Hepatoprotective Effect of the Penthorum Chinense Pursh Extract against the CCl4-Induced Acute Liver Injury via NF-κB and p38-MAPK PATHWAYS in Dogs
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experiment Design and Sample Collection
2.2. Preparation of PCPE
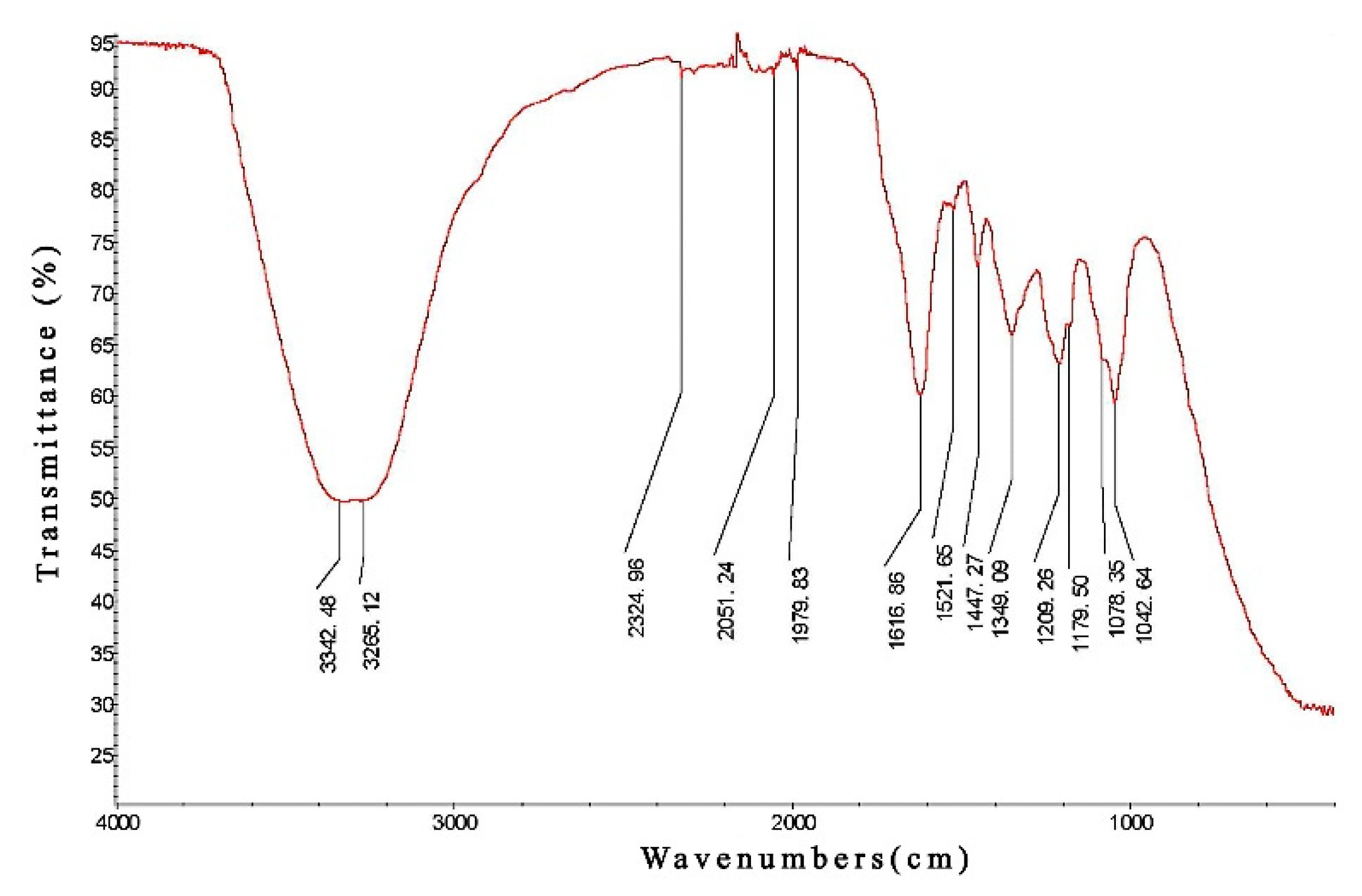
2.3. Identification of Active Ingredients of PCPE by Infrared Spectroscopy
2.4. Criteria for Judging Efficacy
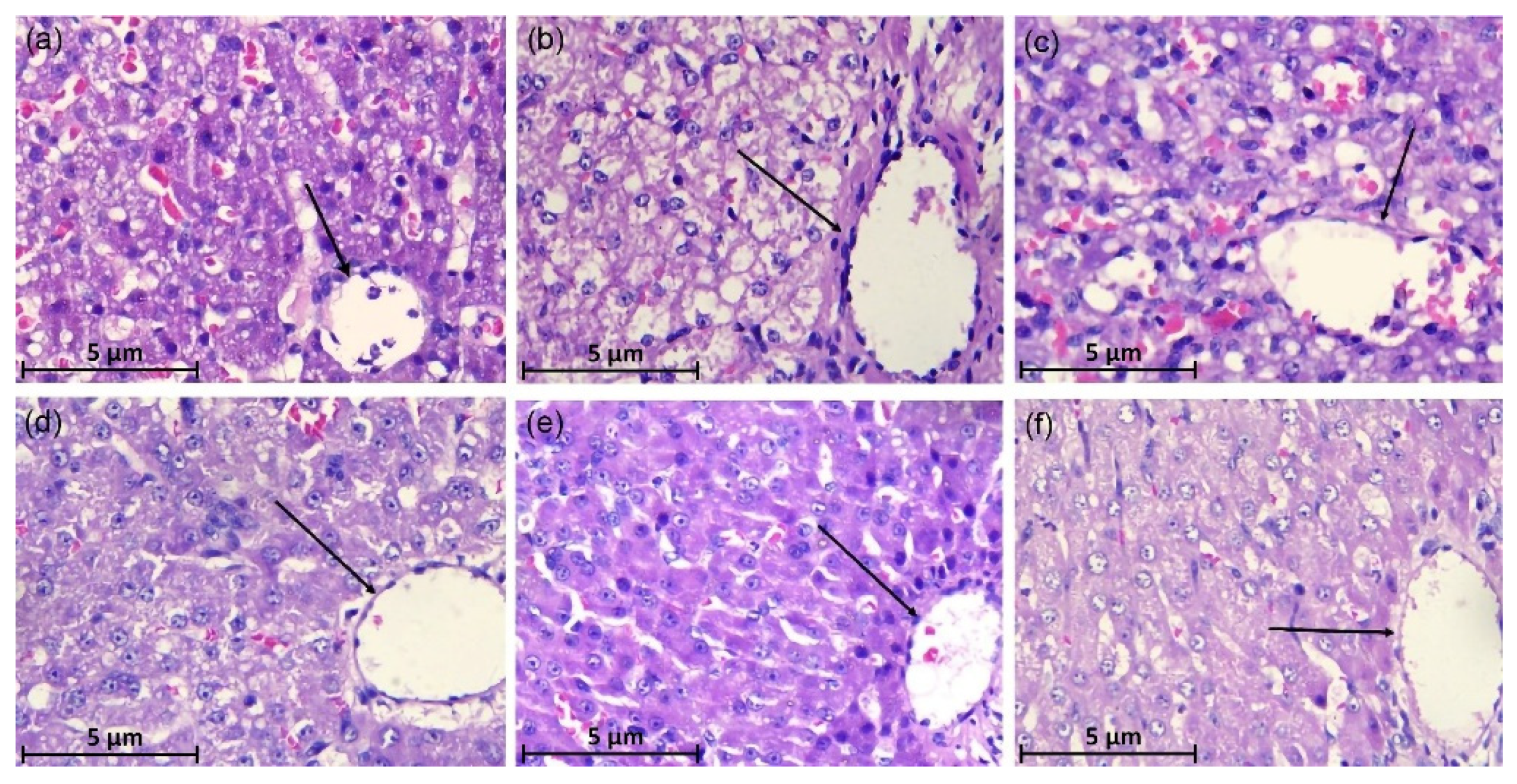
2.5. Histopathology of Liver
2.6. Biochemical Assay
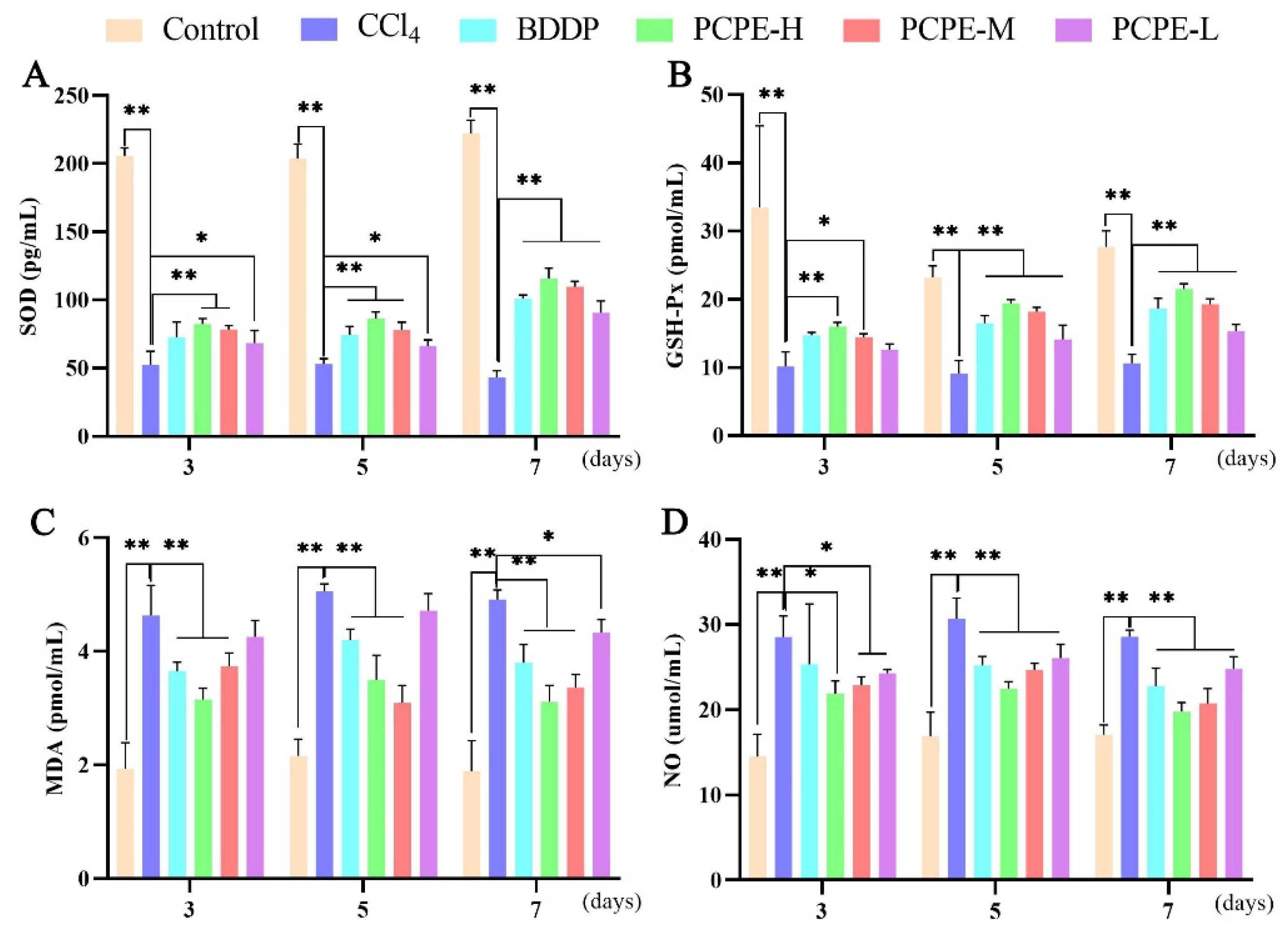
2.7. Oxidative and Inflammatory Assay
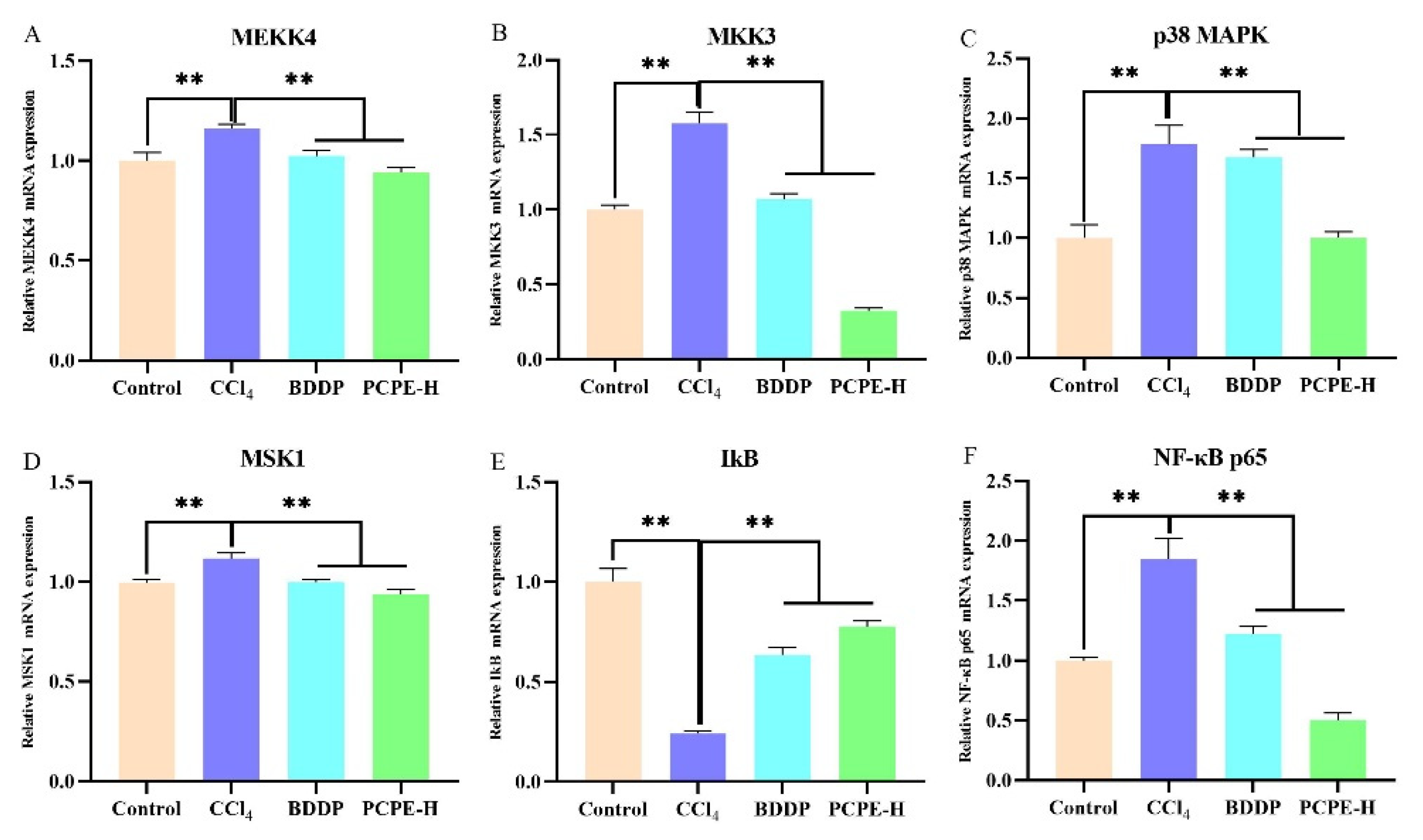
2.8. RT-qPCR
2.9. Statistical Analysis
3. Results
3.1. Infrared Spectroscopy Analysis
3.2. Effect of PCPE on Clinical Symptoms of ALI Dogs
3.3. Histopathological of Liver Tissues
3.4. Biochemical Analysis of Serum
3.5. Serum Antioxidant Indexes
3.6. Analysis of Inflammatory Cytokine
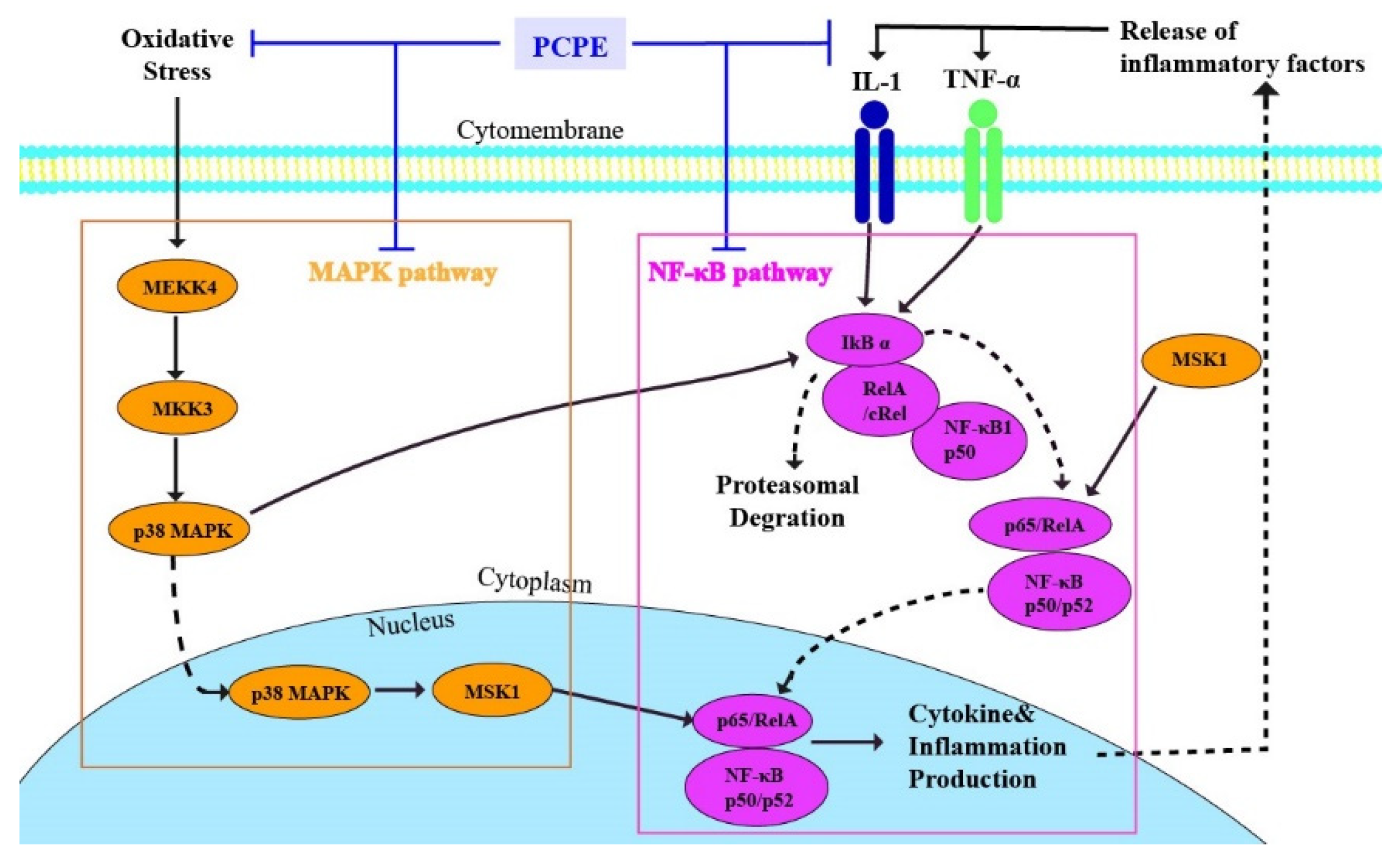
3.7. PCPE Effects on Transcription of MAPK/NF-κB
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kim, S.R.; Park, E.J.; Dusabimana, T.; Je, J.; Jeong, K.; Yun, S.P.; Kim, H.J.; Cho, K.M.; Kim, H.; Park, S.W. Platycodon grandiflorus Fermented Extracts Attenuate Endotoxin-Induced Acute Liver Injury in Mice. Nutrients 2020, 12, 2802. [Google Scholar] [CrossRef] [PubMed]
- Ning, C.; Gao, X.; Wang, C.; Huo, X.; Liu, Z.; Sun, H.; Yang, X.; Sun, P.; Ma, X.; Meng, Q.; et al. Hepatoprotective effect of ginsenoside Rg1 from Panax ginseng on carbon tetrachloride-induced acute liver injury by activating Nrf2 signaling pathway in mice. Environ. Toxicol. 2018, 33, 1050–1060. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Kuang, G.; Wan, J.; Jiang, R.; Ma, L.; Gong, X.; Liu, X. Salidroside protects mice against CCl4-induced acute liver injury via down-regulating CYP2E1 expression and inhibiting NLRP3 inflammasome activation. Int. Immunopharmacol. 2020, 85, 106662. [Google Scholar] [CrossRef]
- Zhao, L.; Jin, Y.; Donahue, K.; Tsui, M.; Fish, M.; Logan, C.Y.; Wang, B.; Nusse, R. Tissue Repair in the Mouse Liver Following Acute Carbon Tetrachloride Depends on Injury-Induced Wnt/β-Catenin Signaling. Hepatology 2019, 69, 2623–2635. [Google Scholar] [CrossRef] [PubMed]
