Development of a Composite Pain Scale in Foals: A Pilot Study
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Development of the Foal Composite Pain Scale
2.2. Animals
2.3. Video Recording
2.4. Observers and Training
2.5. Statistical Analysis
3. Results
3.1. Development of the FCPS
3.1.1. Section I: Facial Expressions
3.1.2. Section II: Behavioural Items
3.1.3. Section III: Physical Items
3.2. FCPS Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brambell, F.W.R. Report of the Technical Committee to Enquire into the Welfare of Animals Kept under Intensive Livestock Hus-Bandry Systems; Command Rep. 2836; Her Majesty’s Stationery Office: London, UK, 1965. [Google Scholar]
- Mullan, S. Assessment of quality of life in veterinary practice. Developing tools for companion animal carers and veterinarians. J. Vet. Med. Res. 2015, 6, 203–210. [Google Scholar]
- Max, M.B.; Donovan, M.; Miaskowski, C.A.; Ward, S.E.; Gordon, D.; Bookbinder, M.; American Pain Society Quality of Care Committee. Quality improvement guidelines for the treatment of acute pain and cancer pain. JAMA 1995, 274, 1874–1880. [Google Scholar]
- Taylor, P.M.; Pascoe, P.J.; Mama, K.R. Diagnosing and treating pain in the horse: Where are we today? Vet. Clin. North. Am. Equine Pract. 2002, 18, 1–19. [Google Scholar] [CrossRef]
- Van Loon, J.P.; Back, W.; Hellebrekers, L.J.; van Weeren, P.R. Application of a composite pain scale to objectively monitor horses with somatic and visceral pain under hospital conditions. J. Equine Vet. Sci. 2010, 30, 641–649. [Google Scholar] [CrossRef]
- Gleerup, K.B.; Lindegaard, C. Recognition and quantification of pain in horses: A tutorial review. Equine Vet. Educ. 2016, 28, 47–57. [Google Scholar] [CrossRef]
- McDonnell, S. Is it psychological, physical, or both? Proc. Am. Ass Equine Pract. 2005, 51, 231–238. [Google Scholar]
- Van Loon, J.P.A.M.; van Dierendonck, M.C. Objective pain assessment in horses (2014–2018). Vet. J. 2018, 242, 1–7. [Google Scholar] [CrossRef]
- Lindegaard, C.; Thomsen, M.H.; Larsen, S.; Andersen, P.H. Analgesic efficacy of intraarticular morphine in experimentally induced radiocarpal synovitis in horses. Vet. Anaesth. Analg. 2010, 37, 171–185. [Google Scholar] [CrossRef] [PubMed]
- Van Loon, J.P.A.M.; van Dierendonck, M.C. Pain assessment in horses after orthopaedic surgery and with orthopaedic trauma. Vet. J. 2019, 246, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Sutton, G.A.; Dahan, R.; Turner, D.; Paltiel, O. A behaviour-based pain scale for horses with acute colic: Scale construction. Vet. J. 2013, 196, 394–401. [Google Scholar] [CrossRef]
- Bussieres, G.; Jacques, C.; Lainay, O.; Beauchamp, G.; Leblond, A.; Cadoré, J.L.; Troncy, E. Development of a composite orthopaedic pain scale in horses. Res. Vet. Sci. 2008, 85, 294–306. [Google Scholar] [CrossRef]
- Pritchett, L.C.; Ulibarri, C.; Roberts, M.C.; Schneider, R.K.; Sellon, D.C. Identification of potential physiological and behavioral indicators of postoperative pain in horses after exploratory celiotomy for colic. Appl. Anim. Behav. Sci. 2003, 80, 31–43. [Google Scholar] [CrossRef]
