Effects of Graded Levels of Mimosa (Acacia mearnsii) Tannin Purified with Organic Solvents on Gas, Methane, and In Vitro Organic Matter Digestibility of Eragrostis curvula Hay
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Materials
2.3. Purification Process
2.4. Tannin Characterization
2.5. Chemical Analysis of Substrate
2.6. In Vitro Incubation
2.6.1. Gas Estimation
2.6.2. Methane Determination
2.7. In Vitro Organic Matter Digestibility Determination (IVOMD)
2.8. Statistical Analysis
3. Results and Discussion
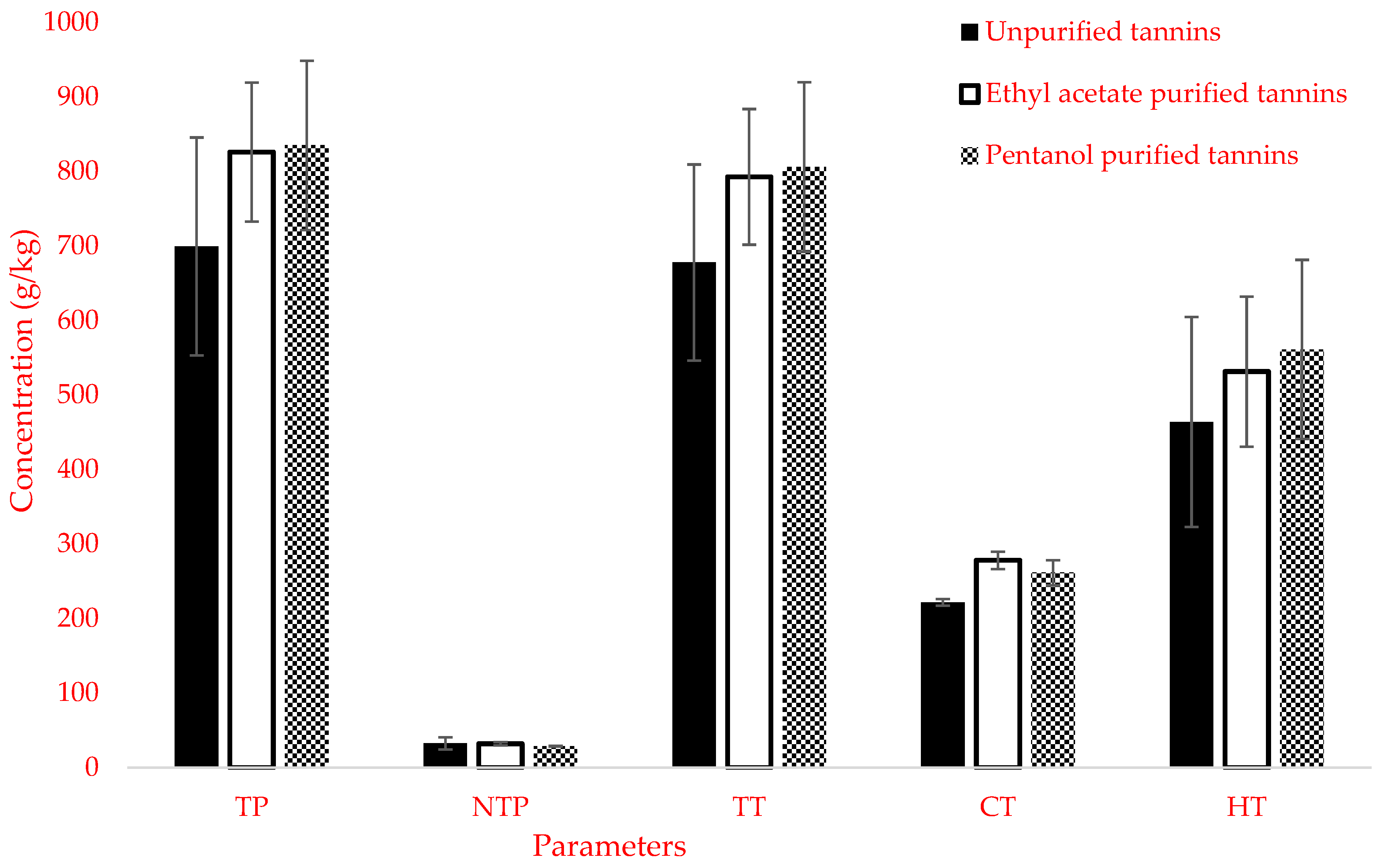
3.1. Characterization of Unpurified and Purified Mimosa Tannins
3.2. Gas, Methane and In Vitro Organic Matter Digestibility
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gerber, P.J.; Steinfeld, H.; Henderson, B.; Mottet, A.; Opio, C.; Dijkman, J.; Falcucci, A.; Tempio, G. Technical options for the mitigation of direct methane and nitrous oxide emissions from livestock: A review. Animal 2013, 7, 220–234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piñeiro-Vázqueza, A.T.; Canul-Solísa, J.R.; Alayón-Gamboab, J.A.; Chay-Canulc, A.J.; Ayala-Burgosa, A.J. Potential of condensed tannins for the reduction of emissions of enteric methane and their effect on ruminant productivity. Arch. Med. Vet. 2015, 47, 263–272. [Google Scholar] [CrossRef] [Green Version]
- Eckard, R.J.; Grainger, C.; de Klein, C.A.M. Options for the abatement of methane and nitrous oxide from ruminant production: A review. Livest. Sci. 2010, 130, 47–56. [Google Scholar] [CrossRef]
- Martin, C.; Morgavi, D.P.; Doreau, M. Methane mitigation in ruminants: From microbe to the farm scale. Animal 2010, 4, 351–365. [Google Scholar] [CrossRef] [Green Version]
- de Wit, M.P.; Crookes, D.J.; Van Wilgen, B.W. Conflicts of interest in environmental management: Estimating. Biol. Invasions 2001, 3, 167–178. [Google Scholar] [CrossRef]
- Galatowitsch, S.; Richardson, D.M. Riparian scrub recovery after clearing of invasive alien trees in headwater streams of the Western Cape, South Africa. Biol. Conserv. 2005, 122, 509–521. [Google Scholar] [CrossRef]
- Carulla, J.E.; Kreuzer, M.; Machmüller, A.; Hess, H.D. Supplementation of Acacia mearnsii tannins decreases methanogenesis and urinary nitrogen in forage-fed sheep. Aust. J. Agric. Res. 2005, 56, 961. [Google Scholar] [CrossRef]
- Grainger, C.; Clarke, T.; Auldist, M.J.; Beauchemin, K.A.; McGinn, S.M.; Waghorn, G.C.; Eckard, R.J. Potential use of Acacia mearnsii condensed tannins to reduce methane emissions and nitrogen excretion from grazing dairy cows. Can. J. Anim. Sci. 2009, 89, 241–251. [Google Scholar] [CrossRef] [Green Version]
