The Candidate Chromosomal Regions Responsible for Milk Yield of Cow: A GWAS Meta-Analysis
Abstract
:Simple Summary
Abstract
1. Introduction
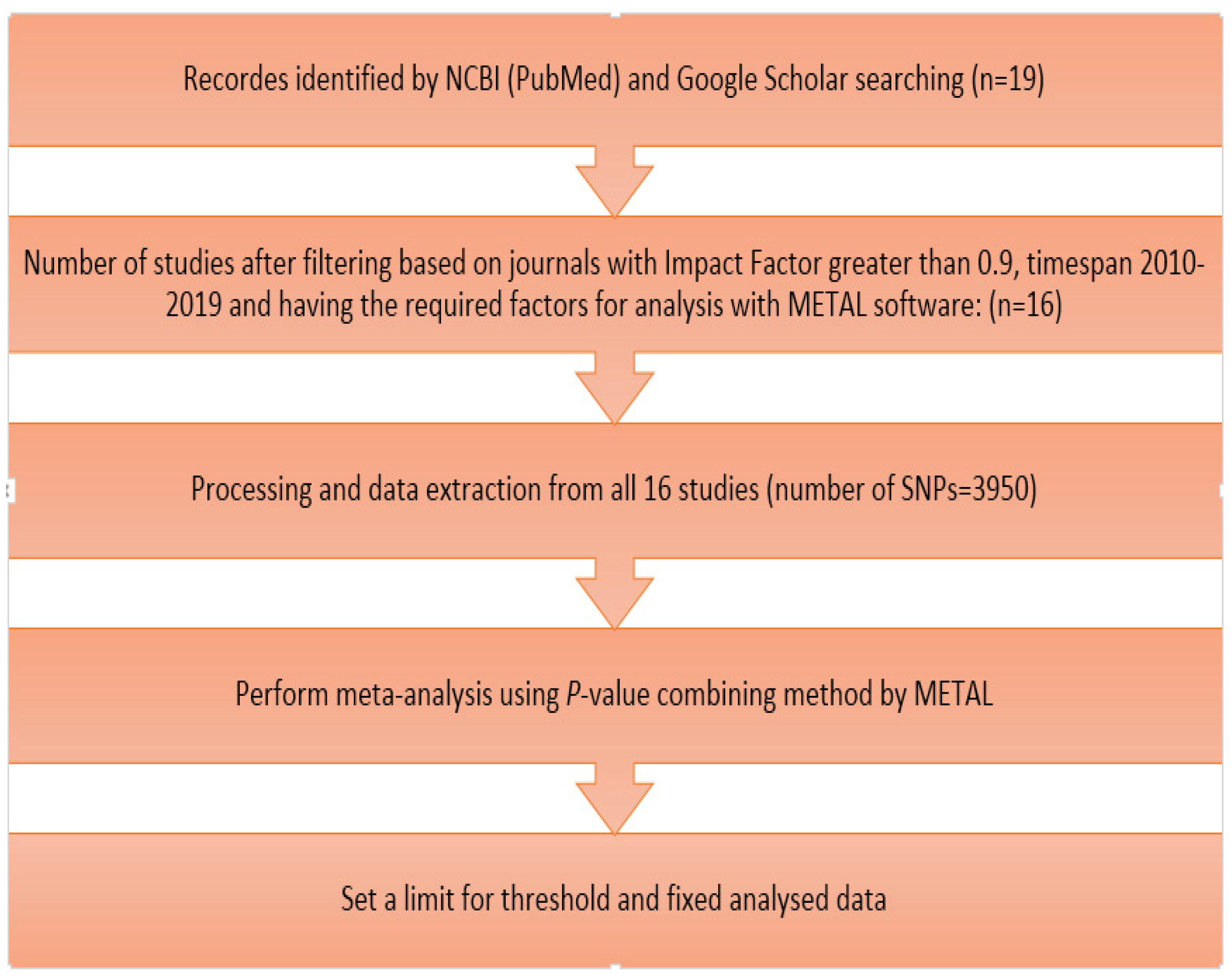
2. Material and Methods
2.1. Data and Literature Review
2.2. Meta-Analysis
2.3. Downstream Analyses
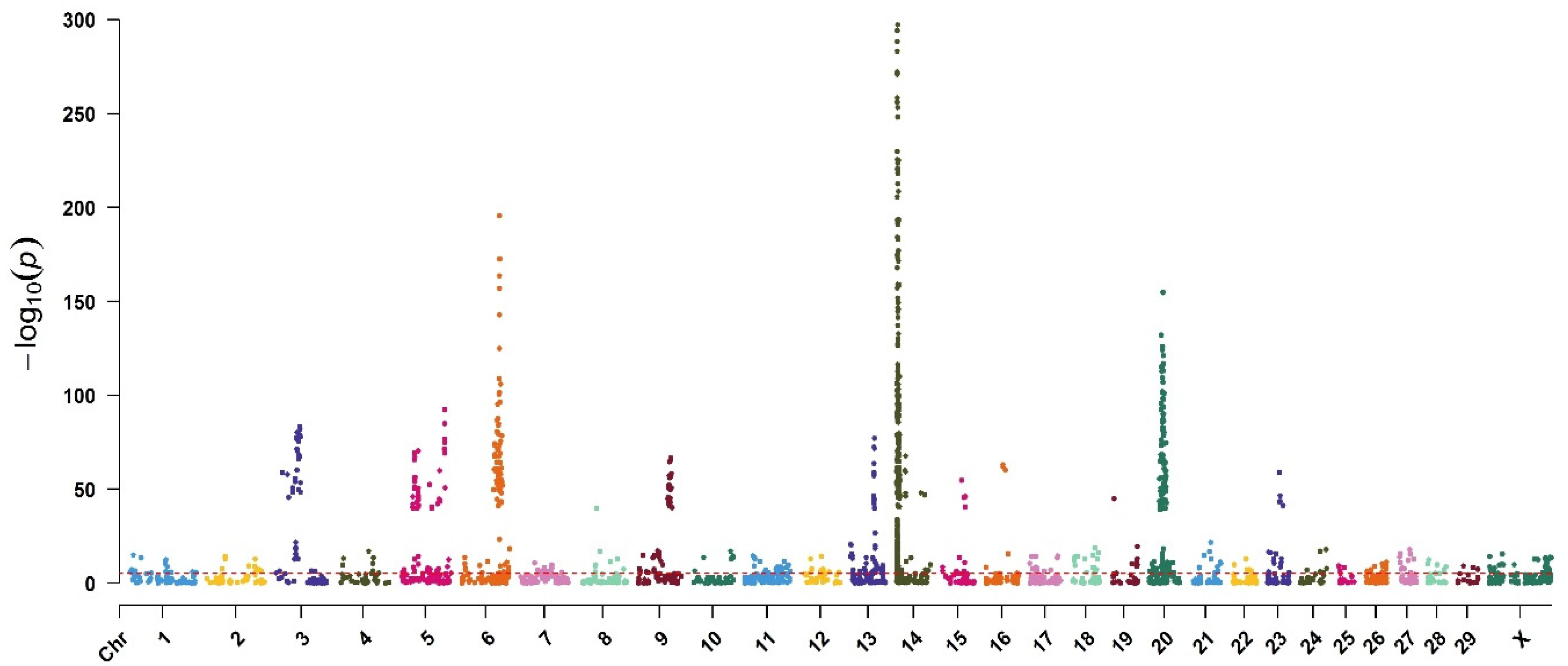
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Goddard, M.E.; Hayes, B.J. Mapping genes for complex traits in domestic animals and their use in breeding programmes. Nat. Rev. Genet. 2009, 10, 381–391. [Google Scholar] [CrossRef] [PubMed]
- Visscher, P.M.; Brown, M.A.; McCarthy, M.I.; Yang, J. Five years of GWAS discovery. Am. J. Hum. Genet. 2012, 90, 7–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gopalakrishnan, S.; Ganeshkumar, P. Systematic reviews and metaanalysis: Understanding the best evidence in primary healthcare. J. Fam. Med. Prim. 2013, 2, 9–14. [Google Scholar]
- Ramasamy, A.; Mondry, A.; Holmes, C.C.; Altman, D.G. Key issues in conducting a meta-analysis of gene expression microarray datasets. PLoS Med. 2008, 5, e184. [Google Scholar] [CrossRef]
- Sharifi, S.; Pakdel, A.; Ebrahimi, M.; Reecy, J.M.; Fazeli Farsani, S.; Ebrahimie, E. Integration of machine learning and meta-analysis identifies the transcriptomic bio-signature of mastitis disease in cattle. PLoS ONE 2018, 13, e0191227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Bakker, P.I.W.; Ferreira, M.A.R.; Jia, X.; Neale, B.M.; Raychaudhuri, S.; Voight, B.F. Practical aspects of imputation-driven meta-analysis of genome-wide association studies. Hum. Mol. Genet. 2008, 17, R122–R128. [Google Scholar] [CrossRef] [Green Version]
- Willer, C.J.; Li, Y.; Abecasis, G.R. METAL: Fast and efficient meta-analysis of genomewide association scans. Bioinformatics 2010, 26, 2190–2191. [Google Scholar] [CrossRef]
- Van den Berg, I.; Xiang, R.; Jenko, J.; Pausch, H.; Boussaha, M.; Schrooten, C.; Tribout, T.; Gjuvsland, A.B.; Boichard, D.; Nordbø, O.; et al. Meta-analysis for milk fat and protein percentage using imputed sequence variant genotypes in 94,321 cattle from eight cattle breeds. Genet. Sel. Evol. 2020, 52, 37. [Google Scholar] [CrossRef]
