The Impacts of Drought on the Health and Demography of Eastern Grey Kangaroos
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Camera Traps
2.3. Body Condition Score and Demography
2.4. Activity Rate
2.5. Parasitology
2.6. Statistical Analysis
3. Results
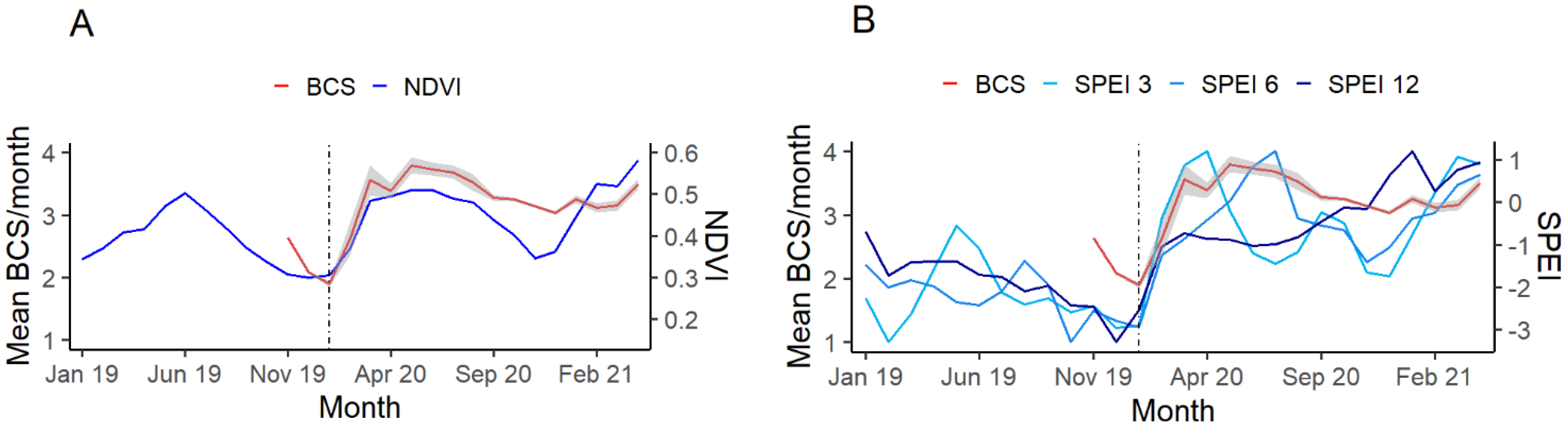
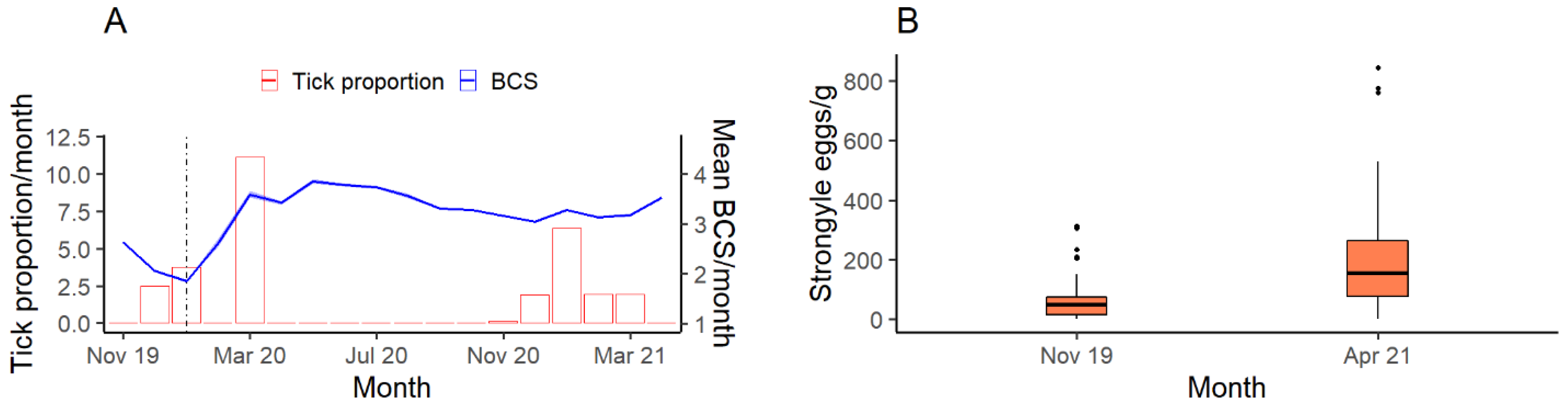
3.1. BCS and Demography
3.2. Activity Rate
3.3. Parasitology
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| BCS | Description | Example Camera Trap Photos |
|---|---|---|
| 5 (Muscular) | Thick tail with a large base. Moderate fat cover on body. Muscle definition highly visible across most of the body, especially biceps and forearms, chest, and legs. |  |
| 4 (Optimal) | Thick base of tail. Hips covered by fatty tissue. Ribs not visible. Hips well covered with no visibly sunken area or bones protruding. Muscle mass well developed with generally minimal fat cover on the body. |  |
| 3 (Thin) | Tail appears thin but no caudal vertebrae are visible. Some ribs may be visible, but the rib cage area is mostly covered. Hips are generally well covered, and muscle mass mostly visible. There is no visible fat cover. |  |
| 2 (Very thin) | Most caudal vertebrae are visible, giving the tail a bony appearance. Tail base is skinny. Hip region is mostly without fat or muscle tissue cover, creating sunken appearance above the femur (as seen in the visual example). Most ribs are visible (and are easily distinguishable on individuals with shorter fur as pictured here). |  |
| 1 (Emaciated) | Caudal vertebrae are visible, giving the tail a bony appearance. Base of tail appears skinny. Hip region is not covered by fatty tissue and is sunken in appearance. All ribs are visible (it may be difficult to distinguish if the individual has longer fur as pictured here). |  |
References
- Hoffmann, A.A.; Sgro, C.M. Climate change and evolutionary adaptation. Nature 2011, 470, 479–485. [Google Scholar] [CrossRef] [PubMed]
- Vicente-Serrano, S.M.; Quiring, S.M.; Peña-Gallardo, M.; Yuan, S.; Domínguez-Castro, F. A review of environmental droughts: Increased risk under global warming? Earth-Sci. Rev. 2020, 201, 102953. [Google Scholar] [CrossRef]