- Munakarmi, S.; Chand, L.; Shin, H.B.; Jang, K.Y.; Jeong, Y.J. Indole-3-Carbinol Derivative DIM Mitigates Carbon Tetrachloride-Induced Acute Liver Injury in Mice by Inhibiting Inflammatory Response, Apoptosis and Regulating Oxidative Stress. Int. J. Mol. Sci. 2020, 21, 2048. [Google Scholar] [CrossRef] [PubMed]
- Ko, I.G.; Jin, J.J.; Hwang, L.; Kim, S.H.; Kim, C.J.; Han, J.H.; Lee, S.; Kim, H.I.; Shin, H.P.; Jeon, J.W. Polydeoxyribonucleotide Exerts Protective Effect Against CCl4-Induced Acute Liver Injury Through Inactivation of NF-κB/MAPK Signaling Pathway in Mice. Int. J. Mol. Sci. 2020, 21, 7894. [Google Scholar] [CrossRef]
- Qian, S.; Li, C.; Liu, X.; Jia, X.; Xiao, Y.; Li, Z. Activation of the JNK/MAPK Signaling Pathway by TGF-β1 Enhances Neonatal Fc Receptor Expression and IgG Transcytosis. Microorganism 2021, 9, 879. [Google Scholar] [CrossRef]
- Yin, P.; Zhang, Z.; Li, J.; Shi, Y.; Jin, N.; Zou, W.; Gao, Q.; Wang, W.; Liu, F. Ferulic acid inhibits bovine endometrial epithelial cells against LPS-induced inflammation via suppressing NK-κB and MAPK pathway. Res. Vet. Sci. 2019, 126, 164–169. [Google Scholar] [CrossRef]
- Xie, C.; Li, X.; Zhu, J.; Wu, J.; Geng, S.; Zhong, C. Magnesium isoglycyrrhizinate suppresses LPS-induced inflammation and oxidative stress through inhibiting NF-κB and MAPK pathways in RAW264.7 cells. Bioorg. Med. Chem. 2019, 27, 516–524. [Google Scholar] [CrossRef]
- Canty, T.G., Jr.; Boyle, E.M., Jr.; Farr, A.; Morgan, E.N.; Verrier, E.D.; Pohlman, T.H. Oxidative stress induces NF-κB nuclear translocation without degradation of IκBα. Circulation 1999, 100, Ii361–Ii364. [Google Scholar] [CrossRef]
- Ali, M.; Khan, T.; Fatima, K.; Ali, Q.U.A.; Ovais, M.; Khalil, A.T.; Ullah, I.; Raza, A.; Shinwari, Z.K.; Idrees, M. Selected hepatoprotective herbal medicines: Evidence from ethnomedicinal applications, animal models, and possible mechanism of actions. Phytother. Res. PTR 2018, 32, 199–215. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Lin, L.; Wang, Y. Traditional Chinese Herbal Medicine Penthorum chinense Pursh: A Phytochemical and Pharmacological Review. Am. J. Chin. Med. 2015, 43, 601–620. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Li, M.; Huang, H.; Xiao, Z.; Shen, J.; Zhao, Y.; Yin, J.; Kaboli, P.J.; Cao, J.; Cho, C.H.; et al. A review of Penthorum chinense Pursh for hepatoprotection: Traditional use, phytochemistry, pharmacology, toxicology and clinical trials. J. Ethnopharmacol. 2020, 251, 112569. [Google Scholar] [CrossRef] [PubMed]
