Factors Associated with Mortality of Lambs Born to Ewe Hoggets
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Lamb Mortality in Lambs Born to Ewe Hoggets (Objective 1)
2.1.1. Farms and Animals
2.1.2. Reproductive and Health Management
2.1.3. Grazing Management
2.1.4. Weight and Body Condition Score (BCS) Data
2.1.5. Lambing Management and Lamb Necropsy
2.2. Ewe Hogget Mortality over the Lambing Period (Objective 2)
2.3. Comparison of Mortality in Lambs Born to Ewe Hoggets and Mature-Age Ewes (Objective 3)
2.4. Statistical Analyses
2.4.1. Causes and Risk Factors for Lamb Mortality for Lambs Born to Ewe Hoggets (Objective 1)
2.4.2. Ewe Hogget Mortality over the Lambing Period (Objective 2)
2.4.3. Comparison of Mortality in Lambs Born to Ewe Hoggets and Mature-Age Ewes (Objective 3)
3. Results
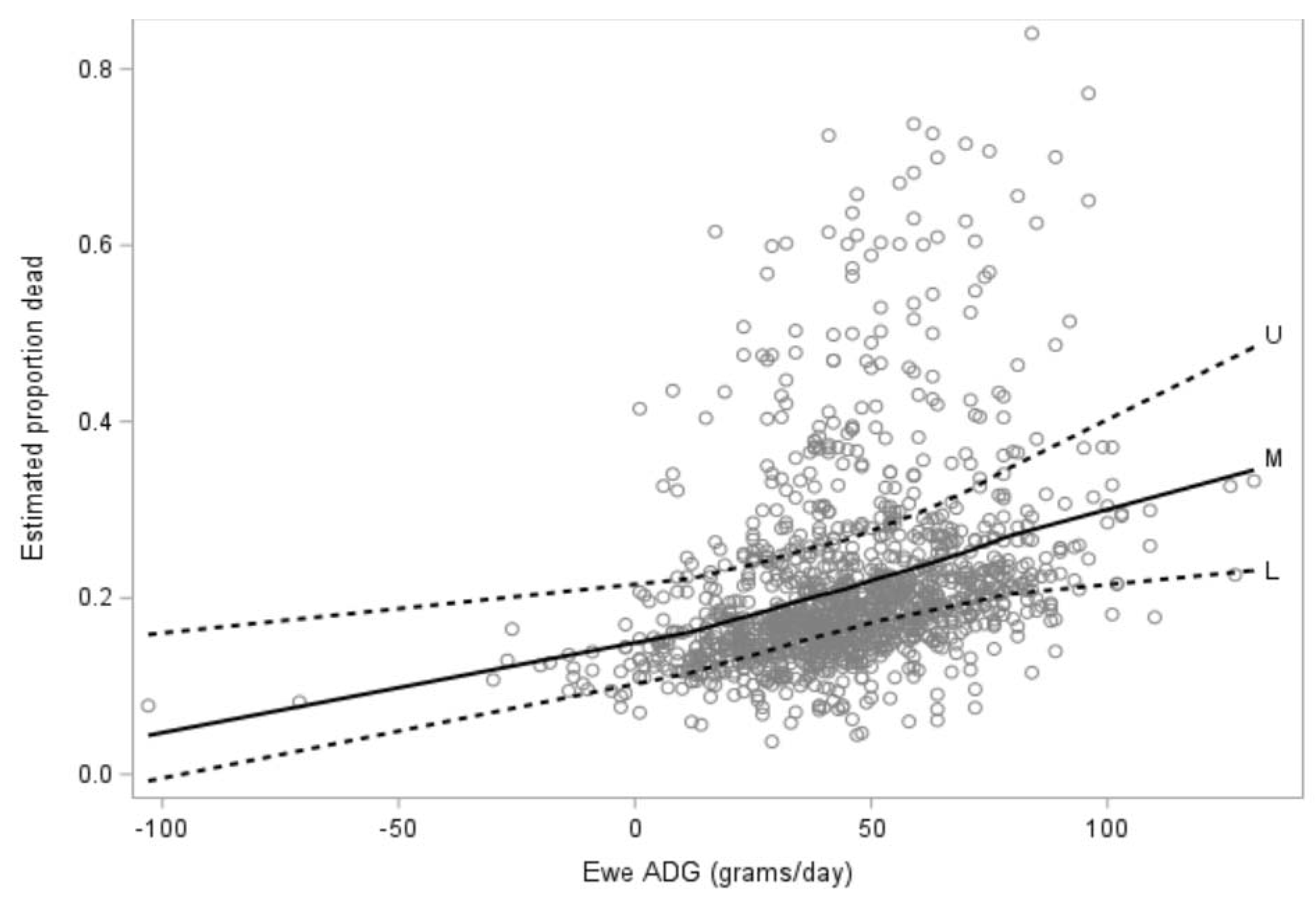
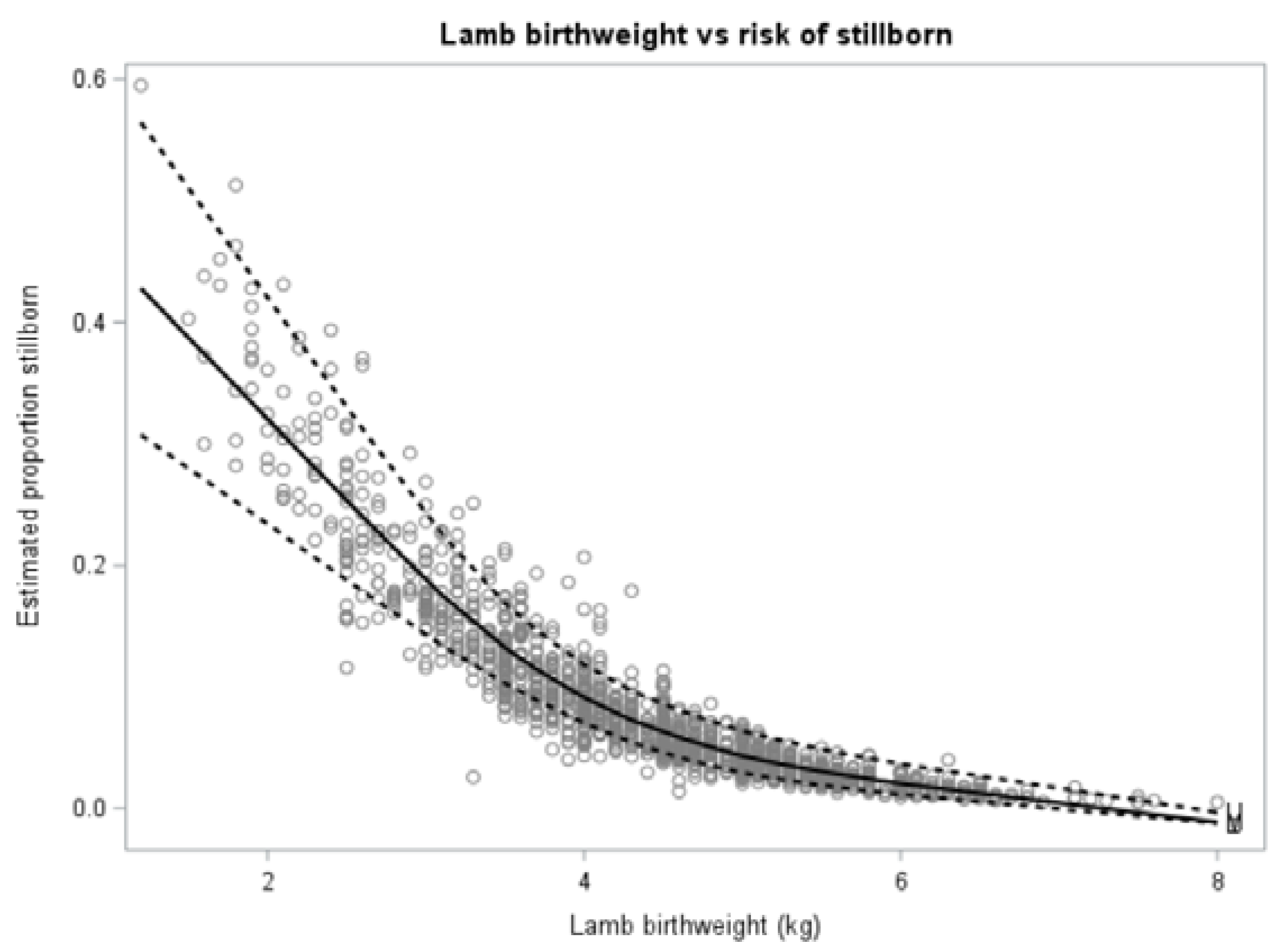
3.1. Risk Factors for Lamb Mortality for Lambs Born to Ewe Hoggets (Objective 1)
3.1.1. Descriptive and Univariate Analysis
3.1.2. Multivariate Analysis
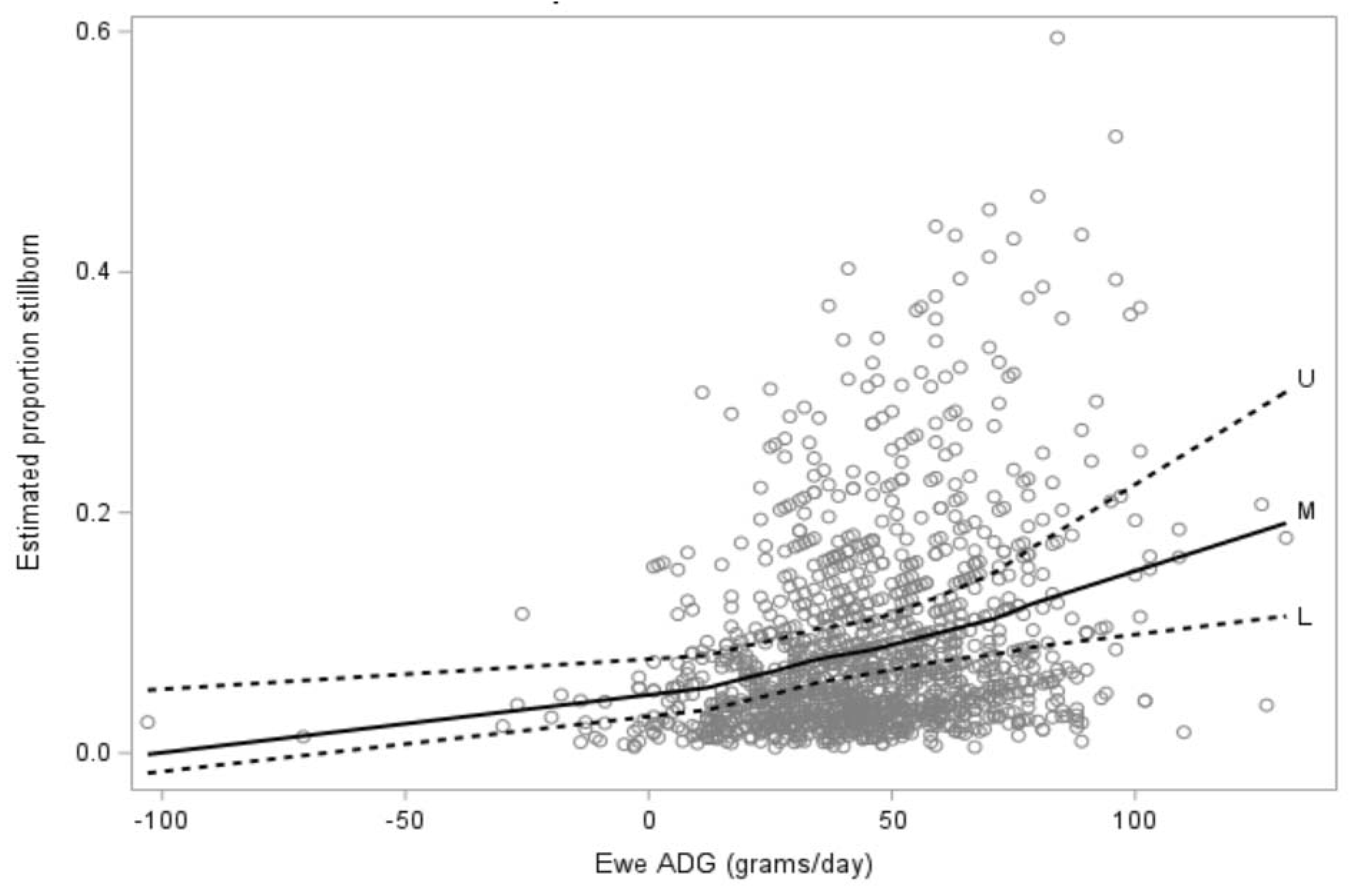
3.2. Causes of Lamb Mortality for Lambs Born to Ewe Hoggets (Objective 1)
3.2.1. Descriptive and Univariate Analysis
3.2.2. Multivariate Analysis
3.3. Ewe Hogget Mortality over the Lambing Period (Objective 2)
3.4. Comparison of Lamb Mortality for Lambs Born to Ewe Hoggets and Mature-Age Ewes (Objective 3)
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kenyon, P.R.; Thompson, A.N.; Morris, S.T. Breeding ewe lambs successfully to improve lifetime performance. Small Rumin. Res. 2014, 118, 2–15. [Google Scholar] [CrossRef]