- Hassanat, F.; Benchaar, C. Assessment of the effect of condensed (acacia and quebracho) and hydrolysable (chestnut and valonea) tannins on rumen fermentation and methane production in vitro. J. Sci. Food Agric. 2013, 93, 332–339. [Google Scholar] [CrossRef]
- Gemeda, B.S.; Hassen, A. Effect of tannin and species variation on in vitro digestibility, gas, and methane production of tropical browse plants. Asian-Australas. J. Anim. Sci. 2015, 28, 188–199. [Google Scholar] [CrossRef] [Green Version]
- Adejoro, F.A.; Hassen, A.; Akanmu, A.M.; Morgavi, D.P. Replacing urea with nitrate as a non-protein nitrogen source increases lamb growth and reduces methane production, whereas mimosa tannin has no effect. Anim. Feed Sci. Technol. 2019, 259, 114360. [Google Scholar] [CrossRef]
- Tondi, G.; Pizzi, A. Tannin-based rigid foams: Characterization and modification. Indust. Crop. Prod. 2009, 9, 356–363. [Google Scholar] [CrossRef]
- Arbenz, A.; Avérous, L. Chemical modi fi cation of tannins to elaborate aromatic biobased macromolecular architectures. Green Chem. 2015, 17, 2626–2646. [Google Scholar] [CrossRef] [Green Version]
- Adejoro, F.A.; Hassen, A.; Thantsha, M.S. Characterization of starch and gum Arabic-maltodextrin microparticles encapsulating Acacia tannin extract and evaluation of their potential use in ruminant nutrition. Asian-Australas J. Anim. Sci. 2019, 32, 977–987. [Google Scholar] [CrossRef]
- Adejoro, F.A.; Hassen, A.; Akanmu, A.M. Effect of lipid-encapsulated acacia tannin extract on feed intake, nutrient digestibility and methane emission in sheep. Animal 2019, 9, 863. [Google Scholar] [CrossRef] [Green Version]
- Missio, A.L.; Tischer, B.; dos Santos, P.S.B.; Codevilla, C.; de Menezes, C.R.; Barin, J.S.; Haselein, C.R.; Labidi, J.; Gatto, D.A.; Petutschnigg, A.; et al. Analytical characterization of purified mimosa (Acacia mearnsii) industrial tannin extract: Single and sequential fractionation. Sep. Purif. Technol. 2017, 186, 218–225. [Google Scholar] [CrossRef]
- Van Der Watt, E.; Pretorius, J.C. Purification and identification of active antibacterial components in Carpobrotus edulis L. J. Ethnopharmacol. 2001, 76, 87–91. [Google Scholar] [CrossRef]
- Seeram, N.; Lee, R.; Hardy, M.; Heber, D. Rapid large scale purification of ellagitannins from pomegranate husk, a by-product of the commercial juice industry. Sep. Purif. Technol. 2005, 41, 49–55. [Google Scholar] [CrossRef] [Green Version]
- Zhou, H.C.; Lin, Y.M.; Wei, S.D.; Tam, N.F.Y. Structural diversity and antioxidant activity of condensed tannins fractionated from mangosteen pericarp. Food Chem. 2011, 129, 1710–1720. [Google Scholar] [CrossRef]