- El-Halawany, N.; Abdel-Shafy, H.; Shawkya, A.E.M.A.; Abdel-Latif, M.A.; Al-Tohamy, A.F.M.; Abd El-Moneim, O.M. Genome-wide association study for milk production in Egyptian buffalo. Livest. Sci. 2017, 198, 10–16. [Google Scholar] [CrossRef]
- Yodklaew, P.; Koonawootrittriron, S.; Elzo, M.A.; Suwanasopee, T.; Laodim, T. Genome-wide association study for lactation characteristics, milkyield and age at first calving in a Thai multibreed dairy cattle population. Agric. Nat. Resour. 2017, 51, 223–230. [Google Scholar]
- Jiang, L.; Liu, J.; Sun, D.; Ma, P.; Ding, X.; Yu, Y.; Zhang, Q. Genome wide association studies for milk production traits in chinese holstein population. PLoS ONE 2010, 5, e13661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ning, C.; Wang, D.; Zheng, X.; Zhang, Q.; Zhang, S.; Mrode, R.; Liu, J.F. Eigen decomposition expedites longitudinal genome-wide association studies for milk production traits in Chinese Holstein. Genet. Sel. Evol. 2018, 50, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yue, S.J.; Zhao, Y.Q.; Gu, X.R.; Yin, B.; Jiang, Y.L.; Wang, Z.H.; Shi, K.R. A genome-wide association study suggests new candidate genes for milk production traits in Chinese Holstein cattle. Anim. Genet. 2017, 48, 677–681. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Liu, X.; Yang, J.; Wang, H.; Jiang, J.; Liu, L.; He, S.; Ding, X.; Liu, J.; Zhang, Q. Targeted resequencing of GWAS loci reveals novel genetic variants for milk production traits. BMC Genom. 2014, 15, 1105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meredith, B.K.; Kearney, F.J.; Finaly, E.K.; Bradly, D.G.; Fahey, A.G.; Berry, D.P.; Lynn, D.J. Genome-wide associations for milk production and somatic cell score in Holstein-Friesian cattle in Ireland. BMC Genet. 2012, 13, 21. [Google Scholar] [CrossRef] [Green Version]
- Iso-Touru, T.; Sahana, G.; Guldbrandtsen, B.; Lund, M.S.; Vilkki, J. Genome-wide association analysis of milk yield traits in Nordic Red Cattle using imputed whole genome sequence variants. BMC Genet. 2016, 17, 55. [Google Scholar] [CrossRef] [Green Version]