- Corner, R.A.; Mulvaney, F.J.; Morris, S.T.; West, D.M.; Morel, P.C.H.; Kenyon, P.R. A comparison of the reproductive performance of ewe lambs and mature ewes. Small Rumin. Res. 2013, 114, 126–133. [Google Scholar] [CrossRef]
- Edwards, S.J.; Juengel, J.L. Limits on hogget lambing: The fertility of the young ewe. N. Z. J. Agric. Res. 2016, 60, 1–22. [Google Scholar] [CrossRef]
- Pettigrew, E.J.; Hickson, R.E.; Blair, H.T.; Griffiths, K.J.; Ridler, A.L.; Morris, S.T.; Kenyon, P.R. Differences in lamb production between ewe lambs and mature ewes. N. Z. J. Agric. Res. 2020, 64, 508–521. [Google Scholar] [CrossRef]
- Young, E.A.; Yuan, J.V.; Everett-Hincks, J. Yearling lambing performance and primary cause of lamb death. Proc. N. Z. Soc. Anim. Prod. 2010, 70, 96–100. [Google Scholar]
- Griffiths, K.J.; Ridler, A.L.; Heuer, C.; Corner-Thomas, R.A.; Kenyon, P.R. The effect of liveweight and body condition score on the ability of ewe lambs to successfully rear their offspring. Small Rumin. Res. 2016, 145, 130–145. [Google Scholar] [CrossRef]
- Clune, T.; Besier, S.; Hair, S.; Hancock, S.; Lockwood, A.; Thompson, A.; Jelocnik, M.; Jacobsen, C. Chlaymdia pecorum detection in aborted and stillborn lambs from Western Australia. Vet. Res. 2021, 52, 84. [Google Scholar] [CrossRef]
- Thompson, A.N.; Bowen, E.; Keiller, J.; Pegler, D.; Kearney, G.; Rosales-Nieto, C.A. The number of offspring weaned from ewe lambs is affected differently by liveweight and age at breeding. Animals 2021, 11, 2733. [Google Scholar] [CrossRef]
- Jacobsen, C.; Bruce, M.; Kenyon, P.R.; Lockwood, A.; Miller, D.; Refshauge, G.; Masters, D.G. A review of dystocia in sheep. Small Rumin. Res. 2020, 192, 106209. [Google Scholar] [CrossRef]