- Kirono, D.G.C.; Round, V.; Heady, C.; Chiew, F.H.S.; Osbrough, S. Drought projections for Australia: Updated results and analysis of model simulations. Weather. Clim. Extrem. 2020, 30, 100280. [Google Scholar] [CrossRef]
- Cai, W.; Santoso, A.; Collins, M.; Dewitte, B.; Karamperidou, C.; Kug, J.-S.; Lengaigne, M.; McPhaden, M.J.; Stuecker, M.F.; Taschetto, A.S.; et al. Changing El Niño–Southern Oscillation in a warming climate. Nat. Rev. Earth Environ. 2021, 2, 628–644. [Google Scholar] [CrossRef]
- Ripple, W.J.; Wolf, C.; Newsome, T.M.; Betts, M.G.; Ceballos, G.; Courchamp, F.; Hayward, M.W.; Van Valkenburgh, B.; Wallach, A.D.; Worm, B. Are we eating the world’s megafauna to extinction? Conserv. Lett. 2019, 12, e12627. [Google Scholar] [CrossRef]
- Milligan, S.R.; Holt, W.V.; Lloyd, R. Impacts of climate change and environmental factors on reproduction and development in wildlife. Philos. Trans. R. Soc. Lond B Biol. Sci. 2009, 364, 3313–3319. [Google Scholar] [CrossRef]
- Ashcroft, M.B.; Gollan, J.R.; Warton, D.I.; Ramp, D. A novel approach to quantify and locate potential microrefugia using topoclimate, climate stability, and isolation from the matrix. Glob. Chang. Biol. 2012, 18, 1866–1879. [Google Scholar] [CrossRef]
- Gollan, J.R.; Ramp, D.; Ashcroft, M.B. Assessing the distribution and protection status of two types of cool environment to facilitate their conservation under climate change. Conserv. Biol. 2014, 28, 456–466. [Google Scholar] [CrossRef]
- Seabrook, L.; McAlpine, C.; Baxter, G.; Rhodes, J.; Bradley, A.; Lunney, D. Drought-driven change in wildlife distribution and numbers: A case study of koalas in south west Queensland. Wildl. Res. 2011, 38, 509–524. [Google Scholar] [CrossRef]
- Acevedo-Whitehouse, K.; Duffus, A.L. Effects of environmental change on wildlife health. Philos. Trans. R. Soc. Lond B Biol. Sci. 2009, 364, 3429–3438. [Google Scholar] [CrossRef]
- Foley, C.; Pettorelli, N.; Foley, L. Severe drought and calf survival in elephants. Biol. Lett. 2008, 4, 541–544. [Google Scholar] [CrossRef] [PubMed]
- Ratnayake, H.U.; Kearney, M.R.; Govekar, P.; Karoly, D.; Welbergen, J.A. Forecasting wildlife die-offs from extreme heat events. Anim. Conserv. 2019, 22, 386–395. [Google Scholar] [CrossRef]
