A Multi-Institutional Collaboration to Understand Neoplasia, Treatment and Survival of Snakes
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Case Selection
2.2. Medical Records Review
2.3. Data Analysis
3. Results
3.1. Study Population
3.2. Neoplasia Information
3.3. Neoplasia Prevalence
3.4. Treatment
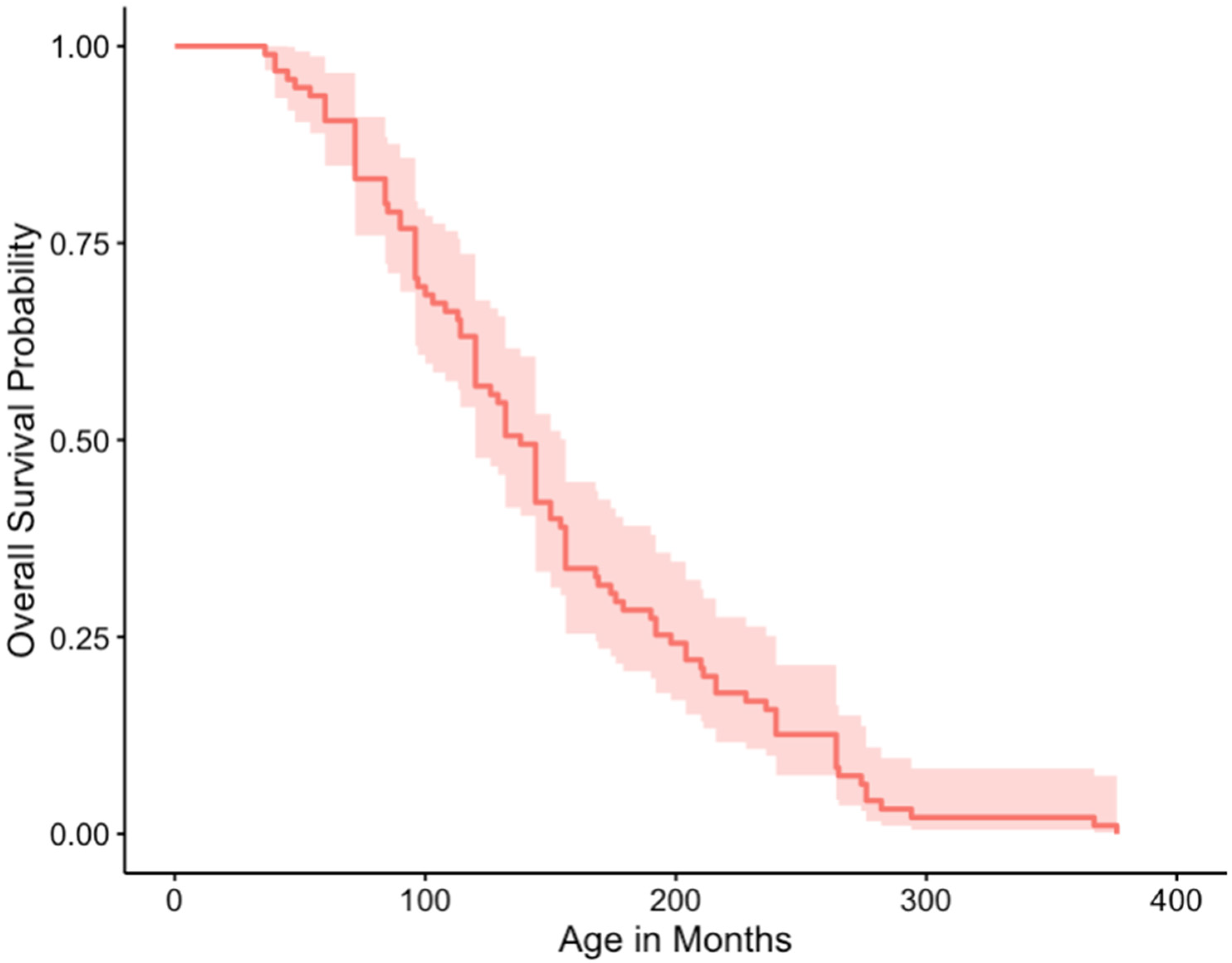
3.5. Outcome and Survival
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Harshbarger, J.C. Registry of Tumors in Lower Animals; Activities Report Supplement (1974–1977); Museum of Natural History, Smithsonian Institution: Washington, DC, USA; Available online: https://library.si.edu/digital-library/book/activitiesrep19741977regi (accessed on 23 November 2021).
