Short Communication: Evaluation of Intestinal Release of Butyric Acid from Sodium Butyrate Protected by Salts of Medium-Chain Fatty Acids in Broiler Chickens
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Housing, Animals, and Experimental Design
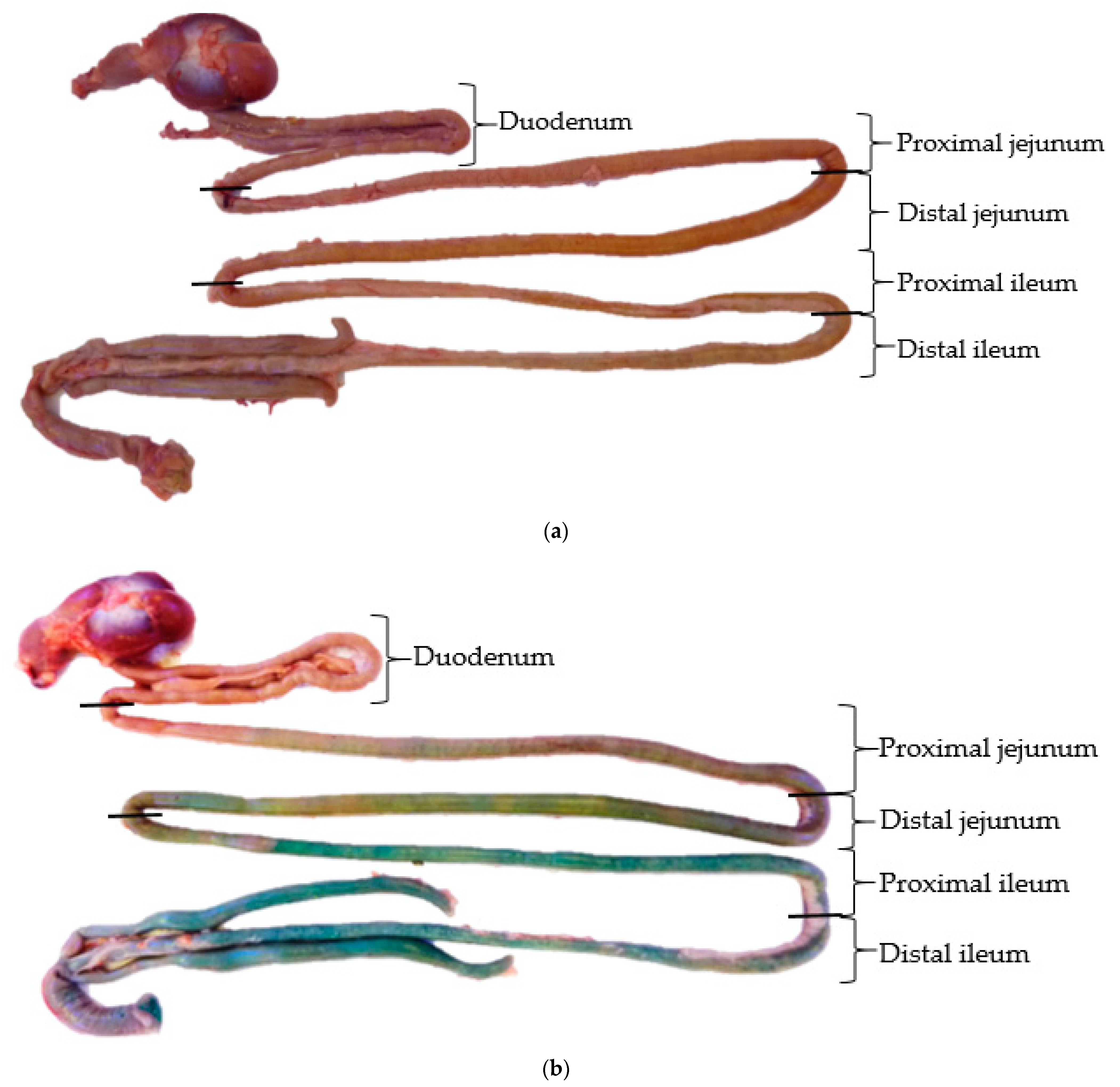
2.2. Sampling and Analytical Determinations
2.3. Statistical Analysis
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- European Parliament and European Council. Regulation (EC) No. 2019/6 of the European Parliament and of the Council of 11 December 2018 on Veterinary Medicinal Products and Repealing Directive 2001/83/EC Off. J. Eur. Union L:4–43; European Parliament and European Council: Brussels, Belgium, 2018. [Google Scholar]
- Rahman, M.R.T.; Fliss, I.; Biron, E. Insights in the development and uses of alternatives to antibiotic growth promoters in poultry and swine production. Antibiotics 2022, 11, 766. [Google Scholar] [CrossRef] [PubMed]
- Bortoluzzi, C.; Pedroso, A.A.; Mallo, J.J.; Puyalto, M.; Kim, W.K.; Applegate, T.J. Sodium butyrate improved performance while modulating the cecal microbiota and regulating the expression of intestinal immune-related genes of broiler chickens. Poult. Sci. 2017, 96, 3981–3993. [Google Scholar] [CrossRef] [PubMed]
- Leeson, S.; Namkung, H.; Antongiovanni, M.; Lee, E.H. Effect of butyric acid on the performance and carcass yield of broiler chickens. Poult. Sci. 2005, 84, 1418–1422. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Xiao, Z.; An, W.; Dong, Y.; Zhang, B. Dietary sodium butyrate improves intestinal development and function by modulating the microbial community in broilers. PLoS ONE 2018, 13, e0197762. [Google Scholar] [CrossRef]
- Melaku, M.; Zhong, R.; Han, H.; Wan, F.; Yi, F.B.; Zhang, H. Butyric and citric acids and their salts in poultry nutrition: Effects on gut health and intestinal microbiota. Int. J. Mol. Sci. 2021, 22, 10392. [Google Scholar] [CrossRef]
- Makowski, Z.; Lipiński, K.; Mazur-Kuśnirek, M. The Effects of sodium butyrate, coated sodium butyrate, and butyric acid glycerides on nutrient digestibility, gastrointestinal function, and fecal microbiota in turkeys. Animals 2022, 12, 1836. [Google Scholar] [CrossRef]
- Ventura, G.; Lima, G.A.; Barbosa, B.F.S.; Polycarpo, G.V.; Denadai, J.C.; Costa, V.E.; Madeira, A.M.B.N.; Malheiros, R.D.; Cruz-Polycarpo, V.C. Microencapsulated and uncoated butyric acid as alternative additives to the regeneration of intestinal mucosa in broilers challenged with Eimeria spp. Br. Poultr. Sci. 2021, 62, 717–725. [Google Scholar] [CrossRef]
- Choi, J.; Wang, L.; Ammeter, E.; Lahaye, L.; Liu, S.; Nyachoti, M.; Yang, C. Evaluation of lipid matrix microencapsulation for intestinal delivery of thymol in weaned pigs. Transl. Anim. Sci. 2019, 4, 411–422. [Google Scholar] [CrossRef]