- Marino, D.J. Ethyl Acetate. Elsevier Inc. 2005, 1, 144. [Google Scholar]
- Cann, A.F.; Liao, J.C. Pentanol isomer synthesis in engineered microorganisms. Appl. Microbiol. Biotechnol. 2010, 85, 893–899. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pattanaik, B.N.; Mandalia, H.C. Ethyl Acetate: Properties, Production Processes and Applications—A Review. Intern. J. Curr. Res. Rev. 2011, 3, 23–40. [Google Scholar]
- van Niekerk, W.A.; Hassen, A.; Snyman, L.D.; Rethman, N.F.G.; Coertze, R.J. Influence of mineral composition and rumen degradability of Atriplex nummularia (Hatfield Select F1) plants on selection preference of sheep. Afr. J. Range Forage Sci. 2009, 26, 91–96. [Google Scholar] [CrossRef]
- Adejoro, F.A.; Hassen, A.; Thantsha, M.S. Preparation of acacia tannin loaded lipid microparticles by solid-in-oil-in-water and melt dispersion methods, their characterization and evaluation of their effect on ruminal gas production in vitro. PLoS ONE 2018, 13, e0206241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Makkar, H.P.S. A laboratory manual for the FAO/IAEA co-ordinated research project on Use of nuclear and related technique to develop simple tannin assays for predicting and improving the safety and efficiency of feeding ruminants on tanniferous tree foliage. In Quantification of Tannins in Tree and Shrub Foliage; IAEA: Vienna, Austria, 2000; 31p. [Google Scholar]
- Porter, L.N.; Hrstich, L.J.; Chans, B.G. The conversion of procyanidins and prodelphinidins to cyanidin and delphinidin. Phytochem. 1986, 2, 223–230. [Google Scholar] [CrossRef] [Green Version]
- Singh, B.; Sahoo, A.; Sharma, R.; Bhat, T.K. Effect of polethylene glycol on gas production parameters and nitrogen disappearance of some tree forages. Anim. Feed Sci. Technol. 2005, 123, 351–364. [Google Scholar] [CrossRef]
- AOAC. Official Method of Analysis; Association of Offcial Analytical Chemists: Washington, DC, USA, 2002. [Google Scholar]
- Van Soest, J.B.; Robertson, P.J.; Lewis, B.A. Methods for dietary fiber neutral detergent fiber, and non starch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Menke, H.; Steingass, K.H. Estimation of the energetic feed value obtained from chemical analysis and in vitro gas production using rumen fluid. Anim. Res. Dev. 1988, 28, 7–55. [Google Scholar]
- Mould, F.L.; Morgan, R.; Kliem, K.E.; Krystallidou, E. A review and simplification of the in vitro incubation medium. Anim. Feed Sci. Technol. 2005, 123-124, 155–172. [Google Scholar] [CrossRef]