- Jeong, D.; Lee, J.; Park, S.H.; Kim, Y.A.; Park, B.J.; Oh, J.; Sung, G.-H.; Aravinthan, A.; Kim, J.-H.; Kang, H.; et al. Antiphotoaging and Antimelanogenic Effects of Penthorum chinense Pursh Ethanol Extract due to Antioxidant- and Autophagy-Inducing Properties. Oxid. Med. Cell. Longev. 2019, 2019, 9679731. [Google Scholar] [CrossRef]
- Zhao, W.W.; Guo, W.; Guo, J.F.; Wang, X.; Chen, X.Q.; Wu, X. Three new flavonoids from Penthorum chinense Pursh and their docking studies. Nat. Prod. Res. 2021, 35, 49–56. [Google Scholar] [CrossRef]
- Sun, Y.; He, L.; Wang, W.; Wang, T.; Hua, W.; Li, T.; Wang, L.; Gao, T.; Chen, F.; Tang, L. Polyphenols from Penthorum chinense Pursh. Attenuates high glucose-induced vascular inflammation through directly interacting with Keap1 protein. J. Ethnopharmacol. 2021, 268, 113617. [Google Scholar] [CrossRef]
- Zhang, T.T.; Xu, X.L.; Jiang, M.H.; Jiang, J.G. Hepatoprotective function of Penthorum chinense Pursh. Food Funct. 2013, 4, 1581–1585. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, X.J.; Feng, R.; Jiang, Y.; Zhang, D.Y.; He, C.; Li, P.; Wan, J.B. Hepatoprotective properties of Penthorum chinense Pursh against carbon tetrachloride-induced acute liver injury in mice. Chin. Med. 2017, 12, 32. [Google Scholar] [CrossRef]
- Cao, Y.W.; Jiang, Y.; Zhang, D.Y.; Wang, M.; Chen, W.S.; Su, H.; Wang, Y.T.; Wan, J.B. Protective effects of Penthorum chinense Pursh against chronic ethanol-induced liver injury in mice. J. Ethnopharmacol. 2015, 161, 92–98. [Google Scholar] [CrossRef]
- Blanchard, O.L.; Smoliga, J.M. Translating dosages from animal models to human clinical trials—Revisiting body surface area scaling. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2015, 29, 1629–1634. [Google Scholar] [CrossRef]
- Wenie, Z.; Jianqin, X. Zhongshouyixue, 4th ed.; China Agriculture Press: Beijing, China, 2014; p. 418. [Google Scholar]
