Symmetry-Breaking Stabilities in Carapace Curvature on Testudo (Reptilia, Testudinidae)
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample
2.2. Imaging
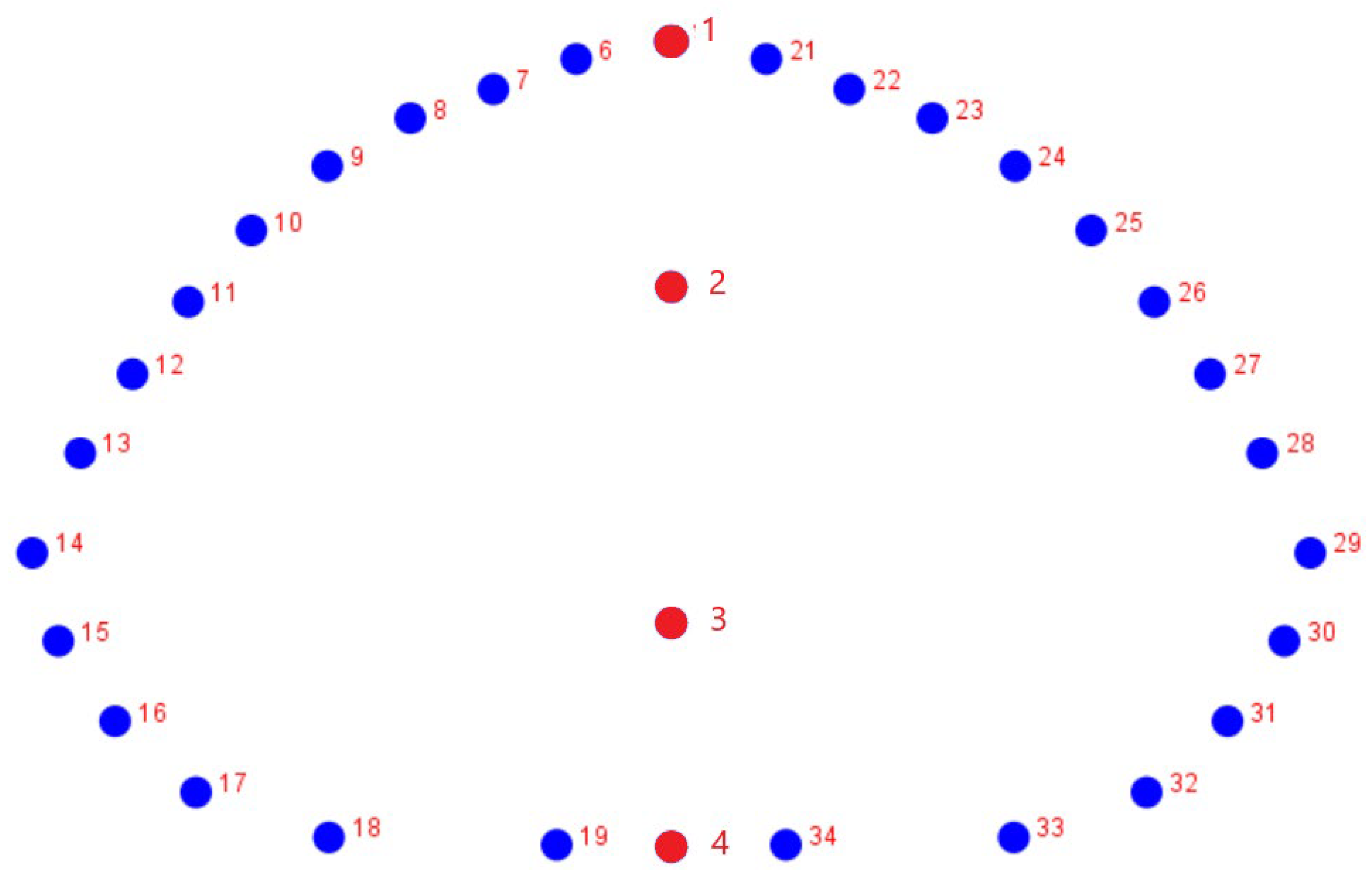
2.3. Geometric Morphometrics
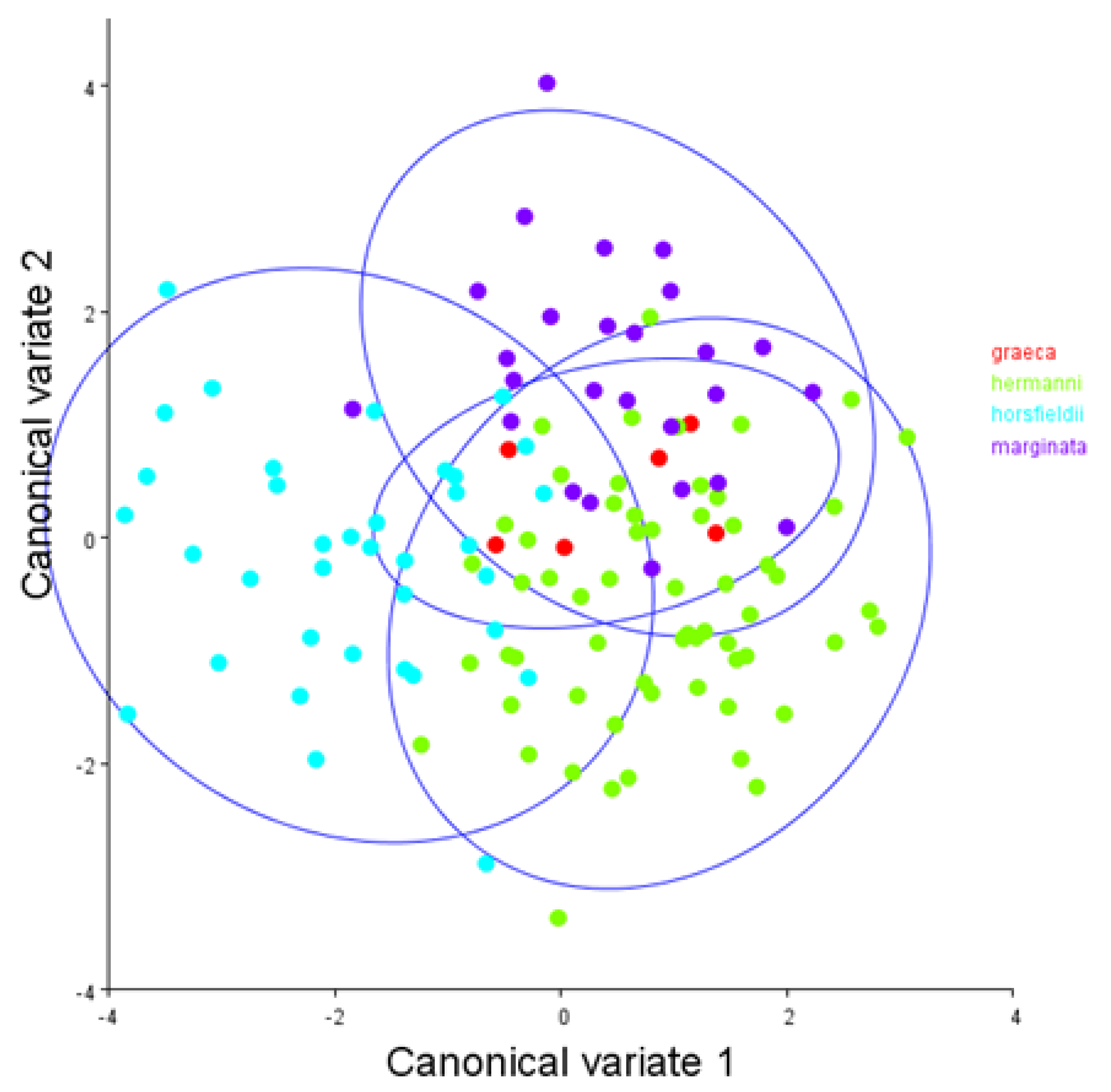
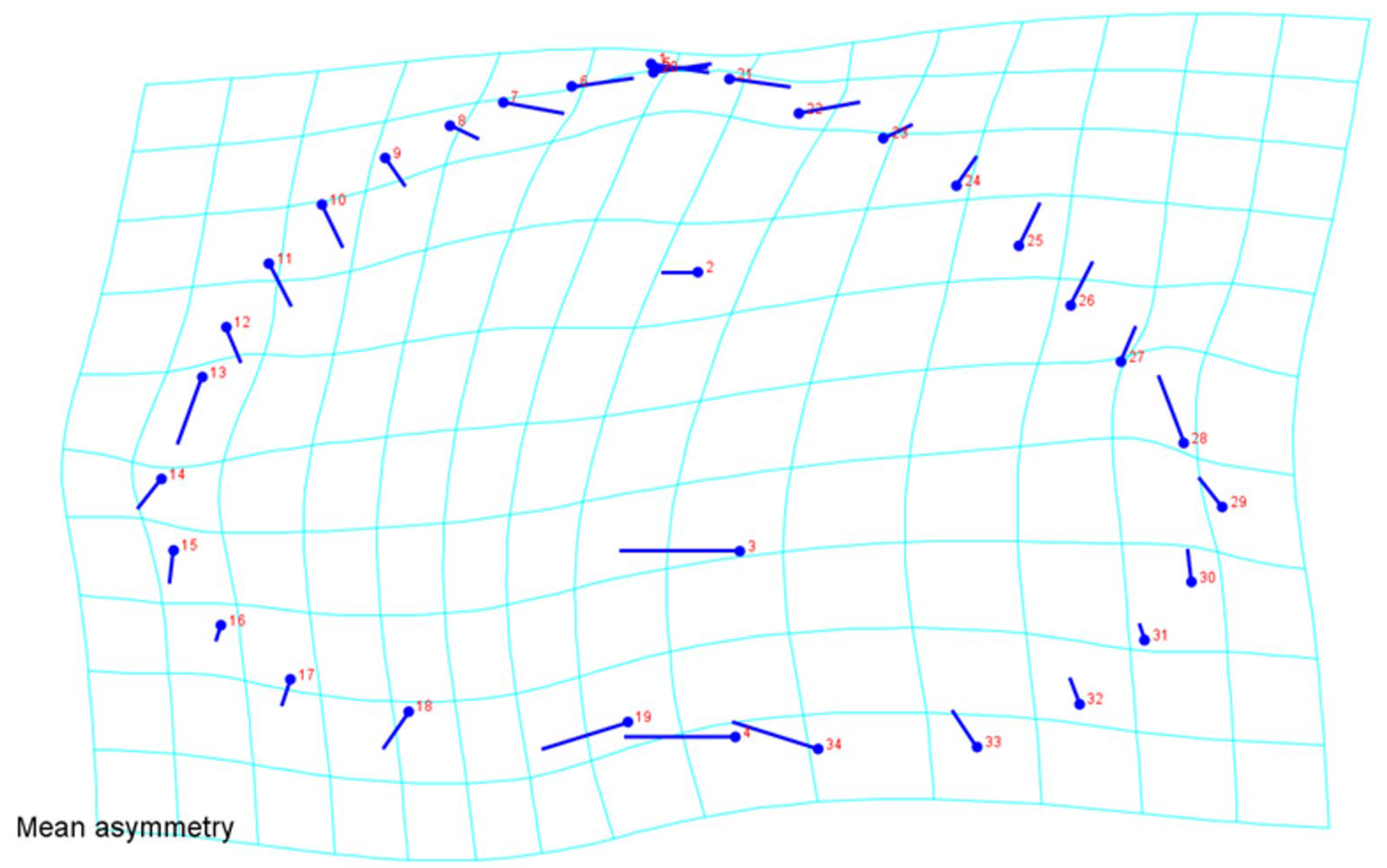
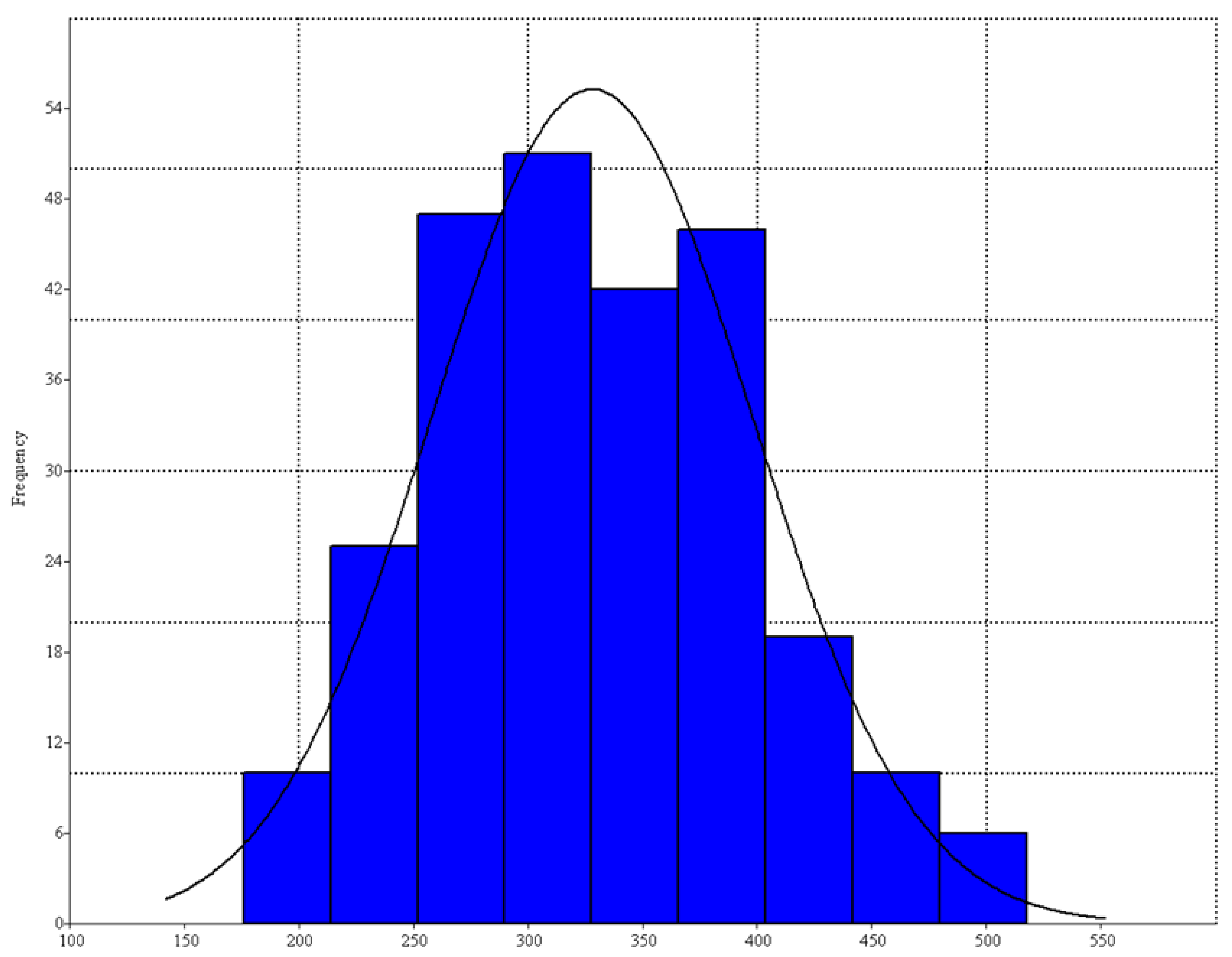
3. Results
4. Discussion
5. Conclusion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Zhu, H. Plastron Reduction and Associated Myology in Turtles, and Its Implications for Functional Morphology and Natural History. Master’s Thesis, Marshall University, Huntington, WV, USA, January 2011. [Google Scholar]
- Chiari, Y.; Van Der Meijden, A.; Caccone, A.; Claude, J.; Gilles, B. Self-righting potential and the evolution of shell shape in Galápagos tortoises. Sci. Rep. 2017, 7, 15828. [Google Scholar] [CrossRef] [PubMed]
- Domokos, G.; Várkonyi, P.L. Geometry and self-righting of turtles. Proc. R. Soc. B Boil. Sci. 2007, 275, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Fernández, C.A.; Rivera, A.C. Asymmetries and accessory scutes in Emys orbicularis from Northwest Spain. Biologia 2004, 59, 85–88. [Google Scholar]
- Băncilă, R.I.; Plăiaşu, R.; Tudor, M.; Samoilă, C.; Cogălniceanu, D. Fluctuating Asymmetry in the Eurasian Spur-Thighed Tortoise, Testudo graeca ibera Linneaus, 1758 (Testudines: Testudinidae), Chelonian Conserv. Biol. 2012, 11, 234–239. [Google Scholar]