- Harshbarger, J.C. Registry of Tumors in Lower Animals; Activities Report Supplement (1978–1981); Museum of Natural History, Smithsonian Institution: Washington, DC, USA; Available online: https://library.si.edu/digital-library/book/activitiesrep19781981regi (accessed on 23 November 2021).
- Harshbarger, J.C. Activities Report: Registry of Tumors in Lower Animals; 1965–1973; National Museum of Natural History: Washington, DC, USA, 1974. [Google Scholar]
- Catão-Dias, J.L.; Nichols, D.K. Neoplasia in snakes at the National Zoological Park, Washington, DC (1978–1997). J. Comp. Pathol. 1999, 120, 89–95. [Google Scholar] [CrossRef]
- Lucke, B.; Schlumberger, H.C. Neoplasia in cold-blooded vertebrates. Physiol. Rev. 1949, 29, 91–126. [Google Scholar] [CrossRef] [PubMed]
- Ramsay, E.C.; Munson, L.; Lowenstine, L.; Fowler, M.E. A Retrospective Study of Neoplasia in a Collection of Captive Snakes. J. Zoo Wildl. Med. March 1996, 27, 28–34. [Google Scholar]
- Christman, J.; Devau, M.; Wilson-Robles, H.; Hoppes, S.; Rech, R.; Russell, K.E.; Heatley, J.J. Oncology of Reptiles: Diseases, Diagnosis, and Treatment. Vet. Clin. N. Am. Exot. Anim. Pract. 2017, 20, 87–110. [Google Scholar] [CrossRef]
- Garner, M.M.; Hernandez-Divers, S.M.; Raymond, J.T. Reptile neoplasia: A retrospective study of case submissions to a specialty diagnostic service. Vet. Clin. N. Am. Exot. Anim. Pract. 2004, 7, 653–671. [Google Scholar] [CrossRef]
- Hernandez-Divers, S.M.; Garner, M.M. Neoplasia of reptiles with an emphasis on lizards. Vet. Clin. N. Am. Exot. Anim. Pract. 2003, 6, 251–273. [Google Scholar] [CrossRef]
- Dietz, J.; Heckers, K.O.; Aupperle, H.; Pees, M. Cutaneous and Subcutaneous Soft Tissue Tumours in Snakes: A Retrospective Study of 33 Cases. J. Comp. Pathol. 2016, 155, 76–87. [Google Scholar] [CrossRef]
- Lamglait, B.; Lemberger, K. Colonic adenocarcinomas in a familial group of captive amur rat snakes (elaphe schrencki). J. Zoo Wildl. Med. 2017, 48, 491–496. [Google Scholar] [CrossRef]
- Sykes, J.M.; Trupkiewicz, J.G. Reptile neoplasia at the Philadelphia Zoological Garden, 1901–2002. J. Zoo Wildl. Med. 2006, 37, 11–19. [Google Scholar] [CrossRef]
- Kaye, S.W.; Daverio, H.; Eddy, R.; Ossiboff, R.J.; Peters-Kennedy, J.; Morrisey, J.K. Surgical resection of an interrenal cell adenocarcinoma in a woma python (Aspidites ramsayi) with 18 month follow-up. J. Herpetol. Med. Surg. 2016, 26, 26–31. [Google Scholar] [CrossRef]
- Summa, N.M.; Guzman, D.S.M.; Hawkins, M.G.; Grosset, C.; Chen, V.S.; Goldsmith, D.; Keel, K.; Woolard, K.; Young, A.C.; Bucy, D.S.; et al. Tracheal and colonic resection and anastomosis in a boa constrictor (Boa constrictor) with T-cell lymphoma. J. Herpetol. Med. Surg. 2015, 25, 87–99. [Google Scholar] [CrossRef]