- Costa, E.D.; Minero, M.; Lebelt, D.; Stucke, D.; Canali, E.; Leach, M.C. Development of the Horse Grimace Scale (HGS) as a pain assessment tool in horses undergoing routine castration. PLoS ONE 2014, 9, e92281. [Google Scholar]
- Costa, E.D.; Stucke, D.; Dai, F.; Minero, M.; Leach, M.C.; Lebelt, D. Using the horse grimace scale (HGS) to assess pain associated with acute laminitis in horses (Equus caballus). Animals 2016, 6, 47. [Google Scholar] [CrossRef]
- Van Loon, J.P.A.M.; Van Dierendonck, M.C. Monitoring equine head-related pain with the Equine Utrecht University scale for facial assessment of pain (EQUUS-FAP). Vet. J. 2017, 220, 88–90. [Google Scholar] [CrossRef] [PubMed]
- Coneglian, M.M.; Borges, T.D.; Weber, S.H.; Bertagnon, H.G.; Michelotto, P.V. Use of the horse grimace scale to identify and quantify pain due to dental disorders in horses. Appl. Anim. Behav. Sci. 2020, 225, 104970. [Google Scholar] [CrossRef]
- van Loon, J.P.; Macri, L. Objective Assessment of Chronic Pain in Horses Using the Horse Chronic Pain Scale (HCPS): A Scale-Construction Study. Animals 2021, 11, 1826. [Google Scholar] [CrossRef]
- Ortolani, F.; Scilimati, N.; Gialletti, R.; Menchetti, L.; Nannarone, S. Development and preliminary validation of a pain scale for ophthalmic pain in horses: The Equine Ophthalmic Pain Scale (EOPS). Vet. J. 2021, 278, 105774. [Google Scholar] [CrossRef] [PubMed]
- Tateo, A.; Maggiolino, A.; Padalino, B.; Centoducati, P. Behavior of artificially suckled foals. J. Vet. Behav. 2013, 8, 162–169. [Google Scholar] [CrossRef]
- Torcivia, C.; McDonnell, S. Equine discomfort ethogram. Animals 2021, 11, 580. [Google Scholar] [CrossRef]
- Robertson, S.A. Analgesia in foals. Compend. Contin. Educ. Pract. Vet. 2012, 34, e3. [Google Scholar]
- van Loon, J.P.A.M.; Verhaar, N.; van den Berg, E.; Ross, S.; de Grauw, J. Objective Assessment of Acute Pain in Foals Using a Facial Expression-Based Pain Scale. Animals 2020, 10, 1610. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010 on the protection of animals used for scientific purposes. Off. J. Eur. Union 2010, 53, 33–79. [Google Scholar]
- Sherwin, C.M.; Christiansen, S.B.; Duncan, I.J.H.; Erhard, H.W.; Lay, D.C.; Mench, J.A. Guidelines for the ethical use of animals in applied animal behaviour research. Appl. Anim. Behav. Sci. 2003, 81, 291–305. [Google Scholar] [CrossRef]
- Landis, J.R.; Koch, G.G. The measurement of observer agreement for categorical data. Biometrics 1977, 33, 159–174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bernard, W. Colic in the foal. Equine Vet. Educ. 2004, 16, 319–323. [Google Scholar] [CrossRef]
- Van Loon, J.P.A.M.; van Dierendonck, M.C. Monitoring acute equine visceral pain with the Equine Utrecht University Scale for Composite Pain Assessment (EQUUS-COMPASS) and the Equine Utrecht University Scale for Facial Assessment of Pain (EQUUS-FAP): A scale-construction study. Vet. J. 2015, 206, 356–364. [Google Scholar] [CrossRef]
- Guide for Veterinary Service and Judging of Equestrian Events, 4th ed.; American Association of Equine Practitioners: Lexington, KY, USA, 1991; Volume 19.
