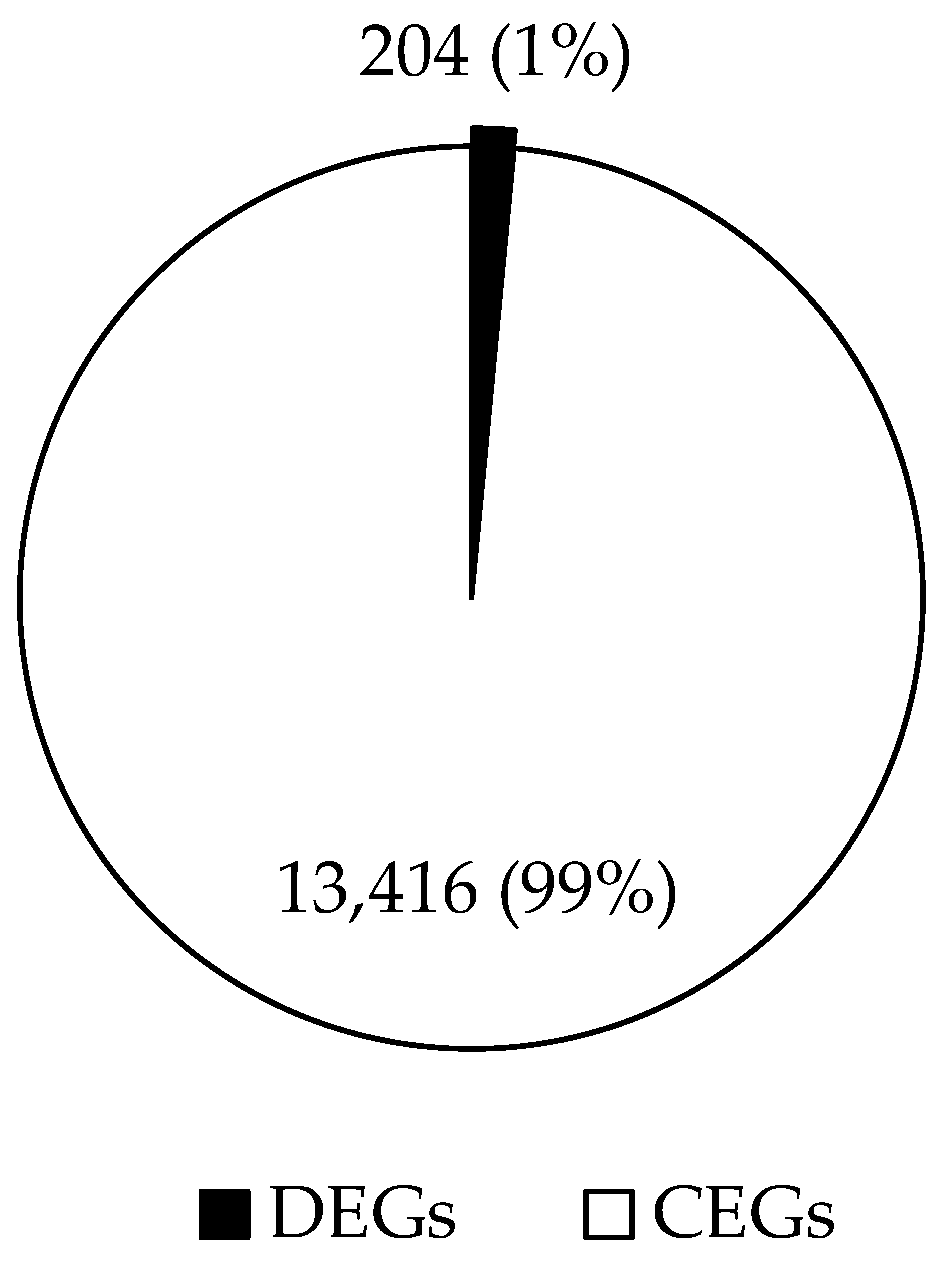
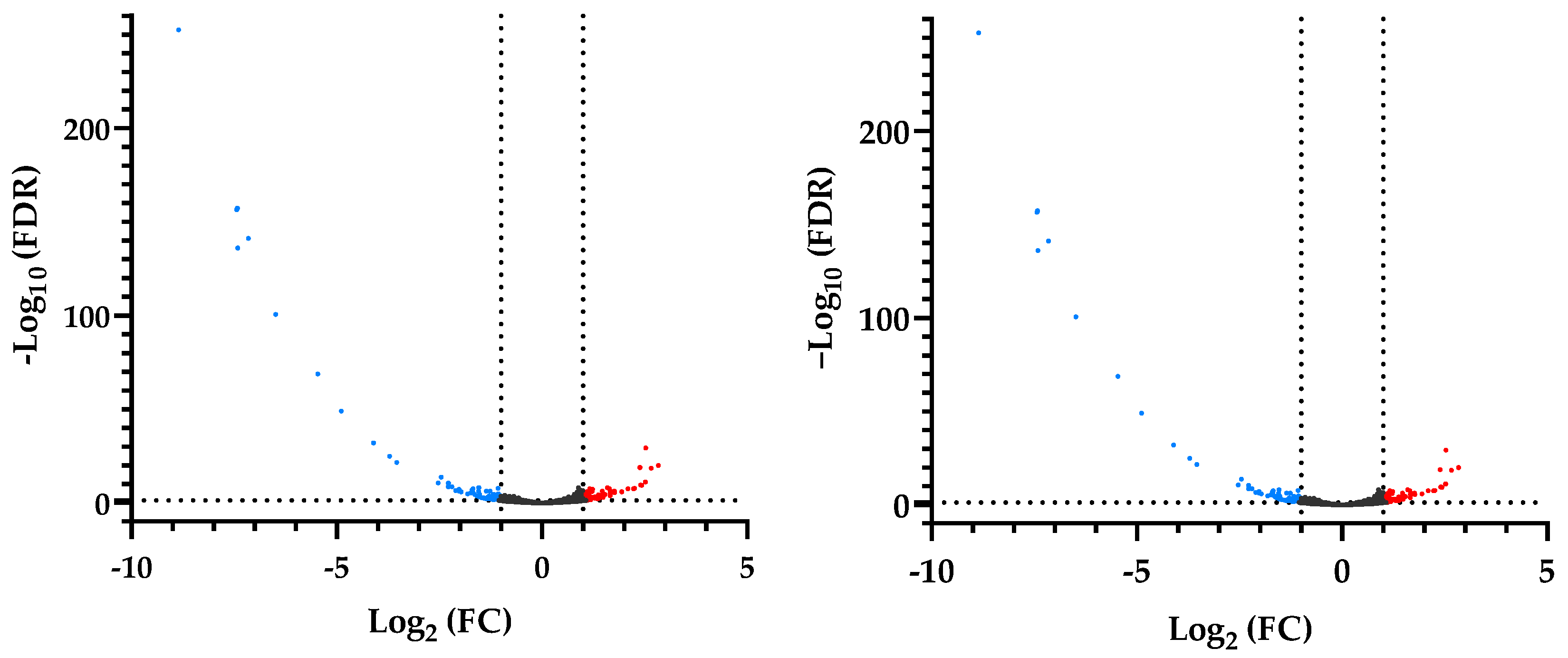
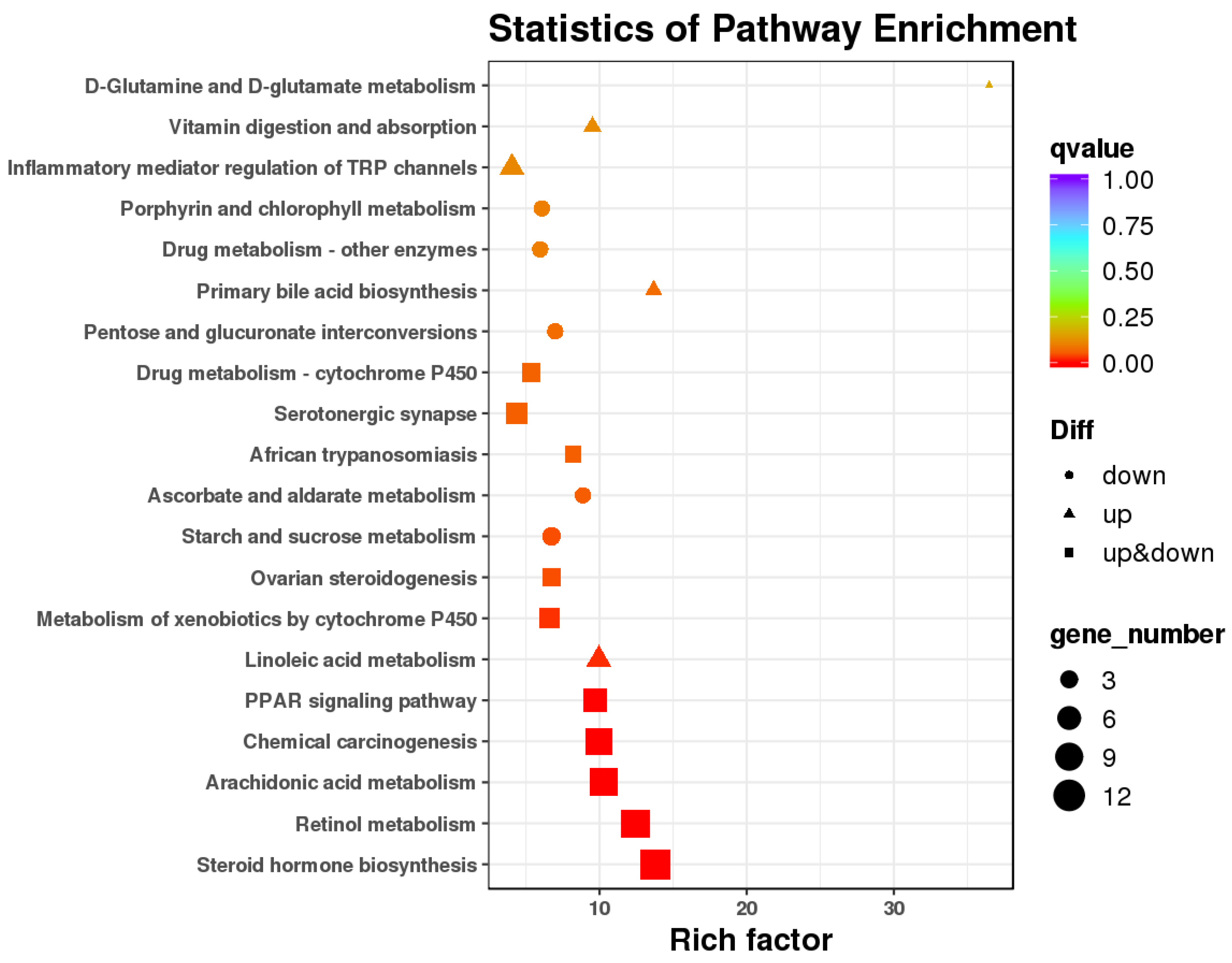

| Necropsy | Control | LBW |
|---|---|---|
| Body weight, kg | 4.67 ± 0.44 | 3.59 ± 0.26 * |
| Carcass weight, kg | 3.40 ± 0.34 | 2.57 ± 0.26 * |
| Brain, g | 65.89 ± 2.93 | 56.62 ± 1.61 ** |
| Heart, g | 30.21 ± 1.85 | 24.88 ± 1.64 * |
| Liver, g | 137.92 ± 10.68 | 108.77 ± 5.92 ** |
| Lungs, g | 81.83 ± 5.65 | 69.17 ± 3.83 * |
| Relative organ mass, g/kg | ||
| Brain | 14.42 ± 0.88 | 16.19 ± 1.32 |
| Heart | 6.55 ± 0.28 | 6.98 ± 0.31 |
| Liver | 29.95 ± 1.90 | 31.01 ± 2.48 |
| Lungs | 17.77 ± 0.98 | 19.62 ± 1.44 |
| Functional Description | KEGG Pathway | KEGG ID | Gene Category |
|---|---|---|---|
| Lipid metabolism | PPAR signaling pathway | KO03320 | FABP3 (fatty acid-binding protein) PLIN5 (perilipin-5 isoform X1) LOC102173339 (7-alpha-diol 12-alpha-hydroxylase) APOA5 (apolipoprotein A-V) LOC102179867 (apolipoprotein A-I) APOA1 (apolipoprotein A-IV) LOC102187785 (cholesterol 7-alpha-monooxygenaseC) |