- Everett-Hincks, J.M.; Dodds, K.G. Management of maternal-offspring behaviour to improve lamb survival in easy care sheep systems. J. Anim. Sci. 2008, 86, E259–E270. [Google Scholar] [CrossRef]
- McHugh, N.; Berry, D.P.; Pabiou, T. Risk factors associated with lambing traits. Animal 2016, 10, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Behrendt, R.; van Burgel, A.J.; Bailey, A.; Barber, P.; Curnow, M.; Gordon, D.J.; Hocking Edwards, J.E.; Oldham, C.M.; Thompson, A.N. On-farm paddock scale comparisons across southern Australia confirm that increasing the nutrition of Merino ewes improves their production and the lifetime performance of their progeny. Anim. Prod. Sci. 2011, 51, 805–812. [Google Scholar] [CrossRef]
- Oldham, C.M.; Thompson, A.N.; Ferguson, M.B.; Gordon, D.J.; Kearney, G.A.; Paganoni, B.L. The birthweight and survival of Merino lambs can be predicted from the profile of liveweight change of their mothers during pregnancy. Anim. Prod. Sci. 2011, 51, 776–783. [Google Scholar] [CrossRef]
- Tarbotton, I.S.; Webby, R.W. Variation in lamb survival within farm and between farms: Results from farmer studies. Proc. N. Z. Soc. Anim. Prod. 1999, 59, 73–75. [Google Scholar]
- Jefferies, B. Body condition scoring and its use in management. Tasman. J. Agric. 1961, 32, 19–21. [Google Scholar]
- Kenyon, P.R.; Maloney, S.; Blache, D. Review of sheep body condition score in relation to production characteristics. N. Z. J. Agric. Res. 2014, 57, 38–64. [Google Scholar] [CrossRef]