- Welbergen, J.A.; Klose, S.M.; Markus, N.; Eby, P. Climate change and the effects of temperature extremes on Australian flying-foxes. Proc. Biol. Sci. 2008, 275, 419–425. [Google Scholar] [CrossRef]
- Marealle, W.N.; Fossøy, F.; Holmern, T.; Stokke, B.G.; Røskaft, E. Does illegal hunting skew Serengeti wildlife sex ratios? Wildl. Biol. 2010, 16, 419–429. [Google Scholar] [CrossRef]
- Gardner, J.L.; Rowley, E.; de Rebeira, P.; de Rebeira, A.; Brouwer, L. Associations between changing climate and body condition over decades in two southern hemisphere passerine birds. Clim. Change Responses 2018, 5, 1–14. [Google Scholar] [CrossRef]
- Pérez-Flores, J.; Calmé, S.; Reyna-Hurtado, R. Scoring body condition in wild baird’s tapir (Tapirus bairdii) using camera traps and opportunistic photographic material. Trop. Conserv. Sci. 2016, 9, 1940082916676128. [Google Scholar] [CrossRef]
- Bassano, B.; Perrone, A.; von Hardenberg, A. Body weight and horn development im Alpine chamois, Rupicapra rupicapra (Bovidae, Caprinae). Mammalia 2003, 67, 65–73. [Google Scholar] [CrossRef]
- Sánchez, C.A.; Becker, D.J.; Teitelbaum, C.S.; Barriga, P.; Brown, L.M.; Majewska, A.A.; Hall, R.J.; Altizer, S. On the relationship between body condition and parasite infection in wildlife: A review and meta—Analysis. Ecol. Lett. 2018, 21, 1869–1884. [Google Scholar] [CrossRef]
- Klasing, K. Nutrition and the immune system. Br. Poult. Sci. 2007, 48, 525–537. [Google Scholar] [CrossRef]
- Becker, D.J.; Streicker, D.G.; Altizer, S. Linking anthropogenic resources to wildlife–pathogen dynamics: A review and meta—Analysis. Ecol. Lett. 2015, 18, 483–495. [Google Scholar] [CrossRef]
- Murray, M.H.; Becker, D.J.; Hall, R.J.; Hernandez, S.M. Wildlife health and supplemental feeding: A review and management recommendations. Biol. Conserv. 2016, 204, 163–174. [Google Scholar] [CrossRef]
- Kiem, A.S.; Johnson, F.; Westra, S.; van Dijk, A.; Evans, J.P.; O’Donnell, A.; Rouillard, A.; Barr, C.; Tyler, J.; Thyer, M.; et al. Natural hazards in Australia: Droughts. Clim. Change 2016, 139, 37–54. [Google Scholar] [CrossRef]
- Epaphras, A.M.; Gereta, E.; Lejora, I.A.; Ole Meing’ataki, G.E.; Ng’umbi, G.; Kiwango, Y.; Mwangomo, E.; Semanini, F.; Vitalis, L.; Balozi, J.; et al. Wildlife water utilization and importance of artificial waterholes during dry season at Ruaha National Park, Tanzania. Wetl. Ecol. Manag. 2007, 16, 183–188. [Google Scholar] [CrossRef]