- Wong, D.M.; Wilkins, P.A. Defining the systemic inflammatory response syndrome in equine neonates. Vet. Clin. N. Am. Equine Pract. 2015, 31, 463–481. [Google Scholar] [CrossRef]
- Bernard, W.V.; Reimer, J.M. Examination of the foal. Vet. Clin. N. Am. Equine Pract. 1994, 10, 37–66. [Google Scholar] [CrossRef]
- Ohmura, H.; Jones, J.H. Changes in heart rate and heart rate variability as a function of age in Thoroughbred horses. J. Equine Vet. Sci. 2017, 28, 99–103. [Google Scholar] [CrossRef] [Green Version]
- McAuliffe, S.B. Neonatal examination, clinical procedures and nursing care. In Color Atlas of Disease and Disorder of the Foal; Mc Auliffe, S.B., Slovis, N.M., Eds.; Elsevier Saunders: Philadelphia, PA, USA, 2008; pp. 43–78. [Google Scholar]
- Nagy, K.; Schrott, A.; Kabai, P. Possible influence of neighbours on stereotypic behaviour in horses. Appl. Anim. Behav. Sci. 2008, 111, 321–328. [Google Scholar] [CrossRef]
- Waring, G. Horse Behaviour, 2nd ed.; Noyes Publications; William Andrew Publishing: New York, NY, USA, 2003; p. 442. [Google Scholar]
- Paradis, M.R. Gastrointestinal disease. In Equine Neonatal Medicine: A Case-Based Approach; Elsevier Saunders: Philadelphia, PA, USA, 2006. [Google Scholar]
- Søndergaard, E.; Jago, J. The effect of early handling of foals on their reaction to handling, humans and novelty, and the foal–mare relationship. Appl. Anim. Behav. Sci. 2010, 123, 93–100. [Google Scholar] [CrossRef]
- Taffarel, M.O.; Luna, S.P.L.; de Oliveira, F.A.; Cardoso, G.S.; de Moura Alonso, J.; Pantoja, J.C.; Brondani, J.T.; Love, E.; Taylor, P.; White, K.; et al. Refinement and partial validation of the UNESP-Botucatu multidimensional composite pain scale for assessing postoperative pain in horses. BMC Vet. Res. 2015, 11, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Da Rocha, P.B.; Driessen, B.; McDonnell, S.M.; Hopster, K.; Zarucco, L.; Gozalo-Marcilla, M.; Hopster-Iversen, C.; Trindade, P.H.E.; da Rocha, T.K.G.; Taffarel, M.O.; et al. A critical evaluation for validation of composite and unidimensional postoperative pain scales in horses. PLoS ONE 2021, 16, e0255618. [Google Scholar]
- Raekallio, M.; Taylor, P.M.; Bennett, R.C. Preliminary investigations of pain and analgesia assessment in horses administered phenylbutazone or placebo after arthroscopic surgery. Vet. Surg. 1997, 26, 150–155. [Google Scholar] [CrossRef] [PubMed]
- Price, J.; Catriona, S.; Welsh, E.M.; Waran, N.K. Preliminary evaluation of a behaviour–based system for assessment of post–operative pain in horses following arthroscopic surgery. Vet. Anaesth. Analg. 2003, 30, 124–137. [Google Scholar] [CrossRef] [PubMed]
