Social Stimulation by the Owner Increases Dogs’ (Canis familiaris) Social Susceptibility in a Food Choice Task—The Possible Effect of Endogenous Oxytocin Release
Abstract
:Simple Summary
Abstract
1. Introduction
2. Experiment I
2.1. Materials and Methods
2.1.1. Ethical Statement
2.1.2. Subjects
2.1.3. Procedure
2.2. Data Analysis
2.3. Results and Discussion
3. Experiment II
3.1. Materials and Methods
3.1.1. Subjects
3.1.2. Procedure
3.1.3. Data Collection and Analysis
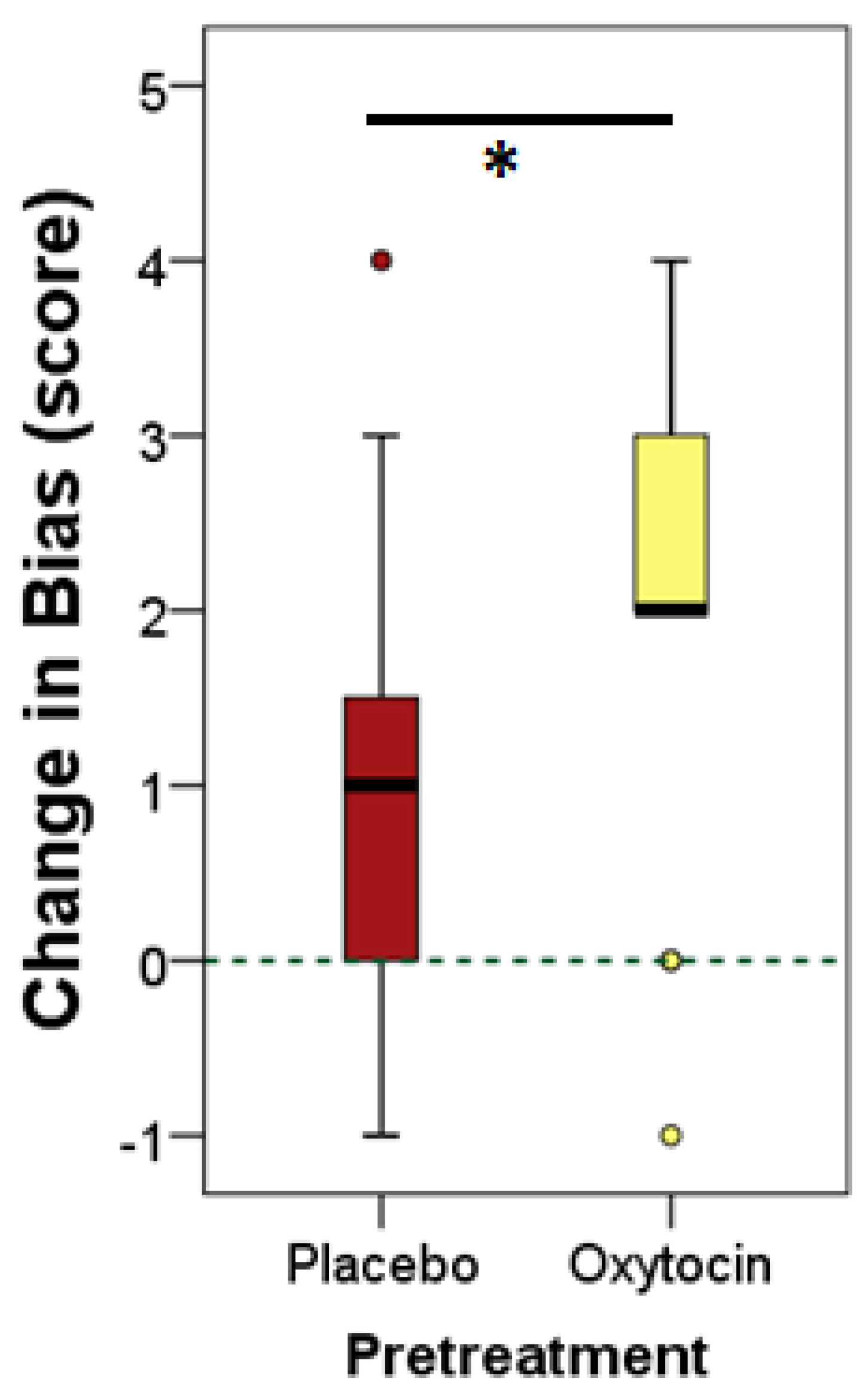
3.2. Results and Discussion
4. General Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cialdini, R.B.; Goldstein, N.J. Social influence: Compliance and conformity. Annu. Rev. Psychol. 2004, 55, 591–621. [Google Scholar] [CrossRef] [PubMed]
- Sinclair, S.; Lowery, B.S.; Hardin, C.D.; Colangelo, A. Social tuning of automatic racial attitudes: The role of affiliative motivation. J. Pers. Soc. Psychol. 2005, 89, 583–592. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sinclair, S.; Huntsinger, J.; Skorinko, J.; Hardin, C.D. Social tuning of the self: Consequences for the self-evaluations of stereotype targets. J. Pers. Soc. Psychol. 2005, 89, 160–175. [Google Scholar] [CrossRef] [Green Version]
- Huber, L.; Range, F.; Voelkl, B.; Szucsich, A.; Virányi, Z.; Miklósi, Á. The evolution of imitation: What do the capacities of non-human animals tell us about the mechanisms of imitation? Philos. Trans. R. Soc. Lond. B. Biol. Sci. 2009, 364, 2299–2309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lakin, J.L.; Chartrand, T.L. Using nonconscious behavioral mimicry to create affiliation and rapport. Psychol. Sci. 2003, 14, 334–339. [Google Scholar] [CrossRef]
- Bargh, J.A.; Gollwitzer, P.M.; Lee-Chai, A.; Barndollar, K.; Trötschel, R. The automated will: Nonconscious activation and pursuit of behavioral goals. J. Pers. Soc. Psychol. 2001, 81, 1014–1027. [Google Scholar] [CrossRef] [PubMed]
- Hare, B.; Tomasello, M. Human-like social skills in dogs? Trends Cogn. Sci. 2005, 9, 439–444. [Google Scholar] [CrossRef] [PubMed]
- Topál, J.; Miklósi, Á.; Gácsi, M.; Dóka, A.; Pongrácz, P.; Kubinyi, E.; Virányi, Z.; Csányi, V. The dog as a model for understanding human social behavior. Adv. Study Behav. 2009, 39, 71–116. [Google Scholar] [CrossRef]
- Tomasello, M.; Kaminski, J. Like Infant, Like Dog. Science 2009, 325, 1213–1214. [Google Scholar] [CrossRef] [Green Version]
- Miklósi, Á.; Topál, J. What does it take to become “best friends”? Evolutionary changes in canine social competence. Trends Cogn. Sci. 2013, 17, 287–294. [Google Scholar] [CrossRef]
- Palagi, E.; Cordoni, G. Intraspecific motor and emotional alignment in dogs and wolves: The basic building blocks of dog–human affective connectedness. Animals 2020, 10, 241. [Google Scholar] [CrossRef] [Green Version]