- Fang, L.; Sahana, G.; Su, G.; Yu, Y.; Zhang, S.; Lund, M.S.; Sorensen, P. Integrating sequence-based GWAS and RNA-Seq provides novel insights into the genetic basis of mastitis and milk production in dairy cattle. Sci. Rep. 2017, 7, 45560. [Google Scholar] [CrossRef]
- Guo, J.; Jorjani, H.; Carlborg, O. A genome-wide association study using international breeding-evaluation data identifies major loci affecting production traits and stature in the Brown Swiss cattle breed. BMC Genet. 2012, 13, 82. [Google Scholar] [CrossRef] [Green Version]
- Deng, T.; Liang, A.; Liang, S.; Ma, X.; Lu, X.; Duan, A.; Pang, C.; Hua, G.; Liu, S.; Campanile, G.; et al. Integrative analysis of transcriptome and GWAS data to identify the hub genes associated with milk yield trait in buffalo. Front. Genet. 2019, 10, 36. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.J.; Liang, A.X.; Campanile, G.; Plastow, G.; Zhang, C.; Wang, Z.; Salzano, A.; Gasparrini, B.; Cassandro, M.; Yang, L.G. Genome-wide association studies to identify quantitative trait loci affecting milk production traits in water buffalo. J. Dairy Sci. 2018, 101, 433–444. [Google Scholar] [CrossRef] [Green Version]
- Jiang, J.; Ma, L.; Prakapenka, D.; VanRaden, P.M.; Cole, J.B.; Da, Y. A large-scale genome-wide association study in U.S. Holstein cattle. Front. Genet. 2019, 10, 412. [Google Scholar] [CrossRef] [PubMed]
- Nayeri, S.; Sargolzaei, M.; Abo-Ismail, M.K.; May, N.; Miller, S.P.; Schenkel, F.; Moore, S.S.; Stothard, P. Genome-wide association for milk production and female fertility traits in Canadian dairy Holstein cattle. BMC Genet. 2016, 17, 75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cole, J.B.; Wiggans, G.R.; Ma, L.; Sonstegard, T.S.; Lawlor, T.J., Jr.; Crooker, B.A.; Tassell, C.P.V.; Yang, J.; Wang, S.; Matukumalli, L.K.; et al. Genome-wide association analysis of thirty one production, health, reproduction and body conformation traits in contemporary U.S. Holstein cows. BMC Genom. 2011, 12, 408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raven, L.A.; Cocks, B.G.; Hayes, B.J. Multibreed genome wide association can improve precision of mapping causative variants underlying milk production in dairy cattle. BMC Genom. 2014, 15, 62. [Google Scholar] [CrossRef] [Green Version]