- Sellon, D.C.; Roberts, M.C.; Blikslager, A.T.; Ulibarri, C.; Papich, M.G. Effects of continuous rate intravenous infusion of butorphanol on physiologic and outcome variables in horses after celiotomy. J. Vet. Intern. 2004, 18, 555–563. [Google Scholar] [CrossRef]
- Gleerup, K.B.; Forkman, B.; Lindegaard, C.; Andersen, P.H. An equine pain face. Vet. Anaesth. Analg. 2015, 42, 103–114. [Google Scholar] [CrossRef] [Green Version]
- Graubner, C.; Gerber, V.; Doherr, M.; Spadavecchia, C. Clinical application and reliability of a post abdominal surgery pain assessment scale (PASPAS) in horses. Vet. J. 2011, 188, 178–183. [Google Scholar] [CrossRef]
- Lindegaard, C.; Vaabengaard, D.; Christophersen, M.T.; Ekstøm, C.T.; Fjeldborg, J. Evaluation of pain and inflammation associated with hot iron branding and microchip transponder injection in horses. Am. J. Vet. Res. 2009, 70, 840–847. [Google Scholar] [CrossRef]
- McCann, J.S.; Heird, J.C.; Bell, R.W.; Lutherer, L.O. Normal and more highly reactive horses. I. Heart rate, respiration rate and behavioral observations. Appl. Anim. Behav. Sci. 1988, 19, 201–214. [Google Scholar]
- Carroll, J.M.; Russell, J.A. Do facial expressions signal specific emotions? Judging emotion from the face in context. J. Pers. Soc. Psychol. 1996, 70, 205. [Google Scholar]
- Siniscalchi, M.; Padalino, B.; Aubé, L.; Quaranta, A. Right-nostril use during sniffing at arousing stimuli produces higher cardiac activity in jumper horses. Laterality 2015, 20, 483–500. [Google Scholar] [CrossRef]
- Corgan, M.E.; Grandin, T.; Matlock, S. Evaluating the Reaction to a Complex Rotated Object in the American Quarter Horse (Equus caballus). Animals 2021, 11, 1383. [Google Scholar] [CrossRef] [PubMed]
- Sutton, G.A.; Paltiel, O.; Soffer, M.; Turner, D. Validation of two behaviour-based pain scales for horses with acute colic. Vet. J. 2013, 197, 646–650. [Google Scholar] [CrossRef] [PubMed]
- Streiner, D.L.; Norman, G.R.; Cainery, J. Health Measurement Scales: A Practical Guide to Their Development and Use, 5th ed.; Oxford University Press: Oxford, UK, 2015; p. 25. [Google Scholar]
- Duhn, L.J.; Medves, J.M. A systematic integrative review of infant pain assessment tools. Adv. Neonatal Care 2004, 4, 126–140. [Google Scholar] [CrossRef]
- von Baeyer, C.L.; Spagrud, L.J. Systematic review of observational (behavioral) measures of pain for children and adolescents aged 3 to 18 year. Pain 2007, 127, 140–150. [Google Scholar] [CrossRef]
