Using Dextran Instead of Egg Yolk in Extender for Cryopreservation of Spermatozoa of Dogs of Different Ages
Abstract
Simple Summary
Abstract
1. Introduction
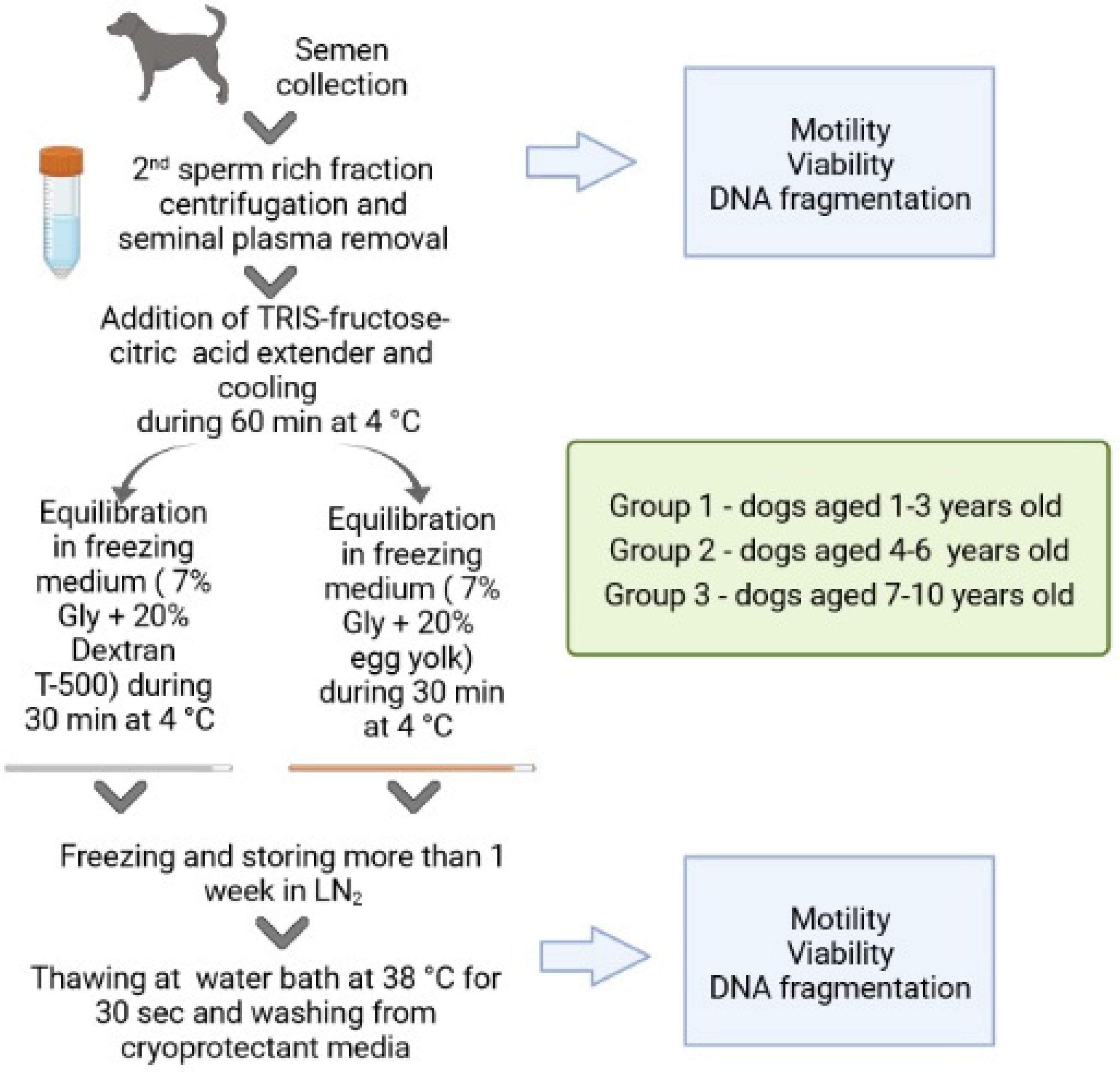
2. Materials and Methods
2.1. Semen Collection and Analysis
2.2. Sperm Preparation and Cryopreservation
2.3. Sperm DNA Fragmentation Assessment
2.4. Statistical Analysis
3. Results
4. Discussion
4.1. Age-Related Cryotolerance of Canin Spermatozoa
4.2. Using Dextran Instead of Egg Yolk as a Component of Cryoprotectant Media
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Quartuccio, M.; Biondi, V.; Liotta, L.; Passantino, A. Legislative and Ethical Aspects on Use of Canine Artificial Insemination in the 21st Century. Ital. J. Anim. Sci. 2020, 19, 630–643. [Google Scholar] [CrossRef]
- Buriak, I.; Fleck, R.A.; Goltsev, A.; Shevchenko, N.; Petrushko, M.; Yurchuk, T.; Puhovkin, A.; Rozanova, S.; Guibert, E.E.; Robert, M.C.; et al. Translation of Cryobiological Techniques to Socially Economically Deprived Populations-Part 1: Cryogenic Preservation Strategies. J. Med. Devices. 2020, 14, 010801. [Google Scholar] [CrossRef]
- Martinez-Rodriguez, J.A.; Carbajal, F.J.; Martinez-De-Anda, R.; Alcantar-Rodriguez, A.; Medrano, A. Melatonin Added to Freezing Diluent Improves Canine (Bulldog) Sperm Cryosurvival. Reprod. Fertil. 2020, 1, 11–19. [Google Scholar] [CrossRef]
- Pavlovych, O.; Hapon, H.; Yurchuk, T.; Repin, M.; Marchenko, L.; Govorukha, T.; Petrushko, M. Ultrastructural and Functional Characteristics of Human Spermatozoa After Cryopreservation by Vitrification. Probl. Cryobiol. Cryomed. 2020, 30, 24–33. [Google Scholar] [CrossRef]
- Yurchuk, T.; Petrushko, M.; Gapon, A.; Piniaiev, V.; Kuleshova, L. The Impact of Cryopreservation on the Morphology of Spermatozoa in Men with Oligoasthenoteratozoospermia. Cryobiology 2021, 100, 117–124. Available online: https://www.sciencedirect.com/science/article/abs/pii/S0011224021000596 (accessed on 3 March 2021). [CrossRef]
- Yurchuk, T.O.; Pavlovich, O.V.; Gapon, G.O.; Pugovkin, A.Y.; Petrushko, M.P. Lipid Peroxidation and DNA Fragmentation in Fresh and Cryopreserved Spermatozoa of Men with Different Spermatogenesis State. Ukr. Biochem. J. 2021, 93, 24–29. [Google Scholar] [CrossRef]