- Angielczyk, K.D.; Feldman, C.R. Are diminutive turtles miniaturized? The ontogeny of plastron shape in emydine turtles. Biol. J. Linn. Soc. 2013, 108, 727–755. [Google Scholar] [CrossRef]
- Ibrahim, R.W. Conformal geometry of the turtle shell. J. King Saud Univ.-Sci. 2020, 32, 2202–2206. [Google Scholar] [CrossRef]
- Parés-Casanova, P.M.; Minoves, J.; Soler, J.; Martínez–Silvestre, A. Divergent scute asymmetry among pure and crossed individuals of Testudo hermanni (Gmelin, 1789). Arx. Miscel·lània Zool. 2020, 18, 43–50. [Google Scholar] [CrossRef][Green Version]
- Cherepanov, G.O. Patterns of scute development in turtle shell: Symmetry and asymmetry. Paléontol. J. 2014, 48, 1275–1283. [Google Scholar] [CrossRef]
- Ana, G.; Ljiljana, T.; Ana, I. Geometry of self righting: The case of Hermann’s tortoises. Zool. Anz.-A. J. Comp. Zool. 2015, 254, 99–105. [Google Scholar] [CrossRef]
- Klingenberg, C.P.; Barluenga, M.; Meyer, A. shape analysis of symmetric structures: Quantifying variation among individuals and asymmetry. Evolution 2002, 56, 1909–1920. [Google Scholar] [CrossRef]
- Lutz, P.L.; Musick, J.A. The Biology of Sea Turtles; CRC Press: Boca Raton, FL, USA, 1996; Volume 1. [Google Scholar]
- Gerlach, J. Effects of diet on the systematic utility of the tortoise carapace. Afr. J. Herpetol. 2004, 53, 77–85. [Google Scholar] [CrossRef]