- Nabi, F.; Shahzad, M.; Liu, J.; Li, K.; Han, Z.; Zhang, D.; Iqbal, M.K.; Li, J. Hsp90 inhibitor celastrol reinstates growth plate angiogenesis in thiram-induced tibial dyschondroplasia. Avian Pathol. 2016, 45, 187–193. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Machado, N.F.L.; de Carvalho, L.A.E.B.; Otero, J.C.; Marques, M.P.M. A conformational study of hydroxyflavones by vibrational spectroscopy coupled to DFT calculations. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2013, 109, 116–124. [Google Scholar] [CrossRef]
- Farzaei, M.H.; Zobeiri, M.; Parvizi, F.; El-Senduny, F.F.; Marmouzi, I.; Coy-Barrera, E.; Naseri, R.; Nabavi, S.M.; Rahimi, R.; Abdollahi, M. Curcumin in Liver Diseases: A Systematic Review of the Cellular Mechanisms of Oxidative Stress and Clinical Perspective. Nutrients 2018, 10, 855. [Google Scholar] [CrossRef] [PubMed]
- Ogaly, H.A.; Eltablawy, N.A.; El-Behairy, A.M.; El-Hindi, H.; Abd-Elsalam, R.M. Hepatocyte Growth Factor Mediates the Antifibrogenic Action of Ocimum bacilicum Essential Oil against CCl4-Induced Liver Fibrosis in Rats. Molecules 2015, 20, 13518–13535. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.-Q.; Li, Z.; Xie, W.-R.; Liu, C.-M.; Liu, S.-S. Quercetin protects mouse liver against CCl4-induced inflammation by the TLR2/4 and MAPK/NF-κB pathway. Int. Immunopharmacol. 2015, 28, 531–539. [Google Scholar] [CrossRef]
- Du, Y.C.; Lai, L.; Zhang, H.; Zhong, F.R.; Cheng, H.L.; Qian, B.L.; Tan, P.; Xia, X.M.; Fu, W.G. Kaempferol from-Penthorum chinense-Pursh suppresses HMGB1/TLR4/NF-κB signaling and NLRP3 inflammasome activation in acetaminophen-induced hepatotoxicity. Food Funct. 2020, 11, 7925–7934. [Google Scholar] [CrossRef]
- Feng, H.; Wang, Z.M.; Dong, G.Y.; Wu, Z. Studies on chemical constitutents from Penthorum chinense Pursh. China J. Chin. Mater. Med. 2001, 26, 260–262. [Google Scholar]
- Ming, F.; Lin, W.; Juan, Y.; Zhaotun, H. Chemical Constituents of Penthorum chinense Pursh. Chin. Pharm. J. 2013, 48, 1911–1914. [Google Scholar]
- He, L.; Zhang, S.; Luo, C.; Sun, Y.; Lu, Q.; Huang, L.; Chen, F.; Tang, L. Functional Teas from the Stems of Penthorum chinense Pursh.: Phenolic Constituents, Antioxidant and Hepatoprotective Activity. Plant Foods Hum. Nutr. 2018, 74, 83–90. [Google Scholar] [CrossRef]