- Pontes-Quero, G.M.; Esteban-Rubio, S.; Pérez Cano, J.; Aguilar, M.R.; Vázquez-Lasa, B. Oregano essential oil micro- and nanoencapsulation with bioactive properties for biotechnological and biomedical applications. Front. Bioeng. Biotechnol. 2021, 9, 703684. [Google Scholar] [CrossRef]
- Zentek, J.; Buchheit-Renko, S.; Ferrara, F.; Vahjen, W.; Van Kessel, A.G.; Pieper, R. Nutritional and physiological role of medium-chain triglycerides and medium-chain fatty acids in piglets. Anim. Health Res. Rev. 2011, 12, 83–93. [Google Scholar] [CrossRef]
- López-Colom, P.; Castillejos, L.; Rodríguez-Sorrento, A.; Puyalto, M.; Mallo, J.J.; Martín-Orúe, S.M. Efficacy of medium-chain fatty acid salts distilled from coconut oil against two enteric pathogen challenges in weanling piglets. J. Anim. Sci. Biotechnol. 2020, 10, 89. [Google Scholar] [CrossRef] [PubMed]
- Hanczakowska, E. The use of medium-chain fatty acids in piglet feeding—A review. Ann. Anim. Sci. 2016, 17, 967–977. [Google Scholar] [CrossRef]
- Mallo, J.J.; Balfagón, A.; Gracia, M.I.; Honrubia, P.; Puyalto, M. Evaluation of different protections of butyric acid aiming for release in the last part of the gastrointestinal tract of piglets. J. Anim. Sci. 2012, 90, 227–229. [Google Scholar] [CrossRef]
- Abdelli, N.; Francisco Pérez, J.; Vilarrasa, E.; Melo-Duran, D.; Cabeza Luna, I.; Karimirad, R.; Solà-Oriol, D. Microencapsulation improved fumaric acid and thymol effects on broiler chickens challenged with a short-term fasting period. Front. Vet. Sci. 2021, 8, 686143. [Google Scholar] [CrossRef]
- European Parliament. Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010 on the Protection of Animals Used for Scientific Purposes. L:276-33; European Parliament: Brussels, Belgium, 2010. [Google Scholar]
- Fundación Española para el Desarrollo de la Nutrición Animal. Necesidades Nutricionales para Avicultura: Pollos de Carne y Aves de Puesta; FEDNA: Madrid, Spain, 2018; ISBN 9788409065295. [Google Scholar]
- European Commission. Regulation (EC) No. 2020/173 of the European Commission of 6 February 2020 Concerning the Authorization of Brilliant Blue FCF as a Feed Additive for Cats and Dogs Off. J. Eur. Union L:35-9; European Commission: Brussels, Belgium, 2020. [Google Scholar]
- Zou, T.; He, P.; Yasen, A.; Li, Z. Determination of seven synthetic dies in animal feeds and meat by high performance liquid chromatography with diode array and tandem mass detectors. Food Chem. 2013, 138, 1742–1748. [Google Scholar] [CrossRef] [PubMed]
- Suarez, R.; Arévalo, E.; Linares, L.; Ustáriz, F.; Hernández, G. Validación de un método analítico para la determinación de magnesio eritrocitario. Avances en Química 2009, 4, 53–62. [Google Scholar]
- Lee, P.S.; Yim, S.G.; Choi, Y.; Van Anh Ha, T.; Ko, S. Physiochemical properties and prolonged release behaviours of chitosan-denatured β-lactoglobulin microcapsules for potential food applications. Food Chem. 2012, 134, 992–998. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: https://www.R-project.org/ (accessed on 9 December 2022).