- Lashley, V.D.; Roe, W.D.; Kenyon, P.R.; Thompson, K.G. Perinatal lamb mortality: An assessment of gross, histological and immunohistochemical changes in the central nervous system. N. Z. Vet. J. 2014, 62, 160–166. [Google Scholar] [CrossRef]
- Morris, S.T.; Kenyon, P.R.; West, D.M. Effect of hogget nutrition in pregnancy on lamb birthweight and survival to weaning. N. Z. J. Agric. Res. 2005, 48, 165–175. [Google Scholar] [CrossRef][Green Version]
- Schreurs, N.M.; Kenyon, P.R.; Mulvaney, F.J.; Morel, P.C.H.; West, D.M.; Morris, S.T. Response of additional ewe lamb liveweight during gestation on birth and weaning weight of offspring and liveweight of the ewe lamb at weaning. Anim. Prod. Sci. 2010, 50, 528–532. [Google Scholar] [CrossRef]
- Dwyer, C.M.; Conington, J.; Corbiere, F.; Holmoy, I.H.; Muri, K.; Nowak, R.; Rooke, J.; Vipond, J.; Gautier, J.M. Invited review: Improving neonatal survival in small ruminants: Science into practice. Animal 2016, 10, 449–459. [Google Scholar] [CrossRef]
- Kenyon, P.R.; Morris, S.T.; Revell, D.K.; McCutcheon, S.N. Shearing during pregnancy—Review of a policy to increase birthweight and survival of lambs in New Zealand pastoral farming systems. N. Z. Vet. J. 2003, 51, 200–207. [Google Scholar] [CrossRef] [PubMed]
- Rosales Nieto, C.A.; Ferguson, M.B.; Macleay, C.A.; Briegel, J.R.; Wood, D.A.; Martin, G.B.; Bencini, R.; Thompson, A.N. Milk production and composition, and progeny performance in young ewes with high merit for rapid growth and muscle and fat accumulation. Animal 2018, 12, 2292–2299. [Google Scholar] [CrossRef] [PubMed]