- Theodorou, M.K.; Williams, B.A.; Dhanoa, M.S.; McAllan, A.B.; France, J. A simple gas production method using a pressure transducer to determine the fermentation kinetics of ruminant feeds. Anim. Feed Sci. Technol. 1994, 48, 185–197. [Google Scholar] [CrossRef]
- Pressure to Gas Production Conversion; Ankom Technology: Mecedon, NY, USA, 2014. Available online: https://www.ankom.com/sites/default/files/document-files/RFS005_Pressure_to_Gas_Production_Conversion.pdf (accessed on 13 July 2018).
- Tavendale, M.H.; Meagher, L.P.; Pacheco, D.; Walker, N.; Attwood, G.T.; Sivakumaran, S. Methane production from in vitro rumen incubations with Lotus pedunculatus and Medicago sativa, and effects of extractable condensed tannin fractions on methanogenesis. Anim. Feed Sci. Technol. 2005, 123, 403–419. [Google Scholar] [CrossRef]
- Tilley, R.A.; Terry, J.M.A. A two-stage technique for the in vitro digestion of forage crops. J. Br. Grassl. Soc. 1963, 18, 104–111. [Google Scholar] [CrossRef]
- Engels, F.J.; Van der Merwe, E.A.N. Application of an in vitro technique to South African forages with special reference to the effect to certain factors on the results. South Afr. J. Agric. Sci. 1967, 10, 983–995. [Google Scholar]
- Schofield, P. Chapter 10—Gas Production Methods. In Farm Animal Metabolism And Nutrition; D’Mello, J.P.F., Ed.; CABI Publishers: New York, NY, USA, 2000; 433p. [Google Scholar]
- Minho, A.P.; Bueno, I.C.D.S.; Gennari, S.M.; Jackson, F.; Abdalla, A.L. In vitro effect of condensed tannin extract from acacia (Acacia mearnsii) on gastrointestinal nematodes of sheep. Rev. Bras. Parasitol. Vet. 2008, 17, 144–148. Available online: http://www.ncbi.nlm.nih.gov/pubmed/20059834 (accessed on 15 January 2018). [PubMed]
- Bhatta, R.; Saravanan, M.; Baruah, L.; Sampath, K.T.; Prasad, C.S. Effect of plant secondary compounds on in vitro methane, ammonia production and ruminal protozoa population. J. Appl. Microbiol. 2013, 115, 455–465. [Google Scholar] [CrossRef]
- Kardel, M.; Taube, F.; Schulz, H. Different approaches to evaluate tannin content and structure of selected plant extracts-review and new aspects. J. Appl. Bot. Food Qual. 2013, 166, 154–166. [Google Scholar] [CrossRef]
- Animut, G.; Puchala, R.; Goetsch, A.L.; Patra, A.K.; Sahlu, T.; Varel, V.H.; Wells, J. Methane emission by goats consuming diets with different levels of condensed tannins from lespedeza. Anim. Feed Sci. Technol. 2008, 144, 212–227. [Google Scholar] [CrossRef]
- Patra, A.K.; Saxena, J. A new perspective on the use of plant secondary metabolites to inhibit methanogenesis in the rumen. Phytochemistry 2010, 71, 1198–1222. [Google Scholar] [CrossRef]