- Jing, L.; Boeren, S.; Hooijdonk, T.V.; Vervoort, J.; Hettinga, K. Effect of the DGAT1 K232A genotype of dairy cows on the milk metabolome and proteome. J. Dairy Sci. 2015, 98, 3460–3469. [Google Scholar]
- Schultz, G.; Rotatori, D.S.; Clark, W. EGF and TGF-α in Wound Healing and Repair. J. Cell. Biochem. 1991, 45, 346–352. [Google Scholar] [CrossRef]
- Hemmati, A.A.; Larki-Harchegani, A.; Shabib, S.; Jalali, A.; Rezaei, A.; Housmand, G. Wound healing property of milk in full thickness wound model of rabbit. Int. J. Surg. 2018, 54, 133–140. [Google Scholar] [CrossRef]
- Katie, A.; Cohen-Fix, F.; Cohen-Fix, O. The metaphase to anaphase transition A case of productive destruction. Eur. J. Biochem. 1999, 263, 14–19. [Google Scholar]
- Friedl, P. Prespecification and plasticity: Shifting mechanisms of cell migration. Cell Biol. 2004, 16, 14–23. [Google Scholar] [CrossRef]
- Friedl, P.; Wolf, K. Tumour-cell invasion and migration: Diversity and escape mechanisms. Nat. Rev. Cancer 2003, 3, 362–374. [Google Scholar] [CrossRef]
- Yue, P.Y.K.; Leung, E.P.Y.; Mak, N.K.; Wong, R.N.S. A Simplified Method for Quantifying Cell Migration/Wound Healing in 96-Well Plates. J. Biomol. Screen. 2010, 15, 427–433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jouanneau, J.; Thiery, J.P. Encyclopedia of Cancer, 2nd ed.; Tumor Cell Motility and Invasion Chapter; Academic Press: Cambridge, MA, USA, 2002; pp. 467–473. [Google Scholar]
- Caliari, S.R.; Harley, B.A.C. Comprehensive Biomaterials, Biologically Inspired and Biomolecular Materials chapter. ScienceDirect 2011, 2, 279–302. [Google Scholar]
- Banabazi, M.H.; Javaremi, A.N.; Imumorin, I.G.; Ghaderi-Zefrei, M.; Miraei Ashtiani, S.R. Single nucleotide polymorphisms (SNP) on transcriptome of Holstein cows shared with Illumina bovine SNP arrays. Online J. Vet. Res. 2016, 20, 177–182. [Google Scholar]
| CHR Number | Length (bp) | No. SNPs on CHR |
|---|---|---|
| 1 | 158,337,067 | 15 |
| 2 | 137,060,424 | 10 |