- Guo, W.W.; Qiu, F.; Chen, X.Q.; Ba, Y.Y.; Wang, X.; Wu, X. In-vivo absorption of pinocembrin-7-O-β-D-glucoside in rats and its in-vitro biotransformation. Sci. Rep. 2016, 6, 29340. [Google Scholar] [CrossRef]
- Frattaruolo, L.; Carullo, G.; Brindisi, M.; Mazzotta, S.; Bellissimo, L.; Rago, V.; Curcio, R.; Dolce, V.; Aiello, F.; Cappello, A.R. Antioxidant and Anti-Inflammatory Activities of Flavanones from Glycyrrhiza glabra L. (licorice) Leaf Phytocomplexes: Identification of Licoflavanone as a Modulator of NF-kB/MAPK Pathway. Antioxidants 2019, 8, 186. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.P.; Son, K.H.; Chang, H.W.; Kang, S.S. Anti-Inflammatory Plant Flavonoids and Cellular Action Mechanisms. J. Pharmacol. Sci. 2004, 96, 229–245. [Google Scholar] [CrossRef] [PubMed]
- Nori, S.L.; Aquino, R.P.; Nicolin, V.; Santoro, A. Flavonoids and flavonoid-rich natural extracts inhibit cytokine release in cystic fibrosis bronchial epithelial cells by regulating NF-kB pathway. Ital. J. Anat. Embryol. 2015, 120, 53. [Google Scholar]
- Trefts, E.; Gannon, M.; Wasserman, D.H. The liver. Curr. Biol. 2017, 27, R1147–R1151. [Google Scholar] [CrossRef] [PubMed]
- Schwabe, R.F.; Luedde, T. Apoptosis and necroptosis in the liver: A matter of life and death. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 738–752. [Google Scholar] [CrossRef]
- Ding, H.-R.; Wang, J.-L.; Ren, H.-Z.; Shi, X.-L. Lipometabolism and Glycometabolism in Liver Diseases. BioMed Res. Int. 2018, 2018, 1287127. [Google Scholar] [CrossRef]
- Sookoian, S.; Pirola, C.J. Liver enzymes, metabolomics and genome-wide association studies: From systems biology to the personalized medicine. World J. Gastroenterol. 2015, 21, 711–725. [Google Scholar] [CrossRef]
- Zheng, J.; Tian, X.; Xu, B.; Yuan, F.; Gong, J.; Yang, Z. Collagen Peptides from Swim Bladders of Giant Croaker (Nibea japonica) and Their Protective Effects against H2O2-Induced Oxidative Damage toward Human Umbilical Vein Endothelial Cells. Mar. Drugs 2020, 18, 430. [Google Scholar] [CrossRef]
- Melekoglu, R.; Ciftci, O.; Eraslan, S.; Cetin, A.; Basak, N. Beneficial effects of curcumin and capsaicin on cyclophosphamide-induced premature ovarian failure in a rat model. J. Ovarian Res. 2018, 11, 33. [Google Scholar] [CrossRef]