- Hall, A.S.; Jacobs, J.L.; Smith, E.N. Possible osteosarcoma reported from a new world elapid snake and review of reptilian bony tumors. Anat. Rec. 2020, 303, 2955–2966. [Google Scholar] [CrossRef]
- Muñoz-Gutiérrez, J.F.; Garner, M.M.; Kiupel, M. Cutaneous Chromatophoromas in Captive Snakes. Vet. Pathol. 2016, 53, 1213–1219. [Google Scholar] [CrossRef]
- Page-Karjian, A.; Hahne, M.; Leach, K.; Murphy, H.; Lock, B.; Rivera, S. Neoplasia in snakes at zoo atlanta during 1992–2012. J. Zoo Wildl. Med. 2017, 48, 521–524. [Google Scholar] [CrossRef]
- Pereira, M.E.; Viner, T.C. Oviduct adenocarcinoma in some species of captive snakes. Vet. Pathol. 2008, 45, 693–697. [Google Scholar] [CrossRef] [Green Version]
- Taggart, P.L.; Woolford, L.; Dunstan, N.; Allen, L.; Buote, M.; Lindsay, S.A. Cutaneous Chromatophoromas in Four Species of Australian Elapid Snake. J. Comp. Pathol. 2021, 183, 33–38. [Google Scholar] [CrossRef]
- Elkan, E.; Cooper, J.E. Tumours and pseudotumours in some reptiles. J. Comp. Pathol. 1976, 86, 337–348. [Google Scholar] [CrossRef]
- Anderson, E.T.; Kennedy-Stoskopf, S.; Sandy, J.R.; Dorn, B.; Boyette, T.; Harms, C.A. Squamous cell carcinoma with vascular invasion in a diamondback rattlesnake (Crotalus adamanteus). J. Zoo Wildl. Med. 2010, 41, 745–748. [Google Scholar] [CrossRef] [PubMed]
- Frye, F.L. Diagnosis and surgical treatment of reptilian neoplasms with a compilation of cases 1966–1993. Vivo 1994, 8, 885–892. [Google Scholar]
- Harris, P.; Taylor, R.; Minor, B.; Elliott, V.; Fernandez, M.; O’Neal, L.; McLeod, L.; Delacqua, G.; Delacqua, F.; Kirby, J.; et al. The REDCap consortium: Building an international community of software partners. J. Biomed. Inform. 2019, 95, 103208. [Google Scholar] [CrossRef] [PubMed]
- Harris, P.; Taylor, R.; Thielke, R.; Payne, J.; Gonzalez, N.; Conde, J. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inform. 2009, 42, 377–381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- IBM Corp. IBM SPSS Statistics for Windows, 26.0; IBM Corp: Armonk, NY, USA, 2019. [Google Scholar]
- Team, R.C. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019. [Google Scholar]
- Kassambara, A.; Kosinski, M.; Biecek, P. Survminer: Drawing Survival Curves Using ‘ggplot2’. R package version 0.4.9. 2021. Available online: https://CRAN.R-project.org/package=survminer (accessed on 18 January 2022).
- Therneau, T. A Package for Survival Analysis in S. version 2.38. 2015. Available online: https://CRAN.R-project.org/package=survival (accessed on 18 January 2022).
- Hothorn, T.; Buehlmann, P.; Kneib, T.; Schmid, M.; Hofner, B. M Boost: Model-Based Boosting. R package version 2.6.0. 2018. Available online: https://CRAN.R-project.org/package=mboost (accessed on 18 January 2022).