| 3 | 121,430,405 | 41 |
| 4 | 120,829,699 | 6 |
| 5 | 121,191,424 | 65 |
| 6 | 119,458,736 | 87 |
| 7 | 112,638,659 | 12 |
| 8 | 113,384,836 | 9 |
| 9 | 105,708,250 | 35 |
| 10 | 104,305,016 | 6 |
| 11 | 107,310,763 | 23 |
| 12 | 91,163,125 | 9 |
| 13 | 84,2403,50 | 45 |
| 14 | 84,648,390 | 950 |
| 15 | 85,296,676 | 13 |
| 16 | 81,724,687 | 5 |
| 17 | 75,158,596 | 13 |
| 18 | 66,004,023 | 14 |
| 19 | 64,057,457 | 12 |
| 20 | 72,042,655 | 224 |
| 21 | 71,599,096 | 10 |
| 22 | 61,435,874 | 5 |
| 23 | 52,530,062 | 17 |
| 24 | 62,714,930 | 16 |
| 25 | 42,904,170 | 13 |
| 26 | 51,681,464 | 11 |
| 27 | 45,407,902 | 11 |
| 28 | 46,312,546 | 5 |
| 29 | 51,505,224 | 3 |
| X | 148,823,899 | 34 |
| 1712 |
| CHR Number | SNP Name | Position | Overlapped Genes | p-Value |
|---|---|---|---|---|
| 14 | rs109421300 | 1801116 | DGAT1 | 2.93 × 10−771 |
| 14 | rs135549651 | 1967325 | ENSBTAG00000015040 | 1.12 × 10−710 |
| 14 | rs109146371 | 1651311 | 1.82 × 10−653 | |
| 14 | rs109350371 | 2054457 | 1.90 × 10−637 | |
| 5 | BovineHD410000357 | 32784231 | RPAP3 | 3.10 × 10−416 |
| 14 | rs109558046 | 2909929 | 1.44 × 10−396 | |
| 14 | rs109752439 | 1489496 | 1.17 × 10−366 | |
| 14 | rs110199901 | 2524432 | 4.10 × 10−298 | |
| 14 | rs110706284 | 2398876 | ZC3H3 | 6.76 × 10−295 |
| 14 | rs41627764 | 2276443 | 5.13 × 10−289 | |
| 14 | rs41629750 | 2002873 | 6.61 × 10−284 | |
| 14 | rs137205809 | 1892559 | MROH1 | 6.36 × 10−273 |
| 14 | rs137787931 | 1880378 | MROH1 | 1.44 × 10−272 |
| 14 | rs133119726 | 1868636 | MROH1 | 9.39 × 10−272 |
| 14 | rs109742607 | 2217163 | 4.47 × 10−259 | |
| 14 | rs41256919 | 1923292 | MAF1 | 3.28 × 10−257 |
| 14 | rs110323635 | 2239085 | MAPK15 | 9.29 × 10−257 |
| 14 | rs109529219 | 2468020 | RHPN1 | 5.37 × 10−254 |
| 14 | rs110060785 | 2553525 | 6.91 × 10−249 | |
| 14 | rs17870736 | 1696470 | VPS28 | 1.86 × 10−230 |
| 14 | rs110892754 | 2117455 | 2.82 × 10−226 | |
| 14 | rs109086264 | 4414829 | TRAPPC9 | 7.94 × 10−226 |
| 14 | rs110174651 | 2754909 | 3.71 × 10−224 | |
| 14 | rs136891853 | 2764862 | 2.95 × 10−221 | |
| 14 | rs110749653 | 2138926 | ENSBTAT00000065585 | 3.89 × 10−221 |