- Letnic, M.; Crowther, M.S. Patterns in the abundance of kangaroo populations in arid Australia are consistent with the exploitation ecosystems hypothesis. Oikos 2013, 122, 761–769. [Google Scholar] [CrossRef]
- Lundgren, E.J.; Ramp, D.; Stromberg, J.C.; Wu, J.; Nieto, N.C.; Sluk, M.; Moeller, K.T.; Wallach, A.D. Equids engineer desert water availability. Science 2021, 372, 491–495. [Google Scholar] [CrossRef] [PubMed]
- Montague-Drake, R.; Croft, D.B. Do kangaroos exhibit water-focused grazing patterns in arid New South Wales? A case study in Sturt National Park. Aust. Mammal. 2004, 26, 87–100. [Google Scholar] [CrossRef]
- Lavery, T.H.; Pople, A.R.; McCallum, H.I. Going the distance on kangaroos and water: A review and test of artificial water point closures in Australia. J. Arid Environ. 2018, 151, 31–40. [Google Scholar] [CrossRef]
- Wallach, A.D.; Johnson, C.N.; Ritchie, E.G.; O’Neill, A.J. Predator control promotes invasive dominated ecological states. Ecol. Lett. 2010, 13, 1008–1018. [Google Scholar] [CrossRef] [PubMed]
- Short, J. The functional response of kangaroos, sheep and rabbits in an arid grazing system. J. Appl. Ecol. 1985, 22, 435–447. [Google Scholar] [CrossRef]
- Croft, D.B.; Witte, I. The perils of being populous: Control and conservation of abundant kangaroo species. Animals 2021, 11, 1753. [Google Scholar] [CrossRef] [PubMed]
- Caughley, G.; Bayliss, P.; Giles, J. Trends in kangaroo numbers in western New South Wales and their relation to rainfall. Aust. Wildl. Res. 1984, 11, 415–422. [Google Scholar] [CrossRef]
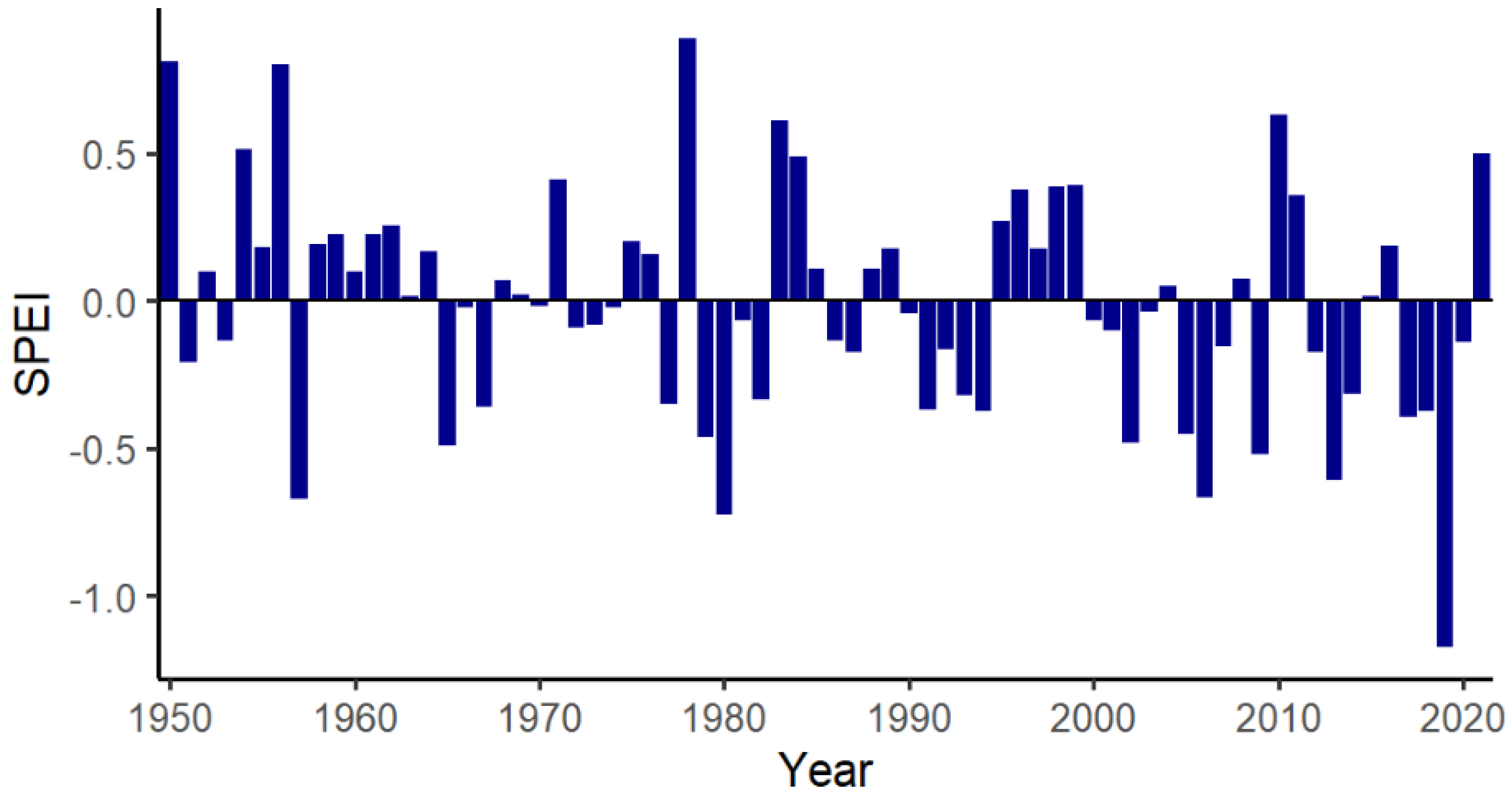
- Vicente-Serrano, S.M.; Beguería, S.; López-Moreno, J.I. A multiscalar drought index sensitive to global warming: The standardized precipitation evapotranspiration index. J. Clim. 2010, 23, 1696–1718. [Google Scholar] [CrossRef]
- Pettorelli, N.; Vik, J.O.; Mysterud, A.; Gaillard, J.-M.; Tucker, C.J.; Stenseth, N.C. Using the satellite-derived NDVI to assess ecological responses to environmental change. Trends Ecol. Evol. 2005, 20, 503–510. [Google Scholar] [CrossRef]