- Mayr, A.; Hofner, B.; Waldmann, E.; Hepp, T.; Meyer, S.; Gefeller, O. An Update on Statistical Boosting in Biomedicine. Comput. Math. Methods Med. 2017, 2017, 6083072. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baron, H.R.; Allavena, R.; Melville, L.M.; Doneley, R.J.T. Gastric adenocarcinoma in a diamond python (Morelia spilota spilota). Aust. Vet. J. 2014, 92, 405–409. [Google Scholar] [CrossRef]
- Steeil, J.C.; Schumacher, J.; Hecht, S.; Baine, K.; Ramsay, E.C.; Ferguson, S.; Miller, D.; Lee, N.D. Diagnosis and treatment of a pharyngeal squamous cell carcinoma in a Madagascar ground boa (Boa madagascariensis). J. Zoo Wildl. Med. 2013, 44, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Diethelm, G.; Stauber, E.; Tillson, M.; Ridgley, S. Tracheal resection and anastomosis for an intratracheal chondroma in a ball python. J. Am. Vet. Med. Assoc. 1996, 209, 786–788. [Google Scholar]
- Hopewell, E.; Harrison, S.H.; Posey, R.; Duke, E.; Troan, B.; Harrison, T.M. Analysis of Published Amphibian Neoplasia Case Reports. J. Herpetol. Med. Surg. 2020, 30, 148–155. [Google Scholar] [CrossRef]
- Bryant, B.R.; Vogelnest, L.; Hulst, F. The use of cryosurgery in a diamond python, Morelia spilota spilota, with fibrosarcoma and radiotherapy in a common death adder, Acanthophis antarcticus, with melanoma. Bull. Assoc. Reptil. Amphib. Vet. 1997, 7, 9–12. [Google Scholar] [CrossRef]

| Family | Species and Prevalence | Common Name | Benign | Malignant | Unspecified |
|---|---|---|---|---|---|
| Boidae | Acrantophis dumerili 2.4% (1/41) | Dumeril’s Ground Boa | (1) Soft Tissue Sarcoma | ||
| Boa constrictor 14.3% (2/14) | Boa Constrictor | (1) Neuroendocrine Tumor (1) Soft Tissue Sarcoma | |||
| Chilabothrus granti 7.7% (1/13) | Virgin Islands Boa | (1) Thyroid Carcinoma (1) Hepatocellular Carcinoma | |||
| Eunectes murinus 4.3% (1/23) | Green Anaconda | (1) Sertoli Cell Tumor | |||
| Lichanura trivirgata 15.8% (3/19) | Rosy Boa | (1) Pancreatic Adenoma | (1) Undifferentiated Carcinoma (1) Histiocytic Sarcoma | ||
| Sanzinia madagascariensis 20.0% (1/5) | Madagascar Tree Boa | (1) Soft Tissue Sarcoma | |||
| Colubridae | Coelognathus radiatus 4% (4/101) | Radiated Ratsnake | (2) Hepatocellular Carcinoma (1) Lymphoma/Leukemia (1) Renal Adenocarcinoma | ||
| Elaphe taeniura 11.1% (1/9) | Taiwan Beauty Snake | (1) Soft Tissue Sarcoma | |||
| Heterodon nasicus 10.3% (3/29) | Western Hog-Nosed Snake | (2) Hepatocellular Carcinoma (1) Soft Tissue Sarcoma | |||
| Heterodon platirhinos 25.0% (2/8) | Eastern Hog-Nosed Snake | (1) Soft Tissue Sarcoma (1) Malignant Chromatophoroma (unspecified) | |||
| Lampropeltis getula 17.9% (5/28) | Kingsnake | (1) Renal Adenoma | (3) Soft Tissue Sarcoma (1) Renal Carcinoma (1) Hepatic Adenocarcinoma | (1) Chromatophoroma (iridophoroma) | |
| Lampropeltis triangulum 1.8% (1/56) | Eastern Milksnake | (1) Gastrointestinal Adencocarcinoma | |||
| Leioheterodon madagascariensis 1.3% (1/78) | Madagascar Giant Hognose Snake | (1) Osteosarcoma | |||
| Nerodia sipedon 30.8% (4/13) | Common Or Northern Watersnake | (3) Soft Tissue Sarcoma (1) Malignant Chromatophoroma (iridophoroma) (1) Malignant Chromatophoroma (Uncharacterized) | |||