| 14 | rs110411273 | 3640788 | 1.20 × 10−219 | |
| 14 | rs110626984 | 2674264 | 8.13 × 10−219 | |
| 14 | rs29024688 | 3297177 | 2.57 × 10−213 | |
| 14 | rs55617160 | 4468478 | TRAPPC9 | 3.02 × 10−209 |
| 14 | rs134974438 | 2150825 | 2.63 × 10−206 | |
| 6 | rs110527224 | 88592295 | 3.23 × 10−196 | |
| 14 | rs110143087 | 4767039 | 1.51 × 10−194 | |
| 14 | rs109530164 | 4456595 | TRAPPC9 | 2.40 × 10−194 |
| 14 | rs137757978 | 2164419 | 6.16 × 10−194 | |
| 14 | rs109225594 | 4848750 | 1.00 × 10−193 | |
| 14 | rs109545018 | 3006509 | ADGRB1 | 6.81 × 10−192 |
| 14 | rs109968515 | 1675278 | CYHR1 | 5.37 × 10−185 |
| 14 | rs110251237 | 4068825 | 1.70 × 10−184 | |
| 14 | rs110185345 | 4043743 | PTK2 | 4.90 × 10−184 |
| 14 | rs111018678 | 4336714 | TRAPPC9 | 7.76 × 10−178 |
| 14 | rs137309662 | 3371507 | 2.57 × 10−176 | |
| 14 | rs135270011 | 2084067 | PLEC | 2.82 × 10−175 |
| 14 | rs108992746 | 2951045 | ADGRB1 | 7.76 × 10−174 |
| 6 | rs137147462 | 88887995 | 2.63 × 10−173 | |
| 6 | rs110694875 | 89139865 | 2.75 × 10−173 | |
| 14 | rs110017379 | 4364952 | TRAPPC9 | 4.07 × 10−172 |
| 14 | rs41602530 | 2194228 | SCRIB | 1.66 × 10−168 |
| 6 | rs42766480 | 88891318 | 2.82 × 10−164 | |
| 14 | rs719209105 | 2741434 | GML | 8.32 × 10−160 |
| 14 | rs110501942 | 5494654 | FAM135B | 1.74 × 10−159 |
| Term ID | Term Name | p-Value (Adj) | Gene Name | Number |
|---|---|---|---|---|
| GO:0042060 | Wound healing | 0.033647963 | ENSBTAT00000065585, PTK2, PLEC, SCRIB | 4 |
| GO:0009611 | Response to wounding | 0.039101239 | ENSBTAT00000065585, PTK2, PLEC, SCRIB | 4 |
| GO:0006903 | Vesicle targeting | 0.048695181 | MAPK15, SCRIB | 2 |
| GO:1905188 | Positive regulation of metaphase/anaphase transition of meiosis I | 0.048695181 | MAPK15 | 1 |
| GO:1905186 | Regulation of metaphase/anaphase transition of meiosis I | 0.048695181 | MAPK15 | 1 |
| GO:1905134 | Positive regulation of meiotic chromosome separation | 0.048695181 | MAPK15 | 1 |
| GO:1902104 | Positive regulation of metaphase/anaphase transition of meiotic cell cycle | 0.048695181 | MAPK15 | 1 |
| GO:0098968 | Neurotransmitter receptor transport postsynaptic membrane to endosome | 0.048695181 | SCRIB | 1 |