| Fat digestion and absorption | KO04975 | LOC102179867 (apolipoprotein A-I) APOA1 (apolipoprotein A-I) | |
| Glycerophospholipid metabolism | KO00564 | LCAT (phosphatidylcholine-sterol acyltransferase precursor) | |
| Non-alcoholic fatty liver disease (NAFLD) | KO04932 | Capra_hircus_newGene_23729 Capra_hircus_newGene_43596 | |
| Fatty acid degradation | KO00071 | LOC102181105 (alcohol dehydrogenase E chain isoform X1) LOC108633240 (cytochrome P450 4A11-like) | |
| FoxO signaling pathway | KO04068 | LOC102172279 (serine protease HTRA3) | |
| Oxidative regulation | Glutathione metabolism | KO00480 | GPX2 (glutathione peroxidase 2) GPX3 (glutathione peroxidase 3) |
| Metabolism of xenobiotics by cytochrome P450 | KO00980 | LOC102170823 (cytochromeP450 1A1) LOC102175204 (UDP-glucuronosyltransferase 2B4) | |
| Drug metabolism - cytochrome P450 | KO00982 | LOC102175204 (UDP-glucuronosyltransferase 2B4) LOC102181105 (alcohol dehydrogenase E chain isoform X1) LOC108635023 (UDP-glucuronosyltransferase 2B18-like) | |