- Duranton, C.; Bedossa, T.; Gaunet, F. Interspecific behavioural synchronization: Dogs exhibit locomotor synchrony with humans. Sci. Rep. 2017, 7, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Wanser, S.H.; MacDonald, M.; Udell, M.A.R. Dog–human behavioral synchronization: Family dogs synchronize their behavior with child family members. Anim. Cogn. 2021, 24, 747–752. [Google Scholar] [CrossRef] [PubMed]
- Topál, J.; Byrne, R.W.; Miklósi, Á.; Csányi, V. Reproducing human actions and action sequences: “Do as I Do!” in a dog. Anim. Cogn. 2006, 9, 355–367. [Google Scholar] [CrossRef]
- Fugazza, C.; Petro, E.; Miklósi, Á.; Pogány, Á. Social learning of goal-directed actions in dogs (Canis familiaris): Imitation or emulation? J. Comp. Psychol. 2019, 133, 244–251. [Google Scholar] [CrossRef] [PubMed]
- Turcsán, B.; Szánthó, F.; Miklósi, Á.; Kubinyi, E. Fetching what the owner prefers? Dogs recognize disgust and happiness in human behaviour. Anim. Cogn. 2014, 18, 83–94. [Google Scholar] [CrossRef] [Green Version]
- Merola, I.; Prato-Previde, E.; Marshall-Pescini, S. Dogs’ social referencing towards owners and strangers. PLoS ONE 2012, 7, e47653. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prato-Previde, E.; Marshall-Pescini, S.; Valsecchi, P. Is your choice my choice? The owners’ effect on pet dogs’ (Canis lupus familiaris) performance in a food choice task. Anim. Cogn. 2008, 11, 167–174. [Google Scholar] [CrossRef]
- Marshall-Pescini, S.; Prato-Previde, E.; Valsecchi, P. Are dogs (Canis familiaris) misled more by their owners than by strangers in a food choice task? Anim. Cogn. 2011, 14, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Marshall-Pescini, S.; Passalacqua, C.; Petrazzini, M.E.M.; Valsecchi, P.; Prato-Previde, E. Do Dogs (Canis lupus familiaris) Make Counterproductive Choices Because They Are Sensitive to Human Ostensive Cues? PLoS ONE 2012, 7, e35437. [Google Scholar] [CrossRef] [Green Version]
- Odendaal, J.S.J. Animal-assisted therapy—Magic or medicine? J. Psychosom. Res. 2000, 49, 275–280. [Google Scholar] [CrossRef]
- Handlin, L.; Hydbring-sandberg, E.; Nilsson, A.; Ejdebäck, M.; Jansson, A.; Uvnäs-moberg, K. Short-Term Interaction between Dogs and Their Owners: Effects on Oxytocin, Cortisol, Insulin and Heart Rate—An Exploratory Study. Anthrozoos 2011, 24, 301–315. [Google Scholar] [CrossRef]
- Nagasawa, M.; Mitsui, S.; En, S.; Ohtani, N.; Ohta, M.; Sakuma, Y.; Onaka, T.; Mogi, K.; Kikusui, T. Oxytocin-gaze positive loop and the co-evolution of human dog bonds. Science 2015, 348, 333–336. [Google Scholar] [CrossRef]
- Wirobski, G.; Range, F.; Schaebs, F.S.; Palme, R.; Deschner, T.; Marshall-Pescini, S. Life experience rather than domestication accounts for dogs’ increased oxytocin release during social contact with humans. Sci. Rep. 2021, 11, 1–12. [Google Scholar] [CrossRef]
- Romero, T.; Nagasawa, M.; Mogi, K.; Hasegawa, T.; Kikusui, T. Oxytocin promotes social bonding in dogs. Proc. Natl. Acad. Sci. USA 2014, 111, 9085–9090. [Google Scholar] [CrossRef] [Green Version]