- Rhodin, A.G.J.; Iverson, J.B.; Bour, R.; Fritz, U.; Georges, A.; Shaffer, H.B.; van Dijk, P.P. Turtles of the World. Annotated Checklist and Atlas of Taxonomy, Synonymy, Distribution, and Conservation Status, 9th ed. In: Conservation Biology of Freshwater Turtles and Tortoises: A Compilation Project of the IUCN/SSC Tortoise and Freshwater Turtle Specialist Group. Rhodin, A.G.J., Iverson, J.B., van Dijk, P.P., Stanford, C.B., Goode, E.V., Buhlmann, K.A., Mittermeier, R.A. Eds. Chelonian Res. Monogr. 2021, 8, 1–472. [Google Scholar] [CrossRef]
- Rohlf, F.J. The tps series of software. Hystrix 2015, 26, 9–12. [Google Scholar] [CrossRef]
- Rohlf, F.J.; Bookstein, F.L. Proceedings of the Michigan Morphometrics Workshop; University of Michigan Museum of Zoology: Ann Arbor, MI, USA, 1990; p. 396. ISBN 0-9628499-0-1. [Google Scholar]
- Adams, D.C.; Rohlf, F.J.; Slice, D.E. A field comes of age: Geometric morphometrics in the 21st century. Hystrix 2013, 24, 7–14. [Google Scholar] [CrossRef]
- Klingenberg, C.P.; McIntyre, G.S. Geometric Morphometrics of Developmental Instability: Analyzing Patterns of Fluctuating Asymmetry with Procrustes Methods. Evolution 1998, 52, 1363. [Google Scholar] [CrossRef]
- Klingenberg, C.P. Analyzing Fluctuating Asymmetry with Geometric Morphometrics: Concepts, Methods, and Applications. Symmetry 2015, 7, 843–934. [Google Scholar] [CrossRef]