- Zwakhalen, S.M.; Hamers, J.P.; Abu-Saad, H.H.; Berger, M.B. Pain in elderly people with severe dementia: A systematic review of behavioural pain assessment tools. BMC Geriatr. 2006, 6, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bradley, C. Importance of differentiating health status from quality of life. Lancet 2001, 357, 7–8. [Google Scholar] [CrossRef]
- Mellor, D.J. Updating animal welfare thinking: Moving beyond the ‘Five Freedoms’ towards ‘A Life Worth Living’. Animals 2016, 6, 21. [Google Scholar] [CrossRef]
- Parker, R.A.; Yeates, J.W. Assessment of quality of life in equine patients. Equine Vet. J. 2012, 44, 244–249. [Google Scholar] [CrossRef]


| Patients’ Data | Control Group (n = 35) | Pain Group (n = 15) |
|---|---|---|
| Age (days) | 33 ± 26 (1–88) | 13 ± 12 (1–47) |
| Males | 17/35 (49%) | 12/15 (80%) |
| Females | 18/35 (51%) | 3/15 (20%) |
| Breeds | 31/35 (88%) Stb 1/35 (3%) Arab 1/35 (3%) QH 2/35 (6%) Oth | 6/15 (40%) Stb 2/15 (13%) Arab 3/15 (20%) QH 4/15 (27%) Oth |
| Type of Analysis | Description | Statistical Test |
|---|---|---|
| Inter-observer reliability | Agreement among the five blinded observers | Fleiss’ kappa and ICC for single measures |
| Intra-observer reliability | Agreement between scores assigned by the same observer to videos viewed twice | Fleiss’ kappa |
| Internal consistency | Agreement between individual items of the scale and value of Cronbach’s alpha if each item is deleted | Cronbach’s alpha |
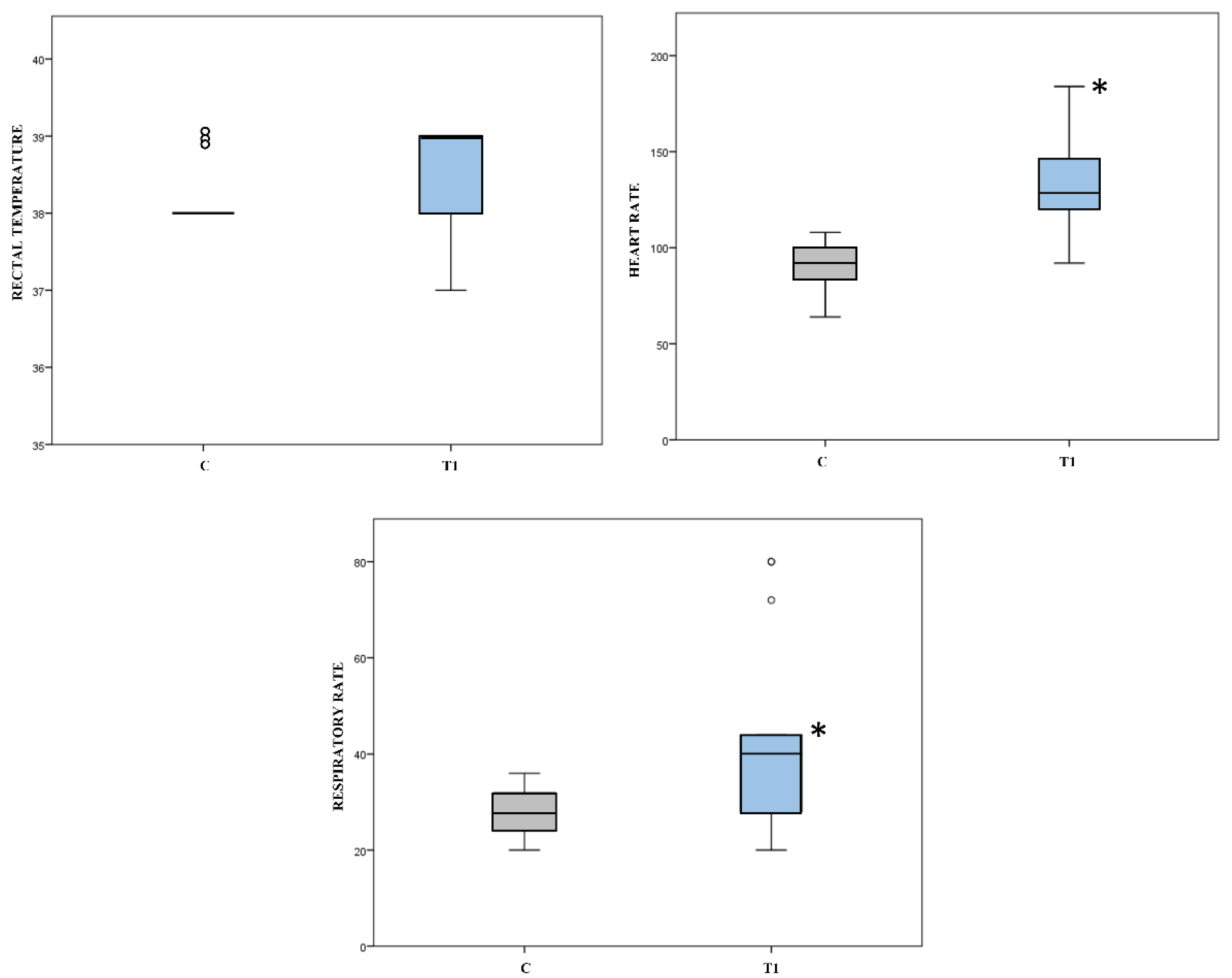
| Construct validity | Degree of correlation of the scale with rectal temperature, heart rate, and respiratory rate theoretically related to pain | Spearman’s rank coefficient of correlation |
| Construct validity | Cut-off value of the scale to discriminate between pain and no-pain | Receiver operating characteristic (ROC) analysis |
| Construct validity (Group effect) | Score variation between Control Group and Pain Group | Mann Whitney U test |
| Construct validity (Time effect in the Pain Group) | Score variation when pain decreases (T1-T2-T3) | Friedman ANOVA test and Tukey’s test |
| Facial Expression Items | Categories | Score |
|---|---|---|
| Head | Normal head movement | 0 |
| Less movement/increased movement (low head carriage/head shaking) | 1 | |
| No movement/strongly increased movement (low head carriage/head shaking) | 2 | |
| Eyelids * | Opened, sclera can be seen in case of eye/head movement | 0 |
| More opened eyes/tightening of eyelids | 1 | |
| Obviously more opened eyes/obvious tightening of eyelids | 2 | |
| Focus | Focused on environment (interacts with the surrounding) | 0 |
| Less focused on environment (sometimes depressed and sometimes alert) | 1 | |
| Not focused on environment (always depressed) | 2 | |