- Wallace, J.M. Competition for nutrients in pregnant adolescents: Consequences for maternal, conceptus and offspring endocrine systems. J. Endocrinol. 2019, 242, T1–T19. [Google Scholar] [CrossRef] [PubMed]
- Refshauge, G.; Brien, F.D.; Hinch, G.N.; van de Ven, R. Neonatal lamb mortality: Factors associated with death of Australian lambs. Anim. Prod. Sci. 2016, 56, 726–735. [Google Scholar] [CrossRef]
- Roberston, S.M.; Boulton, J.; Xie, G.; Neef, A.; Friend, M.A. Usefulness of pathological examinations of the central nervous system for monitoring and controlling perinatal lamb mortality. Animals 2020, 11, 2372–2377. [Google Scholar] [CrossRef]
- Williams, S. The challenges of an unusual abortion outbreak in a ewe flock. Proc. Soc. Sheep Beef Cattle Vet. N. Z. Vet. Assoc. 2019, 2019, 69–74. [Google Scholar]
- West, D.M.; Bruere, A.N.; Ridler, A.L. Exotic Diseases. In The Sheep: Health, Disease and Production, 3rd ed.; Massey University Press: Auckland, New Zealand, 2017; pp. 377–390. [Google Scholar]
- Capdevila-Ospina, K.; Corner-Thomas, R.A.; Flay, K.J.; Kenyon, P.R.; Ridler, A.L. Factors associated with ewe death and casting in a commercially farm sheep flock in New Zealand. Ruminants 2021, 1, 87–99. [Google Scholar] [CrossRef]
- Jackson, R.; Hilson, R.P.N.; Roe, A.R.; Perkins, N.; Heuer, C.; West, D.M. Epidemiology of vaginal prolapse in mixed-age ewes in New Zealand. N. Z. Vet. J. 2014, 62, 328–337. [Google Scholar] [CrossRef]

| Cohort | |||
|---|---|---|---|
| Event | A1 | A2 | B |
| Start of breeding | 7 May 2017 1 | 5 May 2018 1 | 11 April 2016 1 |
| End of breeding | 19 June 2017 | 8 June 2018 | 29 April 2016 |
| Pregnancy diagnosis (mid-pregnancy) | 8 August 2017 1 | 6 August 2018 1 | 15 July 2016 1 |