- Niemeier, S.; Mueller, J.; Roedel, M.O. Fluctuating asymmetry-appearances are deceptive. Comparison of methods for assessing developmental instability in European common frogs (Rana temporaria). Salamandra 2019, 55, 14–26. [Google Scholar]
- Kharlamova, A.V.; Trut, L.N.; Chase, K.; Kukekova, A.V.; Lark, K.G. Directional asymmetry in the limbs, skull and pelvis of the silver fox (V. vulpes). J. Morphol. 2010, 271, 1501–1508. [Google Scholar] [CrossRef]
- Leśniak, K. Directional asymmetry of facial and limb traits in horses and ponies. Vet. J. 2013, 198, e46–e51. [Google Scholar] [CrossRef]
- Parés-Casanova, P.M.; Otin, G. Functional skull asymmetries in Carollia perspicillata (Phyllostomidae Gray, 1825: Carollinae). Acta Biol. Szeged. 2020, 64. [Google Scholar] [CrossRef]
- Klingenberg, C.P. MorphoJ: An integrated software package for geometric morphometrics. Mol. Ecol. Resour. 2011, 11, 353–357. [Google Scholar] [CrossRef] [PubMed]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. PAST v. 2.17c: Paleontological Statistics Software Package for Education and Data Analysis. Palaeontol. Electron. 2001, 4, 1–229. [Google Scholar]
- Parés-Casanova, P.M.; Cladera, M.; Martínez Silvestre, A. Adaptative directional asymmetric shape in Testudo hermanni hermanni Gmelin, 1789 (Reptilia: Testudines: Testudinidae). Herpetol. Notes 2019, 12, 743–747. [Google Scholar]