- Tejero, J.; Shiva, S.; Gladwin, M.T. Sources of Vascular Nitric Oxide and Reactive Oxygen Species and Their Regulation. Physiol. Rev. 2019, 99, 311–379. [Google Scholar] [CrossRef]
- Dai, C.; Xiao, X.; Li, D.; Tun, S.; Wang, Y.; Velkov, T.; Tang, S. Chloroquine ameliorates carbon tetrachloride-induced acute liver injury in mice via the concomitant inhibition of inflammation and induction of apoptosis. Cell Death Dis. 2018, 9, 1164. [Google Scholar] [CrossRef] [PubMed]
- Kany, S.; Vollrath, J.T.; Relja, B. Cytokines in Inflammatory Disease. Int. J. Mol. Sci. 2019, 20, 6008. [Google Scholar] [CrossRef] [PubMed]
- Cavalcanti, M.R.M.; Passos, F.R.S.; Monteiro, B.S.; Gandhi, S.R.; Heimfarth, L.; Lima, B.S.; Nascimento, Y.M.; Duarte, M.C.; Araujo, A.A.S.; Menezes, I.R.A.; et al. HPLC-DAD-UV analysis, anti-inflammatory and anti-neuropathic effects of methanolic extract of Sideritis bilgeriana (lamiaceae) by NF-κB, TNF-α, IL-1β and IL-6 involvement. J. Ethnopharmacol. 2021, 265, 113338. [Google Scholar] [CrossRef] [PubMed]
- Negash, A.A.; Ramos, H.J.; Crochet, N.; Lau, D.T.Y.; Doehle, B.; Papic, N.; Delker, D.A.; Jo, J.; Bertoletti, A.; Hagedorn, C.H.; et al. IL-1β production through the NLRP3 inflammasome by hepatic macrophages links hepatitis C virus infection with liver inflammation and disease. PLoS Pathog. 2013, 9, e1003330. [Google Scholar] [CrossRef]
- Gao, R.Y.; Wang, M.; Liu, Q.; Feng, D.; Wen, Y.; Xia, Y.; Colgan, S.P.; Eltzschig, H.K.; Ju, C. Hypoxia-Inducible Factor-2α Reprograms Liver Macrophages to Protect Against Acute Liver Injury Through the Production of Interleukin-6. Hepatology 2020, 71, 2105–2117. [Google Scholar] [CrossRef]
- Liu, A.; Sun, Y.; Wang, X.; Ihsan, A.; Tao, Y.; Chen, D.; Peng, D.; Wu, Q.; Wang, X.; Yuan, Z. DNA methylation is involved in pro-inflammatory cytokines expression in T-2 toxin-induced liver injury. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2019, 132, 110661. [Google Scholar] [CrossRef]
- Liu, Y.; Wen, P.-H.; Zhang, X.-X.; Dai, Y.; He, Q. Breviscapine ameliorates CCl4-induced liver injury in mice through inhibiting inflammatory apoptotic response and ROS generation. Int. J. Mol. Med. 2018, 42, 755–768. [Google Scholar] [CrossRef]