| Nostrils * | Relaxed | 0 |
| A bit more opened, sometimes relaxed sometimes flared | 1 | |
| Obviously more opened, nostril always flaring and possibly audible breathing | 2 | |
| Corner mouth/lips * | Relaxed | 0 |
| Lifted slightly | 1 | |
| Obviously lifted | 2 | |
| Muscle head tone * | No fasciculations | 0 |
| Mild fasciculations | 1 | |
| Obvious fasciculations | 2 | |
| Yawning | Not seen | 0 |
| Seen, once or more times | 2 | |
| Licking/chewing | Both behaviours are not seen | 0 |
| Seen, one or both behaviours, once or more times | 2 | |
| Teeth grinding | Not heard | 0 |
| Heard, one or more times | 2 | |
| Moaning | Not heard | 0 |
| Heard, once or more times | 2 | |
| Ears | Position: orientation towards sound/clear response with both ears or ear closest to source | 0 |
| Reduced movements and delayed/reduced response to sounds | 1 | |
| No movement, held backwards and no response to sounds | 2 | |
| Score | …/22 |
| Behavioural Items | Categories | Score * |
|---|---|---|
| Signs of abdominal pain | No kicking, quietly standing without pawing/rolling/dropping to the ground | 0 |
| Occasionally (1–2 times) kicking/rolling/dropping to the ground/pawing | 1 | |
| Excessive (>2 times) kicking/rolling/dropping to the ground/pawing | 2 | |
| Posture (Standing and recumbency) | Stands quietly, normal walk; normal recumbency, no weight shift Occasional weight-shift (1–2 times), slight muscle tremors, but normal recumbency | 0 |
| Non-weight bearing, abnormal weight distribution, analgesic posture, attempts to urinate or defecate, prostration, generalized muscle tremors, prolonged or restless recumbency | 1 | |
| Non-weight bearing, abnormal weight distribution, analgesic posture, attempts to urinate or defecate, prostration, generalized muscle tremors, prolonged or restless recumbency | 2 | |
| Appetite * | Feeds normally to the udder, interested in milk | 0 |
| Shows interest in milk, but drinks very little or stimulates the udder but does not drink | 1 | |
| Not interest | 2 | |
| Lameness * | Not seen | 0 |
| Moderate (I–II degree) | 1 | |
| Severe (III–IV degree) | 2 | |
| Score | …/8 |
| Physical Items | Categories | Score |
|---|---|---|
| Rectal temperature | In range (37.2–39.2 °C) [30] | 0 |
| Out of range | 2 | |
| Heart rate | In range (0–30 days 80–100 bpm/min-1–6 months 45–89 bpm/min) [31,32] | 0 |
| Out of range (tachycardia > 115 bpm/min) | 2 | |
| Respiratory rate | In range (20–40 breaths/min) [33] | 0 |
| Out of range (dyspnea or tachypnea > 56 breaths/min) | 2 | |
| Reaction to palpation of the painful area | No reaction to palpation | 0 |