- Pettorelli, N.; Ryan, S.; Mueller, T.; Bunnefeld, N.; Jędrzejewska, B.; Lima, M.; Kausrud, K. The Normalized Difference Vegetation Index (NDVI): Unforeseen successes in animal ecology. Clim. Res. 2011, 46, 15–27. [Google Scholar] [CrossRef]
- Hasselerharm, C.D.; Yanco, E.; McManus, J.S.; Smuts, B.H.; Ramp, D. Wildlife-friendly farming recouples grazing regimes to stimulate recovery in semi-arid rangelands. Sci. Total Environ. 2021, 788, 147602. [Google Scholar] [CrossRef]
- Zemanova, M.A.; Ramp, D. Genetic Structure and Gene Flow in Eastern Grey Kangaroos in an Isolated Conservation Reserve. Diversity 2021, 13, 570. [Google Scholar] [CrossRef]
- Paulo, A.; Rosa, R.; Pereira, L. Climate trends and behaviour of drought indices based on precipitation and evapotranspiration in Portugal. Nat. Hazards Earth Syst. Sci. 2012, 12, 1481–1491. [Google Scholar] [CrossRef]
- Johnston, S.; Blyde, D.; Gamble, J.; Higgins, D.; Field, H.; Cooper, J. Collection and short-term preservation of semen from free—Ranging eastern grey kangaroos (Macropus giganteus: Macropodidae). Aust. Vet. J. 1997, 75, 648–651. [Google Scholar] [CrossRef]
- Austin, C.M.; Ramp, D. Behavioural plasticity by eastern grey kangaroos in response to human behaviour. Animals 2019, 9, 244. [Google Scholar] [CrossRef] [PubMed]
- Jarman, P.; Jones, M.; Johnson, C.; Southwell, C.; Stuartdick, R.; Higginbottom, K.; Clarke, J. Macropod studies at Wallaby Creek. 8. Individual recognition of kangaroos and wallabies. Wildl. Res. 1989, 16, 179–185. [Google Scholar] [CrossRef]
- Power, M.L.; Sangster, N.C.; Slade, M.B.; Veal, D.A. Patterns of Cryptosporidium oocyst shedding by eastern grey kangaroos inhabiting an Australian watershed. Appl. Environ. Microbiol. 2005, 71, 6159–6164. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, M.; Vidyashankar, A.; Andersen, U.; DeLisi, K.; Pilegaard, K.; Kaplan, R. Effects of fecal collection and storage factors on strongylid egg counts in horses. Vet. Parasitol. 2010, 167, 55–61. [Google Scholar] [CrossRef]
- Gordon, H.M.; Whitlock, H.V. A new technique for counting nematode eggs in sheep faeces. J. Scientif. Indust. Res. 1939, 12, 50–52. [Google Scholar]
- Begueria, S.; Latorre, B.; Reig, F.; Vicente-Serrano, S.M. SPEI Global Drought Monitor. Available online: https://spei.csic.es/map/maps.html#months=1#month=7#year=2021 (accessed on 3 July 2021).