| Set-stocking (pre-lambing) | 23 September 2017 1 | 25 September 2018 1 | 23 August 2016 1 |
| Start of lambing | 4 October 2017 | 2 October 2018 | 8 September 2016 |
| End of lambing | 16 November 2017 | 5 November 2018 | 23 September 2016 |
| Cohort | ||||
|---|---|---|---|---|
| Status | A1 | A2 | B | TOTAL |
| Alive | 530 (80%) | 230 (78%) | 291 (74%) | 1051 (78%) |
| Dead | 132 (20%) | 64 (22%) | 101 (26%) | 297 (22%) |
| TOTAL | 662 | 294 | 392 | 1348 |
| Cohort | ||||
|---|---|---|---|---|
| Cause of Mortality | A1 | A2 | B | TOTAL |
| Dystocia | 13 (9.8%) | 19 (29.7%) | 18 (17.8%) | 50 (16.8%) |
| Starvation Mismothering Exposure complex | 49 (37.1%) | 14 (21.9%) | 14 (13.9%) | 77 (25.9%) |
| Stillborn | 60 (45.5%) | 20 (31.3%) | 42 (41.6%) | 122 (41.1%) |
| Unknown | 7 (5.0%) | 8 (12.5%) | 18 (17.8%) | 33 (11.1%) |
| Other | 3 (2.3%) | 3 (4.7%) | 9 (8.9%) | 15 (5.1%) |
| TOTAL | 132 | 64 | 101 | 297 |
| Cohort | ||||
|---|---|---|---|---|
| Lamb Type | A1 | A2 | B | OVERALL |
| All lambs | 4.16 (0.04) | 4.52 (0.06) | 4.78 (0.05) | 4.42 (0.03) |
| Female | 4.08 (0.05) | 4.25 (0.08) | 4.61 (0.06) | 4.27 (0.04) |
| Male | 4.24 (0.06) | 4.80 (0.09) | 4.96 (0.08) | 4.57 (0.05) |
| Single-born | 4.64 (0.05) | 4.98 (0.07) | 4.78 (0.05) | 4.76 (0.03) |
| Multiple-born | 3.58 (0.05) | 3.72 (0.07) | N/A | 3.62 (0.04) |
| Not stillborn | 4.25 (0.04) | 4.61 (0.06) | 4.84 (0.05) | 4.50 (0.03) |
| Stillborn | 3.19 (0.17) | 3.26 (0.26) | 4.28 (0.18) | 3.57 (0.12) |
| Cohort | ||||
|---|---|---|---|---|
| Cause of Mortality | A1 n = 509 | A2 n = 241 | B n = 392 | Total n = 1142 |