| Oxidative phosphorylation | KO00190 | Capra_hircus_newGene_23729 Capra_hircus_newGene_43596 Capra_hircus_newGene_43600 Capra_hircus_newGene_48268 | |
| Inflammation | Leukocyte transendothelial migration | KO04670 | NCF4 (neutrophil cytosol factor 4 isoform X1) PTK2B (protein-tyrosine kinase 2-beta isoform X1) |
| NF-kappa B signaling pathway | KO04064 | LBP (lipopolysaccharide binding protein) Capra_hircus_newGene_7098 | |
| Inflammatory mediator regulation of TRP channels | KO04750 | LOC100861186 (cytochrome P450 2C31) OC102169851 (cytochrome P450 2C31) LOC106503891 (cytochrome P450 2C31) LOC108633308 (cytochrome P450 2C31-like) | |
| TNF signaling pathway | KO04668 | LOC102184244 (interferon-inducible GTPase 1) 102188524 (leukemia inhibitory factor) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, T.; Li, R.; Luo, N.; Lou, P.; Limesand, S.W.; Yang, Y.; Zhao, Y.; Chen, X. Hepatic Lipid Accumulation and Dysregulation Associate with Enhanced Reactive Oxygen Species and Pro-Inflammatory Cytokine in Low-Birth-Weight Goats. Animals 2022, 12, 766. https://doi.org/10.3390/ani12060766
Liu T, Li R, Luo N, Lou P, Limesand SW, Yang Y, Zhao Y, Chen X. Hepatic Lipid Accumulation and Dysregulation Associate with Enhanced Reactive Oxygen Species and Pro-Inflammatory Cytokine in Low-Birth-Weight Goats. Animals. 2022; 12(6):766. https://doi.org/10.3390/ani12060766
Chicago/Turabian StyleLiu, Tingting, Rui Li, Nanjian Luo, Peng Lou, Sean W. Limesand, You Yang, Yongju Zhao, and Xiaochuan Chen. 2022. "Hepatic Lipid Accumulation and Dysregulation Associate with Enhanced Reactive Oxygen Species and Pro-Inflammatory Cytokine in Low-Birth-Weight Goats" Animals 12, no. 6: 766. https://doi.org/10.3390/ani12060766
APA StyleLiu, T., Li, R., Luo, N., Lou, P., Limesand, S. W., Yang, Y., Zhao, Y., & Chen, X. (2022). Hepatic Lipid Accumulation and Dysregulation Associate with Enhanced Reactive Oxygen Species and Pro-Inflammatory Cytokine in Low-Birth-Weight Goats. Animals, 12(6), 766. https://doi.org/10.3390/ani12060766