- Sirohi, S.K.; Goel, N.; Pandey, P. Efficacy of different methanolic plant extracts on anti-methanogenesis, rumen fermentation and gas production kinetics in vitro. Open Vet. J. 2012, 2, 72–77. [Google Scholar]
- Jayanegara, A.; Leiber, F.; Kreuzer, M. Meta-analysis of the relationship between dietary tannin level and methane formation in ruminants from in vivo and in vitro experiments. J. Anim. Physiol. Anim. Nutr. 2012, 96, 365–375. [Google Scholar] [CrossRef]
- Hristov, A.N.; Oh, J.; Firkins, J.L.; Dijkstra, J.; Kebreab, E.; Waghorn, G.; Makkar, H.P.S.; Adesogan, A.T.; Yang, W.; Lee, C.; et al. Special topics—Mitigation of methane and nitrous oxide emissions from animal operations: I. A review of enteric methane mitigation options. J. Anim. Sci. 2013, 91, 5045–5069. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, H.Y.; Sieo, C.C.; Abdullah, N.; Liang, J.B.; Huang, X.D.; Ho, Y.W. Effects of condensed tannins from Leucaena on methane production, rumen fermentation and populations of methanogens and protozoa in vitro. Anim. Feed Sci. Technol. 2011, 169, 185–193. [Google Scholar] [CrossRef]
- Akanmu, A.M.; Hassen, A. The use of certain medicinal plant extracts reduced in vitro methane production while improving in vitro organic matter digestibility. Anim. Prod. Sci. 2017, 58, 900–908. [Google Scholar] [CrossRef] [Green Version]
- Akanmu, A.M.; Hassen, A.; Adejoro, F.A. Gas Production, Digestibility and Efficacy of Stored or Fresh Plant Extracts to Reduce Methane Production on different substrates. Animal 2020, 10, 146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schofield, P.; Pitt, R.E.; Pell, A.N. Kinetics of fiber digestion from in vitro gas production. J. Anim. Sci. 1994, 72, 2980–2991. [Google Scholar] [CrossRef] [PubMed]
- Beauchemin, K.A.; McGinn, S.M.; Martinez, T.F.; McAllister, T.A. Use of condensed tannin extract from quebracho trees to reduce methane emissions from cattle. J. Anim. Sci. 2007, 85, 1990–1996. [Google Scholar] [CrossRef] [Green Version]
- Schofield, J.P.; Pell, A.N. Measurement and kinetic analysis of the neutral detergent-soluble carbohydrate fraction of legumes and grasses. J. Anim. Sci. 1995, 73, 3455–3463. [Google Scholar] [CrossRef]
- Mir, Z.; Acharya, S.N.; Mir, P.S.; Taylor, W.G.; Zaman, M.S.; Mears, G.J.; Goonewardene, L.A. Nutrient composition, in vitro gas production and digestibility of fenugreek (Trigonella foenum-graecum ) and alfalfa forages. Can. J. Anim. Sci. 1997, 77, 119–124. [Google Scholar] [CrossRef] [Green Version]
| Tannin Extracts | Parameters | |||
|---|---|---|---|---|