- Didan, K. MOD13A3 MODIS/Terra vegetation Indices Monthly L3 Global 1km SIN Grid V006. NASA EOSDIS Land Processes DAAC 2015, 10. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 2015, 67. [Google Scholar] [CrossRef]
- Pettorelli, N.; Gaillard, J.M.; Mysterud, A.; Duncan, P.; Stenseth, N.C.; Delorme, D.; Van Laere, G.; Toïgo, C.; Klein, F. Using a proxy of plant productivity (NDVI) to find key periods for animal performance: The case of roe deer. Oikos 2006, 112, 565–572. [Google Scholar] [CrossRef]
- Ryan, S.J.; Cross, P.C.; Winnie, J.; Hay, C.; Bowers, J.; Getz, W.M. The utility of normalized difference vegetation index for predicting African buffalo forage quality. J. Wildl. Manag. 2012, 76, 1499–1508. [Google Scholar] [CrossRef]
- Knight, M. Drought—Related mortality of wildlife in the southern Kalahari and the role of man. Afr. J. Ecol. 1995, 33, 377–394. [Google Scholar] [CrossRef]
- Gaughwin, M.; Judson, G.; Macfarlane, W.; Siebert, B. Effect of drought on the health of wild hairy-nosed wombats. Lasiorhinus Latifrons. Wildl. Res. 1984, 11, 455–463. [Google Scholar] [CrossRef]
- Moss, G.; Croft, D. Body condition of the red kangaroo (Macropus rufus) in arid Australia: The effect of environmental condition, sex and reproduction. Aust. J. Ecol. 1999, 24, 97–109. [Google Scholar] [CrossRef]
- Katona, P.; Katona-Apte, J. The interaction between nutrition and infection. Clin. Infect. Dis. 2008, 46, 1582–1588. [Google Scholar] [CrossRef]
- Houston, A.I.; Stephens, P.A.; Boyd, I.L.; Harding, K.C.; McNamara, J.M. Capital or income breeding? A theoretical model of female reproductive strategies. Behav. Ecol. 2007, 18, 241–250. [Google Scholar] [CrossRef]
- Norbury, G.; Coulson, G.; Walters, B. Aspects of the Demography of the Western Grey-Kangaroo, Macropus-Fuliginosus-Melanops, in Semiarid Northwest Victoria. Wildl. Res. 1988, 15, 257–266. [Google Scholar] [CrossRef]
- Robertson, G. The mortality of kangaroos in drought. Wildl. Res. 1986, 13, 349–354. [Google Scholar] [CrossRef]
- Poole, W.; Catling, P. Reproduction in the two species of grey kangaroo, Macropus giganteus Shaw and M. fuliginosus (Desmarest) I. Sexual maturity and oestrus. Aust. J. Zool. 1974, 22, 277–302. [Google Scholar] [CrossRef]
- Clutton-Brock, T.; Iason, G.; Albon, S.; Guinness, F. Effects of lactation on feeding behaviour and habitat use in wild red deer hinds. J. Zool. 1982, 198, 227–236. [Google Scholar] [CrossRef]
- Jönsson, K.I. Capital and income breeding as alternative tactics of resource use in reproduction. Oikos 1997, 78, 57–66. [Google Scholar] [CrossRef]
- Dawson, T.J. Kangaroos, 2nd ed.; CSIRO Publishing: Melbourne, Australia, 2012. [Google Scholar]
- Ben-Ami, D.; Boom, K.; Boronyak, L.; Townend, C.; Ramp, D.; Croft, D.; Bekoff, M. The welfare ethics of the commercial killing of free-ranging kangaroos: An evaluation of the benefits and costs of the industry. Anim. Welf. 2014, 23, 1–10. [Google Scholar] [CrossRef][Green Version]
- Shepherd, N. Condition and recruitment of kangaroos. In Kangaroos: Their Ecology and Management in the Sheep Rangelands of Australia; Caughley, G., Shepherd, N., Short, J., Eds.; Cambridge University Press: Cambridge, UK, 1987; pp. 135–158. [Google Scholar]