- Smith, D.J.; Barri, A.; Herges, G.; Hahn, J.; Yersin, A.G.; Jourdan, A. In vitro dissolution and in vivo absorption of calcium [1-14C] butyrate in free or protected forms. J. Agric. Food Chem. 2012, 60, 3151–3157. [Google Scholar] [CrossRef]
- van den Borne, J.J.G.C.; Heetkamp, M.J.W.; Buyse, J.; Niewold, T.A. Fat coating of Ca butyrate results in extended butyrate release in the gastrointestinal tract of broilers. Livest. Sci. 2015, 175, 96–100. [Google Scholar] [CrossRef]
- Rodriguez-Sanchez, R.; Tres, A.; Sala, R.; Guardiola, F.; Barroeta, A.C. Evolution of lipid classes and fatty acid digestibility along the gastrointestinal tract of broiler chickens fed different fat sources at different ages. Poult. Sci. 2018, 98, 1341–1353. [Google Scholar] [CrossRef]
- Sacranie, A.; Iji, P.A.; Choct, M. Reflux of digesta and its implications for nutrient digestion and bird health. Proc. Aust. Poult. Sci. Symp. 2005, 17, 171–174. [Google Scholar]
- Angel, R.S.; Kim, W.; Li, W.; Jimenez-Moreno, E. Velocidad de paso y pH Intestinal e Naves: Implicaciones para la Digestión y el Uso de Enzimas; XXIX Curso de Especialización FEDNA; FEDNA: Madrid, Spain, 2013. [Google Scholar]
| Item | Duodenum | Proximal Jejunum | Distal Jejunum | Proximal Ileum | Distal Ileum | RSE | p-Value |
|---|---|---|---|---|---|---|---|
| Butyric acid, % | 1.80 d | 5.74 cd | 15.4 c | 31.1 b | 45.9 a | 4.88 | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sadurní, M.; Barroeta, A.C.; Sol, C.; Puyalto, M.; Castillejos, L. Short Communication: Evaluation of Intestinal Release of Butyric Acid from Sodium Butyrate Protected by Salts of Medium-Chain Fatty Acids in Broiler Chickens. Animals 2022, 12, 3525. https://doi.org/10.3390/ani12243525
Sadurní M, Barroeta AC, Sol C, Puyalto M, Castillejos L. Short Communication: Evaluation of Intestinal Release of Butyric Acid from Sodium Butyrate Protected by Salts of Medium-Chain Fatty Acids in Broiler Chickens. Animals. 2022; 12(24):3525. https://doi.org/10.3390/ani12243525
Chicago/Turabian StyleSadurní, Meritxell, Ana Cristina Barroeta, Cinta Sol, Mónica Puyalto, and Lorena Castillejos. 2022. "Short Communication: Evaluation of Intestinal Release of Butyric Acid from Sodium Butyrate Protected by Salts of Medium-Chain Fatty Acids in Broiler Chickens" Animals 12, no. 24: 3525. https://doi.org/10.3390/ani12243525
APA StyleSadurní, M., Barroeta, A. C., Sol, C., Puyalto, M., & Castillejos, L. (2022). Short Communication: Evaluation of Intestinal Release of Butyric Acid from Sodium Butyrate Protected by Salts of Medium-Chain Fatty Acids in Broiler Chickens. Animals, 12(24), 3525. https://doi.org/10.3390/ani12243525