- Kis, A.; Hernádi, A.; Kanizs, O.; Gácsi, M.; Topál, J. Oxytocin induces positive expectations about ambivalent stimuli (cognitive bias) in dogs. Horm. Behav. 2015, 69, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Oliva, J.L.; Rault, J.-L.; Appleton, B.; Lill, A. Oxytocin enhances the appropriate use of human social cues by the domestic dog (Canis familiaris) in an object choice task. Anim. Cogn. 2015, 18, 767–775. [Google Scholar] [CrossRef] [PubMed]
- Kis, A.; Hernádi, A.; Miklósi, B.; Kanizsár, O.; Topál, J. The way dogs (Canis familiaris) look at human emotional faces is modulated by oxytocin. an eye-tracking study. Front. Behav. Neurosci. 2017, 11, 210. [Google Scholar] [CrossRef] [PubMed]
- Topál, J.; György, G.; Erdőhelyi, Á. Differential Sensitivity to Human Communication in Dogs, Wolves, and Human Infants. Science 2009, 325, 27–30. [Google Scholar] [CrossRef] [Green Version]
- Hritcu, L.D.; Horhogea, C.; Ciobica, A.; Spataru, M.C.; Spataru, C.; Kis, A. Conceptual replication of canine serum oxytocin increase following a positive dog-human interaction. Rev. Chim. 2019, 70, 1579–1581. [Google Scholar] [CrossRef]
- Over, H.; Carpenter, M. Eighteen-Month-Old Inf Show Increased Helping F Priming With Affiliation. Psychol. Sci. 2009, 20, 1189–1193. [Google Scholar] [CrossRef]
- Souza, G.G.L.; Pereira, M.G.; Vila, J.; Oliveira, L.; Volchan, E. Affiliative Stimuli as Primers to Prosocial Predispositions. Span. J. Psychol. 2012, 15, 237–243. [Google Scholar] [CrossRef]
- De Dreu, C.K.W. Oxytocin modulates cooperation within and competition between groups: An integrative review and research agenda. Horm. Behav. 2012, 61, 419–428. [Google Scholar] [CrossRef] [PubMed]
- Stallen, M.; De Dreu, C.K.W.; Shalvi, S.; Smidts, A.; Sanfey, A.G. The herding hormone: Oxytocin stimulates in-group conformity. Psychol. Sci. 2012, 23, 1288–1292. [Google Scholar] [CrossRef]
- Uvnäs-Moberg, K. Oxytocin may mediate the benefits of positive social interaction and emotions. Psychoneuroendocrinology 1998, 23, 819–835. [Google Scholar] [CrossRef]
- Odendaal, J.S.J.; Meintjes, R.A. Neurophysiological correlates of affiliative behaviour between humans and dogs. Vet. J. 2003, 165, 296–301. [Google Scholar] [CrossRef]
- Mitsui, S.; Yamamoto, M.; Nagasawa, M.; Mogi, K.; Kikusui, T.; Ohtani, N.; Ohta, M. Urinary oxytocin as a noninvasive biomarker of positive emotion in dogs. Horm. Behav. 2011, 60, 239–243. [Google Scholar] [CrossRef]
- Kis, A.; Bence, M.; Lakatos, G.; Pergel, E.; Turcsán, B.; Pluijmakers, J.; Vas, J.; Elek, Z.; Brúder, I.; Földi, L.; et al. Oxytocin receptor gene polymorphisms are associated with human directed social behavior in dogs (Canis familiaris). PLoS ONE 2014, 9, e83993. [Google Scholar] [CrossRef] [Green Version]
- Born, J.; Lange, T.; Kern, W.; McGregor, G.P.; Bickel, U.; Fehm, H.L. Sniffing neuropeptides: A transnasal approach to the human brain. Nat. Neurosci. 2002, 5, 514–516. [Google Scholar] [CrossRef] [PubMed]