- Van Valen, L. A study of fluctuating asymmetry. Evolution 1962, 16, 125–142. [Google Scholar] [CrossRef]
- Parés-Casanova, P.M.; Brando, P.; Caviedes, D.; Salamanca-Carreño, A. Scutation asymmetries in red-footed tortoise Chelonoidis carbonaria Spix, 1824 (Testudines: Testudinidae). Pap. Avulsos Zool. 2020, 60, 1–6. [Google Scholar] [CrossRef]
- Stancher, G.; Clara, E.; Regolin, L.; Vallortigara, G. Lateralized righting behavior in the tortoise (Testudo hermanni). Behav. Brain Res. 2006, 173, 315–319. [Google Scholar] [CrossRef]
- Romeijer, C.; Beaufrère, H.; Laniesse, D.; Birch, S.M.; MacKenzie, S.; Melville, L.; Moens, N. Vomiting and Gastrointestinal Obstruction in a Red-Footed Tortoise (Chelonoidis carbonaria). J. Herpetol. Med. Surg. 2016, 26, 32–35. [Google Scholar] [CrossRef]
- Varlet, I.; Robertson, E.J. Left—Right asymmetry in vertebrates. Curr. Opin. Genet. Dev. 1997, 7, 519–523. [Google Scholar] [CrossRef]
- Barboza, P. Digesta passage and functional anatomy of the digestive tract in the desert tortoise (Xerobates agassizii). J. Comp. Physiol. B 1995, 165. [Google Scholar] [CrossRef]
- Barone, R. Anatomie Comparée des Mammifères Domestiques. Tome Troisième, Splanchnologie I: Appareil Digestif, Appareil Respiratoire, 4th ed.; Vigot: Paris, France, 2009. (In French) [Google Scholar]
| Effect | SS | MS | Degrees of Freedom | Fisher Test | p-Value |