| Level (g/kg DM) | Gas (mL/g DM) | CH4 (mL/g DM) | IVOMD (g/kg) | |
| Unpurified Tannin | 0 | 155.2 a | 7.9 a | 600.7 a |
| 10 | 149.8 ab | 7.4 ab | 592.5 bc | |
| 20 | 145.0 bc | 7.1 ab | 592.9 b | |
| 30 | 142.5 c | 6.8 ab | 589.0 bc | |
| 40 | 138.2 c | 6.2 b | 582.5 def | |
| SEM | 1.59 | 0.33 | 14.50 | |
| Linear | <0.0.01 | 0.01 | <0.01 | |
| Quadratic | 0.45 | 0.95 | 0.99 | |
| Cubic | 0.65 | 0.65 | 0.81 | |
| Ethyl acetate purified Tannin | 0 | 155.2 a | 7.9 a | 600.7 a |
| 10 | 150.1 ab | 7.3 ab | 592.9 b | |
| 20 | 144.5 bc | 6.9 ab | 584.7 cde | |
| 30 | 139.7 cd | 6.3 ab | 582.4 def | |
| 40 | 137.3 d | 6.1 b | 576.3 f | |
| SEM | 1.63 | 0.41 | 13.73 | |
| Linear | <0.01 | 0.01 | <0.01 | |
| Quadratic | 0.33 | 0.73 | 0.86 | |
| Cubic | 0.58 | 0.94 | 0.94 | |
| Pentanol purified Tannin | 0 | 155.2 a | 7.9 a | 600.7 a |
| 10 | 149.4 ab | 7.1 ab | 592.5 bc | |
| 20 | 143.7 bc | 6.6 ab | 585.4 cde | |
| 30 | 141.5 c | 6.40 ab | 582.0 def | |
| 40 | 139.6 c | 6.1 b | 578.9 ef | |
| SEM | 1.43 | 0.39 | 13.84 | |
| Linear | <0.0.01 | 0.01 | <0.01 | |
| Quadratic | 0.05 | 0.42 | 0.79 | |
| Cubic | 0.96 | 0.77 | 0.98 | |
| SEM | 0.414 | 0.387 | 0.696 | |
| p values | ||||
| T | 0.25 | <0.01 | <0.01 | |
| L | <0.01 | <0.01 | <0.01 | |
| T * L | 0.25 | 0.10 | 0.05 | |
| Tannin Extracts | Parameters | |||
|---|---|---|---|---|
| Level (g/kg DM) | CH4/Gas | CH4/IVOMD (mL/kg) | Gas/IVOMD (mL/kg) | |
| Unpurified tannin | 0 | 0.051 a | 0.013 a | 0.259 a |
| 10 | 0.050 ab | 0.013 a | 0.254 ab | |
| 20 | 0.049 abc | 0.012 ab | 0.245 b–e | |
| 30 | 0.048 a–d | 0.012 ab | 0.243 de | |
| 40 | 0.045 de | 0.011 b | 0.238 e | |
| SEM | 0.002 | 0.005 | 0.008 | |
| Linear | <0.01 | 0.003 | <0.01 | |
| Quadratic | 0.724 | 0.793 | 0.832 | |
| Cubic | 0.732 | 0.877 | 0.969 | |
| Ethyl acetate purified tannin | 0 | 0.051 a | 0.013 a | 0.259 a |
| 10 | 0.049 abc | 0.012 ab | 0.254 ab | |
| 20 | 0.048 a–d | 0.012 ab | 0.248 bcd | |
| 30 | 0.045 de | 0.011 b | 0.240 de | |
| 40 | 0.044 e | 0.011 b | 0.239 e | |
| SEM | 0.003 | 0.001 | 0.008 | |
| Linear | <0.01 | 0.010 | <0.01 | |
| Quadratic | 0.959 | 0.837 | 0.864 | |
| Cubic | 0.951 | 0.808 | 0.784 | |
| Pentanol purified tannin | 0 | 0.051 a | 0.013 a | 0.259 a |
| 10 | 0.048 a–d | 0.012 ab | 0.253 abc | |
| 20 | 0.046 cde | 0.011 bc | 0.246 b–e | |
| 30 | 0.045 de | 0.011 bc | 0.244 cde | |
| 40 | 0.044 e | 0.010 c | 0.242 e | |
| SEM | 0.003 | 0.001 | 0.008 | |
| Linear | <0.01 | 0.007 | <0.01 | |
| Quadratic | 0.669 | 0.688 | 0.700 | |
| Cubic | 0.822 | 0.721 | 0.955 | |
| SEM | 0.0003 | 0.0001 | 0.0008 | |
| p-values | ||||