| Oreocryptophis porphyraceus 5.3% (1/19) | Black-Banded Trinket Snake | (1) Hepatocellular Carcinoma | |||
| Pantherophis guttatus 9.6% (11/115) | Corn snake | (1) Granulosa Cell Tumor (1) Lymphoma/Leukemia (7) Soft Tissue Sarcoma (1) Osteosarcoma (2) Hemangiosarcoma | |||
| Pantherophis obsoletus (Or) alleghaniensis 7.4% (14/189) | Ratsnake | (5) Soft Tissue Sarcoma (1) Gastrointestinal Adencocarcinoma (1) Renal Carcinoma (1) Chondrosarcoma (4) Lymphoma/leukemia (1) Osteosarcoma | (1) Granulosa Cell Tumor | ||
| Pituophis catenifer 5.4% (2/37) | Gophersnake (or) Bullsnake | (2) Hepatobiliary Carcinoma | |||
| Pituophis lineaticollis 25.0% (1/4) | Middle American Gophersnake | (1) Undifferentiated Carcinoma | |||
| Pituophis ruthveni 2.4% (1/42) | Louisiana Pinesnake | (1) Soft Tissue Sarcoma | |||
| Rhamphiophis oxyrhynchus 14.3% (1/7) | Rufous-Beaked Snake | (1) Neuroendocrine Tumor | |||
| Rhinocheilus lecontei 16.7% (1/6) | Long-Nosed Snake | (1) Gastrointestinal Adencocarcinoma | |||
| Rhynchophis boulengeri 5.3% (1/19) | Rhinoceros Snake | (1) Lymphoma/Leukemia | |||
| Salvadora bairdi 4.8% (1/21) | Baird’s Patchnose Snake | (1) Renal Adenocarcinoma | |||
| Thamnophis cyrtopsis 100.0% (1/1) | Black-Necked Gartersnake | (1) Malignant Chromatophoroma (Melanoma) | |||
| Thamnophis exsul 10.5% (2/19) | Exiled Gartersnake | (2) Biliary Cystadenoma | |||
| Thamnophis radix (4) 0.8% (4/500) | Plains Gartersnake | (1) Malignant Chromatophoroma (Melanoma) (1) Granulosa Cell Tumor (1) Undifferentiated Carcinoma (1) Hepatocellular Carcinoma | |||
| Thamnophis sirtalis 100.0% (1/1) | Common Gartersnake | (1) Biliary Cystadenoma | (1) Squamous Cell Carcinoma | ||
| Trimorphodon lambda 25.0% (1/4) | Sonoran Lyresnake | (1) Renal Carcinoma | |||
| Elapidae | Hemachatus haemachatus 14.3% (2/14) | Ringhals Cobra | (1) Lymphoma/Leukemia (1) Gastrointestinal Adencocarcinoma | ||
| Hydrodynastes gigas 1.4% (1/72) | False Water Cobra | (1) Renal Adenocarcinoma (1) Pancreatic Adenocarcinoma | |||
| Naja pallida 3.8% (1/26) | Red Spitting Cobra | (1) Renal Adenocarcinoma | |||
| Ophiophagus hannah 15.4% (2/13) | King Cobra | (1) Granulosa Cell Tumor | (1) Lymphoma/Leukemia (1) Undifferentiated Adenocarcinoma | ||
| Pythonidae | Antaresia childreni 2.3% (1/44) | Children’s Python | (1) Lymphoma/Leukemia (1) Soft Tissue Sarcoma | ||
| Liasis mackloti 50.0% (1/2) | Macklot’s Python | (1) Lymphoma/Leukemia | |||
| Malayopython reticulatus 25.0% (1/4) | Reticulated Python | (1) Hepatocellular Carcinoma | |||
| Morelia spilota 12.5% (1/8) | Carpet Python | (1)Esophageal Carcinoma | |||
| Morelia viridis 1.7% (1/59) | Green Tree Python | (1) Squamous Cell Carcinoma | |||
| Python bivittatus 12.5% (1/8) | Burmese Python | (1) Soft Tissue Sarcoma | |||
| Python regius 10.5% (2/19) | Ball Python | (1) Squamous Cell Carcinoma (1) Gastrointestinal Adenocarcinoma | (1) Chromatophoroma (melanophoroma) | ||
| Viperidae | Agkistrodon contortrix 37.5% (3/8) | Copperhead | (2) Hepatocellular Adenoma | (1) Lymphoma/Leukemia | |
| Agkistrodon piscivorus 18.2% (2/11) | Cottonmouth | (1) Soft Tissue Sarcoma (1) Lymphoma/Leukemia | |||
| Atheris squamigera 7.4% (2/27) | African Bush Viper | (1) Hepatic Cystadenocarcinoma (1) Oviduct Adenocarcinoma | |||