- He, Y.; Hwang, S.; Ahmed, Y.A.; Feng, D.; Li, N.; Ribeiro, M.; Lafdil, F.; Kisseleva, T.; Szabo, G.; Gao, B. Immunopathobiology and therapeutic targets related to cytokines in liver diseases. Cell. Mol. Immunol. 2021, 18, 18–37. [Google Scholar] [CrossRef]
- Carvalho, A.M.S.; Heimfarth, L.; Pereira, E.W.M.; Oliveira, F.S.; Menezes, I.R.A.; Coutinho, H.D.M.; Picot, L.; Antoniolli, A.R.; Quintans, J.S.S.; Quintans-Júnior, L.J. Phytol, a Chlorophyll Component, Produces Antihyperalgesic, Anti-inflammatory, and Antiarthritic Effects: Possible NFκB Pathway Involvement and Reduced Levels of the Proinflammatory Cytokines TNF-α and IL-6. J. Nat. Prod. 2020, 83, 1107–1117. [Google Scholar] [CrossRef]
- An, S.Y.; Petrescu, A.D.; DeMorrow, S. Targeting Certain Interleukins as Novel Treatment Options for Liver Fibrosis. Front. Pharmacol. 2021, 12, 645703. [Google Scholar] [CrossRef]
- Sun, X.; Wu, A.; Law, B.Y.K.; Liu, C.; Zeng, W.; Qiu, A.C.L.; Han, Y.; He, Y.; Wong, V.K.W. The active components derived from Penthorum chinense Pursh protect against oxidative-stress-induced vascular injury via autophagy induction. Free Radic. Biol. Med. 2020, 146, 160–180. [Google Scholar] [CrossRef] [PubMed]
- Hammouda, M.B.; Ford, A.E.; Liu, Y.; Zhang, J.Y. The JNK Signaling Pathway in Inflammatory Skin Disorders and Cancer. Cells 2020, 9, 857. [Google Scholar] [CrossRef] [PubMed]
- Coulthard, L.R.; White, D.E.; Jones, D.L.; McDermott, M.F.; Burchill, S.A. p38MAPK: Stress responses from molecular mechanisms to therapeutics. Trends Mol. Med. 2009, 15, 369–379. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; She, H.; Zhang, T.; Xu, H.; Cheng, L.; Yepes, M.; Zhao, Y.; Mao, Z. p38 MAPK inhibits autophagy and promotes microglial inflammatory responses by phosphorylating ULK1. J. Cell Biol. 2018, 217, 315–328. [Google Scholar] [CrossRef] [PubMed]
- Nie, Y.; Wang, Z.; Chai, G.; Xiong, Y.; Li, B.; Zhang, H.; Xin, R.; Qian, X.; Tang, Z.; Wu, J.; et al. Dehydrocostus Lactone Suppresses LPS-induced Acute Lung Injury and Macrophage Activation through NF-κB Signaling Pathway Mediated by p38 MAPK and Akt. Molecules 2019, 24, 1510. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.C. The non-canonical NF-κB pathway in immunity and inflammation. Nat. Rev. Immunol. 2017, 17, 545–558. [Google Scholar] [CrossRef]