- Frith, H.; Sharman, G. Breeding in wild populations of the red kangaroo. Megal. Rufa. CSIRO Wildl. Res. 1964, 9, 86–114. [Google Scholar] [CrossRef]
- Renfree, M.B.; Tyndale-Biscoe, C. Intrauterine development after diapause in the marsupial Macropus eugenii. Dev. Biol. 1973, 32, 28–40. [Google Scholar] [CrossRef]
- Dawson, T.J.; Blaney, C.E.; McCarron, H.C.; Maloney, S.K. Dehydration, with and without heat, in kangaroos from mesic and arid habitats: Different thermal responses including varying patterns in heterothermy in the field and laboratory. J. Comp. Physiol. B 2007, 177, 797–807. [Google Scholar] [CrossRef]
- Dawson, T.J.; Denny, M.; Russell, E.M.; Ellis, B. Water usage and diet preferences of free ranging kangaroos, sheep and feral goats in the Australian arid zone during summer. J. Zool. 1975, 177, 1–23. [Google Scholar] [CrossRef]
- Mooring, M.S.; McKenzie, A.A.; Hart, B.L. Grooming in impala: Role of oral grooming in removal of ticks and effects of ticks in increasing grooming rate. Physiol. Behav. 1996, 59, 965–971. [Google Scholar] [CrossRef]
- King, W.J.; Festa-Bianchet, M.; Coulson, G.; Goldizen, A.W. Long-term consequences of mother-offspring associations in eastern grey kangaroos. Behav. Ecol. Sociobiol. 2017, 71, 77. [Google Scholar] [CrossRef]
- Ladds, P. Pathology of Australian Native Wildlife; Csiro Publishing: Clayton, Australia, 2009. [Google Scholar]
- Cripps, J.; Beveridge, I.; Coulson, G. The efficacy of anthelmintic drugs against nematodes infecting free-ranging eastern grey kangaroos, Macropus giganteus. J. Wildl. Dis. 2013, 49, 535–544. [Google Scholar] [CrossRef] [PubMed]
- Beveridge, I.; Arundel, J. Helminth parasites of grey kangaroos, Macropus giganteus Shaw and M. fuliginosus (Desmarest), in eastern Australia. Wildl. Res. 1979, 6, 69–77. [Google Scholar] [CrossRef]
- Cripps, J.; Beveridge, I.; Martin, J.K.; Borland, D.; Coulson, G. Temporal dynamics of helminth infections in eastern grey kangaroos (Macropus giganteus) in Victoria. Aust. J. Zool. 2015, 63, 163–174. [Google Scholar] [CrossRef]
- Beveridge, I.; Spratt, D.M. Biodiversity and parasites of wildlife: Helminths of Australasian marsupials. Trends Parasitol. 2015, 31, 142–148. [Google Scholar] [CrossRef]
- Ramp, D. Bringing compassion to the ethical dilemma in killing kangaroos for conservation. J. Bioeth. Inq. 2013, 10, 267–272. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Juillard, L.Q.; Ramp, D. The Impacts of Drought on the Health and Demography of Eastern Grey Kangaroos. Animals 2022, 12, 256. https://doi.org/10.3390/ani12030256
Juillard LQ, Ramp D. The Impacts of Drought on the Health and Demography of Eastern Grey Kangaroos. Animals. 2022; 12(3):256. https://doi.org/10.3390/ani12030256
Chicago/Turabian StyleJuillard, Loic Quentin, and Daniel Ramp. 2022. "The Impacts of Drought on the Health and Demography of Eastern Grey Kangaroos" Animals 12, no. 3: 256. https://doi.org/10.3390/ani12030256
APA StyleJuillard, L. Q., & Ramp, D. (2022). The Impacts of Drought on the Health and Demography of Eastern Grey Kangaroos. Animals, 12(3), 256. https://doi.org/10.3390/ani12030256