| Bitis arietans 50.0% (2/4) | Puff Adder | (1) Renal Carcinoma (1) Soft Tissue Sarcoma | |||
| Bitis nasicornis 5.9% (3/51) | Rhinoceros Viper | (1) Renal Adenocarcinoma (1) Cholangiocellular carcinoma (1) Renal Carcinoma (1) Lymphoma/Leukemia | |||
| Bothriechis rowleyi 4.2% (2/48) | Rowley’s Palm Pit Viper | (1) Soft Tissue Sarcoma (1) Squamous Cell Carcinoma | |||
| Bothriechis schlegelii 4.4% (2/45) | Eyelash Viper | (2) Soft Tissue Sarcoma | |||
| Bothrops asper 2.4% (1/41) | Terciopelo | (1) Renal Cystadenoma | (1) Soft Tissue Sarcoma | ||
| Crotalus adamanteus 26.3% (5/19) | Eastern Diamond-Backed Rattlesnake | (2) Soft Tissue Sarcoma (1) Lymphoma/Leukemia (1) Squamous Cell Carcinoma (1) Hemangiosarcoma | (1) Sertoli Cell Tumor | ||
| Crotalus atrox 9.5% (2/21) | Western Diamond-Backed Rattlesnake | (1) Renal Adenocarcinoma (1) Cholangiocellular Carcinoma | (1) Sertoli Cell Tumor | ||
| Crotalus cerastes 20.0% (1/5) | Sidewinder | (1) Hepatic Adenoma | (1) Chromatophoroma (Melanophoroma) | ||
| Crotalus culminatus 3.6% (2/55) | Northwestern Neotropical Rattlesnake | (1) Soft Tissue Sarcoma (1) Biliary Adenocarcinoma | |||
| Crotalus horridus 22.7% (5/22) | Timber Rattlesnake | (1) Lipoma | (3) Soft Tissue Sarcoma (1) Ovarian Carcinoma | ||
| Crotalus lepidus 14.3% (1/7) | Rock Rattlesnake | (1) Hepatic Adenoma | (1) Malignant Chromatophoroma (Melanophoroma) | ||
| Crotalus molossus 20.0% (1/5) | Black-Tailed Rattlesnake | (1) Soft Tissue Sarcoma (1) Hepatocellular Carcinoma | |||
| Crotalus viridis 20.0% (1/5) | Prairie Rattlesnake | (1) Hepatocellular Carcinoma (1) Soft Tissue Sarcoma | |||
| Lachesis muta 15.4% (2/13) | South American Bushmaster | (2) Hepatocellular Carcinoma | |||
| Montivipera raddei 7.7% (1/13) | Armenian Viper | (1) Pancreatic Carcinoma | |||
| Protobothrops flavoviridis 16.7% (1/6) | Habu | (1) Soft Tissue Sarcoma | |||
| Sistrurus catenatus 25.0% (1/4) | Eastern Massasauga | (1) Osteosarcoma | |||
| Sistrurus miliarius 14.3% (2/14) | Pygmy Rattlesnake | (1) Pancreatic Adenoma | (1) Biliary Adenocarcinoma | ||
| Trimeresurus flavomaculatus 10.0% (1/10) | Philippine Pit Viper | (1) Lymphoma/Leukemia | |||
| Trimeresurus mcgregori 2.3% (1/44) | McGregor’s Tree Viper | (1) Soft Tissue Sarcoma | |||
| Trimeresurus sumatranus 1.4% (1/74) | Sumatran Pit Viper | (1) Lymphoma/Leukemia | |||
| Vipera transcaucasiana 5.3% (1/19) | Transcaucasian Long-Nosed Viper | (1) Soft Tissue Sarcoma |
| Institution | Total Snakes with Neoplasia | Total Number of Snakes of Affected Species | Number of Different Affected Species | Prevalence |
|---|---|---|---|---|
| 1 | 32 | 162 | 20 | 19.8% |
| 2 | 10 | 72 | 4 | 13.9% |
| 3 | 16 | 77 | 9 | 20.8% |
| 4 | 19 | 250 | 14 | 7.6% |
| 5 | 31 | 651 | 26 | 4.8% |
| 6 | 25 | 1049 | 16 | 2.4% |
| Family | Common Name/Scientific Name | Tumor (s) | Survival in Months | Treatment ± Supportive Care |
|---|---|---|---|---|
| Boidae | Boa constrictor Boa constrictor | Benign carcinoid tumor | 0.25 | none |
| Boa constrictor Boa constrictor | Soft tissue sarcoma | 1 | surgical excision only | |
| Rosy boa Lichanura trivirgata | Histiocytic sarcoma | 7 | surgical excision only | |
| Madagascar tree boa Sanzinia madagascariensis | Soft tissue sarcoma | 22 | surgical excision only | |