- Liang, W.-J.; Yang, H.-W.; Liu, H.-N.; Qian, W.; Chen, X.-L. HMGB1 upregulates NF-kB by inhibiting IKB-α and associates with diabetic retinopathy. Life Sci. 2020, 241, 117146. [Google Scholar] [CrossRef]


| Target Gene | Primer Sequence (5′-3′) | Product Length (bp) |
|---|---|---|
| MEK4 | F: ATGGGAGCTTTGCCTTTGTTA R: GCTCCATTATCCGCTTTACCTG | 21 22 |
| MKK3 | F: AAGCCACCTGTACCCAACC R: GGCATGTCGCACCTTCTCTAC | 19 21 |
| P38 MAPK | F: TTGGACTGGCCCGACATAC R: GCCATTATGCATCCCACTGAC | 19 21 |
| MSK1 | F: GGTTAGTGGCCTAGCACGAC R: AAACCTGCGACCGACTCAGTA | 20 21 |
| NF-κB p65 | F: GCGCTTGTCACCTGTCCTC R: AGCCTGGTCCCGTGAAATAC | 19 21 |
| IκB | F: GGCCATCACGAGAGATCAATG R: TCCTGTTGGTAGCGGTAGAAG | 21 21 |
| GAPDH | F: ATGGTGAAGGTCGGAGTGAA R: GGAATTTGCCGTGGGTAGAAT | 20 21 |
| Group | Numbers | Cure Rate (%) | Improvement Rate (%) | Efficient Rate (%) |
|---|---|---|---|---|
| Control | 8 | - | - | - |
| CCl4 | 8 | - | - | - |
| BDDP | 8 | 12.5 | 62.5 | 75 |
| PCPE-H | 8 | 25 | 62.5 | 87.5 |
| PCPE-M | 8 | 12.5 | 50 | 62.5 |
| PCPE-L | 8 | 0 | 37.5 | 37.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tao, W.; Yue, X.; Ye, R.; Nabi, F.; Shang, Y.; Zhu, Z.; Ahmed, B.Z.; Liu, J. Hepatoprotective Effect of the Penthorum Chinense Pursh Extract against the CCl4-Induced Acute Liver Injury via NF-κB and p38-MAPK PATHWAYS in Dogs. Animals 2022, 12, 569. https://doi.org/10.3390/ani12050569
Tao W, Yue X, Ye R, Nabi F, Shang Y, Zhu Z, Ahmed BZ, Liu J. Hepatoprotective Effect of the Penthorum Chinense Pursh Extract against the CCl4-Induced Acute Liver Injury via NF-κB and p38-MAPK PATHWAYS in Dogs. Animals. 2022; 12(5):569. https://doi.org/10.3390/ani12050569
Chicago/Turabian StyleTao, Weilai, Xin Yue, Ruiling Ye, Fazul Nabi, Yangfei Shang, Zhaorong Zhu, Bhutto Zohaib Ahmed, and Juan Liu. 2022. "Hepatoprotective Effect of the Penthorum Chinense Pursh Extract against the CCl4-Induced Acute Liver Injury via NF-κB and p38-MAPK PATHWAYS in Dogs" Animals 12, no. 5: 569. https://doi.org/10.3390/ani12050569
APA StyleTao, W., Yue, X., Ye, R., Nabi, F., Shang, Y., Zhu, Z., Ahmed, B. Z., & Liu, J. (2022). Hepatoprotective Effect of the Penthorum Chinense Pursh Extract against the CCl4-Induced Acute Liver Injury via NF-κB and p38-MAPK PATHWAYS in Dogs. Animals, 12(5), 569. https://doi.org/10.3390/ani12050569