| Mild reaction to palpation | 1 | |
| Severe reaction to palpation | 2 | |
| Intestinal motility | Normal motility | 0 |
| Not normal motility (increase/decrease/absent) | 2 | |
| Score | …/10 |
| Time Points | Section I Score (0–22) | Section II Score (0–8) | Section III Score (0–10) | Subtotal Score (I + II; 0–30) | Total Score (I + II + III; 0–40) |
|---|---|---|---|---|---|
| C | 1 (0–2) | 0 | 1 (0–1) | 1 (0–2) | 2 (1–2) |
| T1 | 7 (6–9) | 3 (3–5) | 4 (4–7) | 11 (9–13) | 16 (13–18) |
| T2 | 6 (3–8) | 3 (1–4) | 2 (1–3) | 10 (5–10) | 11 (6–13) |
| T3 | 1 (1–1) | 0 | 0 | 1 (1–2) | 3 (2–4) |
| Items | Scores | C | T1 | T2 | T3 |
|---|---|---|---|---|---|
| Reaction to palpation of the painful area | 0 | 19/35 (54%) | 4/15 (27%) | 6/13 (46%) | 5/6 (83%) |
| 1 | 15/35 (43%) | 4/15 (27%) | 4/13 (31%) | 1/6 (17%) | |
| 2 | 1/35 (3%) | 7/15 (46%) | 3/13 (23%) | 0/6 (0%) | |
| Intestinal motility | 0 | 33/35 (94%) | 12/15 (80%) | 13/13 (100%) | 6/6 (100%) |
| 2 | 2/35 (6%) | 3/15 (20%) | 0/13 (0%) | 0/6 (0%) |
| Items | Corrected Item–Total Correlation | Cronbach’s Alpha if the Item Is Removed |
|---|---|---|
| Head | 0.623 | 0.823 |
| Eyelids | 0.566 | 0.828 |
| Focus | 0.489 | 0.835 |
| Corner mouth/lips | 0.623 | 0.827 |
| Nostrils | 0.545 | 0.828 |
| Muscle head tone | 0.187 | 0.844 |
| Yawning | 0.119 | 0.845 |
| Liking/chewing | 0.439 | 0.854 |
| Teeth grinding | 0.431 | 0.835 |
| Moaning | 0.383 | 0.838 |
| Ears | 0.637 | 0.823 |
| Signs of abdominal pain | 0.390 | 0.838 |
| Posture | 0.839 | 0.803 |
| Appetite | 0.146 | 0.845 |
| Lameness | 0.737 | 0.813 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lanci, A.; Benedetti, B.; Freccero, F.; Castagnetti, C.; Mariella, J.; van Loon, J.P.A.M.; Padalino, B. Development of a Composite Pain Scale in Foals: A Pilot Study. Animals 2022, 12, 439. https://doi.org/10.3390/ani12040439
Lanci A, Benedetti B, Freccero F, Castagnetti C, Mariella J, van Loon JPAM, Padalino B. Development of a Composite Pain Scale in Foals: A Pilot Study. Animals. 2022; 12(4):439. https://doi.org/10.3390/ani12040439
Chicago/Turabian StyleLanci, Aliai, Beatrice Benedetti, Francesca Freccero, Carolina Castagnetti, Jole Mariella, Johannes P. A. M. van Loon, and Barbara Padalino. 2022. "Development of a Composite Pain Scale in Foals: A Pilot Study" Animals 12, no. 4: 439. https://doi.org/10.3390/ani12040439
APA StyleLanci, A., Benedetti, B., Freccero, F., Castagnetti, C., Mariella, J., van Loon, J. P. A. M., & Padalino, B. (2022). Development of a Composite Pain Scale in Foals: A Pilot Study. Animals, 12(4), 439. https://doi.org/10.3390/ani12040439