| Colubridae | Western hog-nosed snake Heterodon nasicus | Hepatocellular carcinoma | 10 | none |
| Western hog-nosed snake Heterodon nasicus | Soft tissue sarcoma | 6 | surgical excision * | |
| Eastern hog-nosed snake Heterodon platirhinos | Soft tissue sarcoma | 15.5 | none | |
| Kingsnake Lampropeltis getula | Soft tissue sarcoma, Hepatocellular adenocarcinoma | 10.5 | none | |
| Kingsnake Lampropeltis getula | Soft tissue sarcoma, Chromatophoroma (uncharacterized) | 8 | surgical excision only | |
| Common or northern watersnake Nerodia sipedon | Soft tissue sarcoma, Malignant chromatophoroma | 5 | surgical excision * | |
| Common or northern watersnake Nerodia sipedon | Malignant chromatophoroma | 1 | surgical excision only | |
| Common or northern watersnake Nerodia sipedon | Soft tissue sarcoma | 0.5 | surgical excision only | |
| Corn snake Pantherophis guttatus | Soft tissue sarcoma | 2 | none | |
| Corn snake Pantherophis guttatus | Soft tissue sarcoma | 12 | surgical excision and radiation and chemotherapy (Piroxicam) * | |
| Corn snake Pantherophis guttatus | Hemangiosarcoma | 2 | surgical excision only | |
| Corn snake Pantherophis guttatus | Soft tissue sarcoma | 108 | surgical excision only * | |
| Corn snake Pantherophis guttatus | Soft tissue sarcoma | 18 | surgical excision and radiation | |
| Corn snake Pantherophis guttatus | Soft tissue sarcoma | 5.5 | surgical excision and chemotherapy (Cyclophosphamide and Piroxicam) | |
| Ratsnake Pantherophis obsoletus or alleghaniensis | Colonic adenocarcinoma | 7 | surgical excision only * | |
| Ratsnake Pantherophis obsoletus or alleghaniensis | Chondrosarcoma | 15 | none | |
| Ratsnake Pantherophis obsoletus or alleghaniensis | Leukemia | 1 | none | |
| Ratsnake Pantherophis obsoletus or alleghaniensis | Osteosarcoma | 6 | supportive care only * | |
| Ratsnake Pantherophis obsoletus or alleghaniensis | Soft tissue sarcoma | 4 | surgical excision only | |
| Gophersnake(or) Bullsnake Pituophis catenifer | Hepatocellular carcinoma | 1 | none | |
| Black-necked gartersnake Thamnophis cyrtopsis | Malignant chromatophoroma | 5.5 | surgical excision only | |
| Plains gartersnake Thamnophis radix | Malignant chromatophoroma | 13 | surgical excision and radiation | |
| Plains gartersnake Thamnophis radix | Malignant granulosa cell tumor | 1 | surgical excision only | |
| Common gartersnake Thamnophis sirtali | Squamous cell carcinoma | 9 | not known | |
| Elapidae | Ringhals cobra Hemachatus haemachatus | Lymphoma | 0.75 | none |
| False water cobra Hydrodynastes gigas | Renal and Pancreatic adenocarcinoma | 71 | surgical excision only | |
| Red spitting cobra Naja pallida | Renal carcinoma/adenocarcinoma | 35 | surgical excision only | |
| King cobra Ophiophagus hannah | Lymphoma | 11 | chemotherapy only (Lomustine) | |
| Pythonidae | Children’s python Antaresia childreni | Lymphoma, Soft tissue sarcoma | 3 | surgical excision only |
| Carpet python Morelia spilota | Esophageal carcinoma | 2.5 | surgical excision only | |
| Green tree python Morelia viridis | Squamous cell carcinoma | 7 | not known | |
| Burmese python Python bivittatus | Soft tissue sarcoma | 1 | surgical excision only | |