- Kis, A.; Kanizsár, O.; Gácsi, M.; Topál, J. Intranasally administered oxytocin decreases heart rate and increases heart rate variability in dogs. J. Vet. Behav. Clin. Appl. Res. 2014, 9, e15. [Google Scholar] [CrossRef]
- Temesi, A.; Thuróczy, J.; Balogh, L.; Mikósi, Á. Increased serum and urinary oxytocin concentrations after nasal administration in beagle dogs. Front. Psychol. 2017, 4, 147. [Google Scholar] [CrossRef] [Green Version]
- Nagasawa, M.; Kikusui, T.; Onaka, T.; Ohta, M. Dog’s gaze at its owner increases owner’s urinary oxytocin during social interaction. Horm. Behav. 2009, 55, 434–441. [Google Scholar] [CrossRef]
- Coppola, C.L.; Grandin, T.; Enns, R.M. Human interaction and cortisol: Can human contact reduce stress for shelter dogs? Physiol. Behav. 2006, 87, 537–541. [Google Scholar] [CrossRef] [PubMed]
- Galambos, Á.; Gergely, A.; Kovács, A.B.; Topál, J. Affect matters: Positive and negative social stimulation influences dogs’ behaviour in a subsequent situation involving an out-of-reach object. Appl. Anim. Behav. Sci. 2021, 236, 105242. [Google Scholar] [CrossRef]
- Kupán, K.; Miklósi, Á.; Gergely, G.; Topál, J. Why do dogs (Canis familiaris) select the empty container in an observational learning task? Anim. Cogn. 2011, 14, 259–268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kis, A.; Topál, J.; Gácsi, M.; Range, F.; Huber, L.; Miklósi, Á.; Virányi, Z. Does the A-not-B error in adult pet dogs indicate sensitivity to human communication? Anim. Cogn. 2012, 15, 737–743. [Google Scholar] [CrossRef]
- Topál, J.; Kis, A.; Oláh, K. Dogs’ Sensitivity to Human Ostensive Cues: A Unique Adaptation? In The Social Dog; Academic Press: Cambridge, MA, USA, 2014; ISBN 9780124078185. [Google Scholar]
- Kaminski, J. Dogs (Canis familiaris) are adapted to receive human communication. In Neurobiology of “Umwelt”: How Living Beings Percive the World; Berthoz, A., Christensen, Y., Eds.; Spinger: Berlin/Heidelberg, Germany, 2009; pp. 103–107. [Google Scholar]
- Kaminski, J.; Schulz, L.; Tomasello, M. How dogs know when communication is intended for them. Dev. Sci. 2012, 15, 222–232. [Google Scholar] [CrossRef]
- Téglás, E.; Gergely, A.; Kupán, K.; Miklósi, Á.; Topál, J.; Kupa, K.; Behavioural, C.; Street, V.H. Dogs’ gaze following is tuned to human communicative signals. Curr. Biol. 2012, 22, 209–212. [Google Scholar] [CrossRef] [Green Version]
- Kirchhofer, K.K.C.; Zimmermann, F.; Kaminski, J.; Tomasello, M. Dogs (Canis familiaris), but not chimpanzees (Pan troglodytes), understand imperative pointing. PLoS ONE 2012, 7, e30913. [Google Scholar] [CrossRef] [Green Version]
- Mikolajczak, M.; Pinon, N.; Lane, A.; de Timary, P.; Luminet, O. Oxytocin not only increases trust when money is at stake, but also when confidential information is in the balance. Biol. Psychol. 2010, 85, 182–184. [Google Scholar] [CrossRef]