| GO:0051271 | Negative regulation of cellular component movement | 0.048695181 | MAPK15, ENSBTAT00000065585, ADGRB1 | 3 |
| GO:0045104 | Intermediate filament cytoskeleton organization | 0.048695181 | ENSBTAT00000065585, PLEC | 2 |
| GO:0045103 | Intermediate filament-based process | 0.048695181 | ENSBTAT00000065585, PLEC | 2 |
| GO:0040013 | Negative regulation of locomotion | 0.048695181 | MAPK15, ENSBTAT00000065585, ADGRB1 | 3 |
| GO:0030336 | Negative regulation of cell migration | 0.048695181 | MAPK15, ENSBTAT00000065585, ADGRB1 | 3 |
| GO:1990949 | Metaphase/anaphase transition of meiosis I | 0.048695181 | MAPK15 | 1 |
| GO:2000146 | Negative regulation of cell motility | 0.048695181 | MAPK15, ENSBTAT00000065585, ADGRB1 | 3 |
| Continent | Studies | N 1 | No. SNPs 2 | Refs. |
|---|---|---|---|---|
| Africa | 1 | 250 | 20 | [9] |
| Asia | 5 | 13,188 | 74 | [10,11,12,13,14] |
| Europe | 5 | 22,384 | 1542 | [15,16,17,18,19] |
| North America | 4 | 299,951 | 2309 | [20,21,22,23] |
| Australia | 1 | 17,925 | 5 | [24] |
| Global | 16 | 353,698 | 3950 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taherkhani, L.; Banabazi, M.H.; EmamJomeh-Kashan, N.; Noshary, A.; Imumorin, I. The Candidate Chromosomal Regions Responsible for Milk Yield of Cow: A GWAS Meta-Analysis. Animals 2022, 12, 582. https://doi.org/10.3390/ani12050582
Taherkhani L, Banabazi MH, EmamJomeh-Kashan N, Noshary A, Imumorin I. The Candidate Chromosomal Regions Responsible for Milk Yield of Cow: A GWAS Meta-Analysis. Animals. 2022; 12(5):582. https://doi.org/10.3390/ani12050582
Chicago/Turabian StyleTaherkhani, Lida, Mohammad Hossein Banabazi, Nasser EmamJomeh-Kashan, Alireza Noshary, and Ikhide Imumorin. 2022. "The Candidate Chromosomal Regions Responsible for Milk Yield of Cow: A GWAS Meta-Analysis" Animals 12, no. 5: 582. https://doi.org/10.3390/ani12050582
APA StyleTaherkhani, L., Banabazi, M. H., EmamJomeh-Kashan, N., Noshary, A., & Imumorin, I. (2022). The Candidate Chromosomal Regions Responsible for Milk Yield of Cow: A GWAS Meta-Analysis. Animals, 12(5), 582. https://doi.org/10.3390/ani12050582