| Ball python Python regius | Squamous cell carcinoma | 84 | surgical excision and chemotherapy (Carboplatin) * | |
| Viperidae | Cottonmouth Agkistrodon piscivorus | Soft tissue sarcoma | 0.25 | surgical excision only |
| Eyelash viper Bothriechis schlegelii | Soft tissue sarcoma | 16 | surgical excision only | |
| Eyelash viper Bothriechis schlegelii | Soft tissue sarcoma | 5 | none | |
| Eastern diamond-backed rattlesnake Crotalus adamanteus | Lymphoma | 20 | surgical excision and chemotherapy (Cyclophosphamide) | |
| Eastern diamond-backed rattlesnake Crotalus adamanteus | Squamous cell carcinoma | 18.5 | surgical excision only | |
| Eastern diamond-backed rattlesnake Crotalus adamanteus | Hemangiosarcoma, Sertoli cell tumor (uncharacterized) | 7 | surgical excision only | |
| Sidewinder Crotalus cerastes | Chromatophoroma (uncharacterized), Hepatobiliary adenoma | 1 | none | |
| Timber rattlesnake Crotalus horridus | Ovarian carcinoma | 0.25 | none | |
| Timber rattlesnake Crotalus horridus | Soft tissue sarcoma | 0.25 | none | |
| Timber rattlesnake Crotalus horridus | Soft tissue sarcoma | 1 | surgical excision only | |
| Timber rattlesnake Crotalus horridus | Lipoma | 24 | surgical excision only | |
| Rock rattlesnake Crotalus lepidus | Malignant chromatophoroma, Hepatobiliary adenoma | 1 | none | |
| Prairie rattlesnake Crotalus viridis | Hepatocellular carcinoma, Soft tissue sarcoma | 1 | none | |
| South american bushmaster Lachesis muta | Hepatocellular carcinoma/adenocarcinoma | 3 | none | |
| Eastern massasauga Sistrurus catenatus | Osteosarcoma | 3.5 | surgical excision * | |
| Sumatran pit viper Trimeresurus sumatranus | Lymphoma | 0.36 | none |
| Predictor Variable | Predictor Variable Value | Sample Size a | Outcome Effect b |
|---|---|---|---|
| Type of Neoplasm | Malignant | 112 | 0.71 |
| Type of Neoplasm | Benign | 9 | −0.71 |
| Metastasis (Yes/No) | Yes | 56 | 0.060 |
| Metastasis (Yes/No) | Unknown Metastasis | 2 | 0.060 |
| Metastasis (Yes/No) | No | 63 | −0.055 |
| Tumor Type | Chromatophoroma | 4 | −0.20 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duke, E.G.; Harrison, S.H.; Moresco, A.; Trout, T.; Troan, B.V.; Garner, M.M.; Smith, M.; Smith, S.; Harrison, T.M. A Multi-Institutional Collaboration to Understand Neoplasia, Treatment and Survival of Snakes. Animals 2022, 12, 258. https://doi.org/10.3390/ani12030258
Duke EG, Harrison SH, Moresco A, Trout T, Troan BV, Garner MM, Smith M, Smith S, Harrison TM. A Multi-Institutional Collaboration to Understand Neoplasia, Treatment and Survival of Snakes. Animals. 2022; 12(3):258. https://doi.org/10.3390/ani12030258
Chicago/Turabian StyleDuke, Elizabeth G., Scott H. Harrison, Anneke Moresco, Tim Trout, Brigid V. Troan, Michael M. Garner, Madison Smith, Sidney Smith, and Tara M. Harrison. 2022. "A Multi-Institutional Collaboration to Understand Neoplasia, Treatment and Survival of Snakes" Animals 12, no. 3: 258. https://doi.org/10.3390/ani12030258
APA StyleDuke, E. G., Harrison, S. H., Moresco, A., Trout, T., Troan, B. V., Garner, M. M., Smith, M., Smith, S., & Harrison, T. M. (2022). A Multi-Institutional Collaboration to Understand Neoplasia, Treatment and Survival of Snakes. Animals, 12(3), 258. https://doi.org/10.3390/ani12030258