- Declerck, C.H.; Boone, C.; Kiyonari, T. Oxytocin and cooperation under conditions of uncertainty: The modulating role of incentives and social information. Horm. Behav. 2010, 57, 368–374. [Google Scholar] [CrossRef]
- Theodoridou, A.; Rowe, A.C.; Penton-Voak, I.S.; Rogers, P.J. Oxytocin and social perception: Oxytocin increases perceived facial trustworthiness and attractiveness. Horm. Behav. 2009, 56, 128–132. [Google Scholar] [CrossRef] [PubMed]
- Churchland, P.S.; Winkielman, P. Modulating social behavior with oxytocin: How does it work? What does it mean? Horm. Behav. 2012, 61, 392–399. [Google Scholar] [CrossRef] [Green Version]
- Neumann, I.D.; Maloumby, R.; Beiderbeck, D.I.; Lukas, M.; Landgraf, R. Increased brain and plasma oxytocin after nasal and peripheral administration in rats and mice. Psychoneuroendocrinology 2013, 38, 1985–1993. [Google Scholar] [CrossRef]
- Kemp, A.H.; Quintana, D.S.; Kuhnert, R.-L.; Griffiths, K.; Hickie, I.B.; Guastella, A.J. Oxytocin increases heart rate variability in humans at rest: Implications for social approach-related motivation and capacity for social engagement. PLoS ONE 2012, 7, e44014. [Google Scholar]
- Weisman, O.; Schneiderman, I.; Zagoory-Sharon, O.; Feldman, R. Salivary Vasopressin Increases Following Intranasal Oxytocin Administration. Peptides 2012, 40, 99–103. [Google Scholar] [CrossRef]
- Kis, A.; Kemerle, K.; Hernádi, A.; Topál, J. Oxytocin and social pretreatment have similar effects on processing of negative emotional faces in healthy adult males. Front. Psychol. 2013, 4, 532. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Freund-Mercier, M.; Stoeckel, M. Somatodendritic autoreceptors on oxytocin neurones. Adv. Exp. Med. Biol. 1995, 395, 185–194. [Google Scholar] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kis, A.; Bolló, H.; Gergely, A.; Topál, J. Social Stimulation by the Owner Increases Dogs’ (Canis familiaris) Social Susceptibility in a Food Choice Task—The Possible Effect of Endogenous Oxytocin Release. Animals 2022, 12, 296. https://doi.org/10.3390/ani12030296
Kis A, Bolló H, Gergely A, Topál J. Social Stimulation by the Owner Increases Dogs’ (Canis familiaris) Social Susceptibility in a Food Choice Task—The Possible Effect of Endogenous Oxytocin Release. Animals. 2022; 12(3):296. https://doi.org/10.3390/ani12030296
Chicago/Turabian StyleKis, Anna, Henrietta Bolló, Anna Gergely, and József Topál. 2022. "Social Stimulation by the Owner Increases Dogs’ (Canis familiaris) Social Susceptibility in a Food Choice Task—The Possible Effect of Endogenous Oxytocin Release" Animals 12, no. 3: 296. https://doi.org/10.3390/ani12030296
APA StyleKis, A., Bolló, H., Gergely, A., & Topál, J. (2022). Social Stimulation by the Owner Increases Dogs’ (Canis familiaris) Social Susceptibility in a Food Choice Task—The Possible Effect of Endogenous Oxytocin Release. Animals, 12(3), 296. https://doi.org/10.3390/ani12030296