|---|---|---|---|---|---|
| Size | |||||
| Individuals | 1,784,840.5892940 | 28,330.8030050 | 63 | 47.17 | <0.0001 |
| Error | 38,436.907440000 | 600.576679000 | 64 | ||
| Shape | |||||
| Individuals | 0.451068730 | 0.000223744 | 2016 | 3.69 | <0.0001 |
| DA | 0.013539950 | 0.000423124 | 32 | 6.99 | <0.0001 |
| FA | 0.122113590 | 0.000060572 | 2016 | 0.32 | 1 |
| Error | 0.770446940 | 0.000188097 | 4096 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parés-Casanova, P.M.; Soler, J.; Buisán, T.; Martínez-Silvestre, A. Symmetry-Breaking Stabilities in Carapace Curvature on Testudo (Reptilia, Testudinidae). Animals 2022, 12, 471. https://doi.org/10.3390/ani12040471
Parés-Casanova PM, Soler J, Buisán T, Martínez-Silvestre A. Symmetry-Breaking Stabilities in Carapace Curvature on Testudo (Reptilia, Testudinidae). Animals. 2022; 12(4):471. https://doi.org/10.3390/ani12040471
Chicago/Turabian StyleParés-Casanova, Pere M., Joaquim Soler, Tania Buisán, and Albert Martínez-Silvestre. 2022. "Symmetry-Breaking Stabilities in Carapace Curvature on Testudo (Reptilia, Testudinidae)" Animals 12, no. 4: 471. https://doi.org/10.3390/ani12040471
APA StyleParés-Casanova, P. M., Soler, J., Buisán, T., & Martínez-Silvestre, A. (2022). Symmetry-Breaking Stabilities in Carapace Curvature on Testudo (Reptilia, Testudinidae). Animals, 12(4), 471. https://doi.org/10.3390/ani12040471