| Dystocia | 4 (0.8%) | 1 (0.4%) | 5 (1.3%) | 10 (0.9%) |
| Vaginal prolapse | 3 (0.6%) | 1 (0.4%) | 4 (1.0%) | 8 (0.7%) |
| Uterine prolapse | 0 (0.0%) | 0 (0.0%) | 6 (1.5%) | 6 (0.5%) |
| Unknown | 1 (0.2%) | 1 (0.4%) | 1 (0.3%) | 3 (0.5%) |
| Other | 0 (0.0%) | 2 (0.8%) | 0 (0.0%) | 2 (0.2%) |
| Total | 8 (1.6%) | 5 (2.1%) | 16 (4.1%) | 29 (2.5%) |
| Cohort | ||
|---|---|---|
| Cause of Mortality | A1 Ewe Hoggets | Farm A Mature-Age Ewes |
| Dystocia | 13 (9.8%) | 45 (16.5%) |
| SME | 49 (37%) | 117 (42.9%) |
| Stillborn | 60 (45.5%) * | 18 (6.6%) * |
| Unknown | 7 (5.0%) * | 56 (20.5%) * |
| Other | 3 (2.3%) * | 37 (13.5%) * |
| Total | 132 | 273 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ridler, A.L.; Flay, K.J.; Kenyon, P.R.; Blair, H.T.; Corner-Thomas, R.A.; Pettigrew, E.J. Factors Associated with Mortality of Lambs Born to Ewe Hoggets. Animals 2022, 12, 319. https://doi.org/10.3390/ani12030319
Ridler AL, Flay KJ, Kenyon PR, Blair HT, Corner-Thomas RA, Pettigrew EJ. Factors Associated with Mortality of Lambs Born to Ewe Hoggets. Animals. 2022; 12(3):319. https://doi.org/10.3390/ani12030319
Chicago/Turabian StyleRidler, Anne L., Kate J. Flay, Paul R. Kenyon, Hugh T. Blair, Rene A. Corner-Thomas, and Emma J. Pettigrew. 2022. "Factors Associated with Mortality of Lambs Born to Ewe Hoggets" Animals 12, no. 3: 319. https://doi.org/10.3390/ani12030319
APA StyleRidler, A. L., Flay, K. J., Kenyon, P. R., Blair, H. T., Corner-Thomas, R. A., & Pettigrew, E. J. (2022). Factors Associated with Mortality of Lambs Born to Ewe Hoggets. Animals, 12(3), 319. https://doi.org/10.3390/ani12030319