| T | <0.01 | 0.01 | 0.48 | |
| L | <0.01 | <0.01 | <0.01 | |
| T * L2 | 0.59 | 0.40 | 0.46 | |
| Tannin Extracts | Parameters | |||
|---|---|---|---|---|
| Level (g/kg DM) | v (mL/g DM) | k (mL/h) | l (h) | |
| Unpurified tannin | 0 | 195.7 a | 0.034 a | 0.017 |
| 10 | 193.6 a | 0.032 ab | 0.079 | |
| 20 | 188.6 ab | 0.031 ab | 0.221 | |
| 30 | 187.9 ab | 0.031 ab | 0.249 | |
| 40 | 180.7 b | 0.030 b | 0.086 | |
| SEM | 3.566 | 0.002 | 0.171 | |
| Linear | 0.007 | 0.022 | 0.578 | |
| Quadratic | 0.659 | 0.495 | 0.394 | |
| Cubic | 0.749 | 1.000 | 0.626 | |
| Ethyl acetate purified tannin | 0 | 195.7 a | 0.034 a | 0.017 |
| 10 | 192.5 ab | 0.032 ab | 0.150 | |
| 20 | 186.7 ab | 0.031 ab | 0.238 | |
| 30 | 182.7 b | 0.030 b | 0.177 | |
| 40 | 180.4 b | 0.030 b | 0.144 | |
| SEM | 2.881 | 0.001 | 0.158 | |
| Linear | 0.001 | 0.02 | 0.585 | |
| Quadratic | 0.748 | 0.640 | 0.429 | |
| Cubic | 0.636 | 0.911 | 0.885 | |
| Pentanol purified tannin | 0 | 195.7 a | 0.034 a | 0.017 |
| 10 | 192.1 ab | 0.032 ab | 0.255 | |
| 20 | 187.6 ab | 0.031 ab | 0.132 | |
| 30 | 187.4 b | 0.030 b | 0.087 | |
| 40 | 186.7 b | 0.029 b | 0.083 | |
| SEM | 2.700 | 0.001 | 0.191 | |
| Linear | 0.024 | 0.022 | 0.551 | |
| Quadratic | 0.220 | 0.813 | 0.318 | |
| Cubic | 0.769 | 0.779 | 0.701 | |
| SEM | 0.703 | 0.0003 | 0.039 | |
| p-values | ||||
| T | 0.02 | 0.43 | 0.32 | |
| L | <0.01 | 0.01 | 0.11 | |
| T * L | 0.03 | 0.73 | 0.26 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ibrahim, S.L.; Hassen, A. Effects of Graded Levels of Mimosa (Acacia mearnsii) Tannin Purified with Organic Solvents on Gas, Methane, and In Vitro Organic Matter Digestibility of Eragrostis curvula Hay. Animals 2022, 12, 562. https://doi.org/10.3390/ani12050562
Ibrahim SL, Hassen A. Effects of Graded Levels of Mimosa (Acacia mearnsii) Tannin Purified with Organic Solvents on Gas, Methane, and In Vitro Organic Matter Digestibility of Eragrostis curvula Hay. Animals. 2022; 12(5):562. https://doi.org/10.3390/ani12050562
Chicago/Turabian StyleIbrahim, Shehu Lurwanu, and Abubeker Hassen. 2022. "Effects of Graded Levels of Mimosa (Acacia mearnsii) Tannin Purified with Organic Solvents on Gas, Methane, and In Vitro Organic Matter Digestibility of Eragrostis curvula Hay" Animals 12, no. 5: 562. https://doi.org/10.3390/ani12050562
APA StyleIbrahim, S. L., & Hassen, A. (2022). Effects of Graded Levels of Mimosa (Acacia mearnsii) Tannin Purified with Organic Solvents on Gas, Methane, and In Vitro Organic Matter Digestibility of Eragrostis curvula Hay. Animals, 12(5), 562. https://doi.org/10.3390/ani12050562