- De la Fuente-Lara, A.; Hesser, A.; Christensen, B.; Gonzales, K.; Meyers, S. Effects from Aging on Semen Quality of Fresh and Cryopreserved Semen in Labrador Retrievers. Theriogenology 2019, 132, 164–171. [Google Scholar] [CrossRef]
- Lechner, D.; Aurich, J.; Schäfer-Somi, S.; Aurich, C. Effects of Age, Size and Season on Cryotolerance of Dog Semen—A Retrospective Analysis. Anim. Reprod. Sci. 2022, 236, 106912. [Google Scholar] [CrossRef]
- Brito, M.M.; Lúcio, C.F.; Angrimani, D.S.; Losano, J.D.; Dalmazzo, A.; Nichi, M.; Vannucchi, C.I. Comparison of Cryopreservation Protocols (Single and Two-steps) and Thawing (Fast and Slow) for Canine Sperm. Anim. Biotechnol. 2017, 28, 67–73. [Google Scholar] [CrossRef]
- Yang, M.; Wang, B.; Liu, J.; Li, Y.; He, S.; Kuang, H.; Li, Y.; Peng, J. Investigation of Various Cryopreservation Methods for Improvement of Canine Sperm Survival. Isr. J. Vet. Med. 2018, 73, 24–32. [Google Scholar]
- Blanch, E.; Tomás, C.; Hernández, M.; Roca, J.; Martínez, E.A.; Vázquez, J.M.; Mocé, E. Egg Yolk and Glycerol Requirements for Freezing Boar Spermatozoa Treated with Methyl β-cyclodextrin or Cholesterol-loaded Cyclodextrin. J. Reprod. Dev. 2014, 60, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Lopes, G.; Soares, L.; Ferreira, P.; Rocha, A. Tris-Egg Yolk-Glycerol (TEY) Extender Developed for Freezing Dog Semen is a Good Option to Cryopreserve Bovine Epididymal Sperm Cells. Reprod. Dom. Anim. 2015, 50, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.R.; de Cássia Soares Cardoso, R.; Machado da Silva, L.D. Canine Semen Cryopreservation with Different Glycerol. Rev. Bras. Med. Vet. 2002, 9, 25–28. [Google Scholar] [CrossRef]
- Talha, N.A.H.; Jeon, Y.; Yu, I.J. Cryopreservation of Dog Spermatozoa Using Essential and Non-Essential Amino Acids Solutions in An Egg Yolk-Free Polyvinyl Alcohol Extender. CryoLetters 2021, 42, 44–52. [Google Scholar]
- Anzar, M.; Rajapaksha, K.; Boswall, L. Egg Yolk-Free Cryopreservation of Bull Semen. PLoS ONE 2019, 14, e0223977. [Google Scholar] [CrossRef]
- Kimizuka, N.; Viriyarattanasak, C.; Suzuki, T. Ice Nucleation and Supercooling Behavior of Polymer Aqueous Solutions. Cryobiology 2008, 56, 80–87. [Google Scholar] [CrossRef]
- Simonik, O.; Bubenickova, F.; Tumova, L.; Frolikova, M.; Sur, V.P.; Beran, J.; Havlikova, K.; Hackerova, L.; Spevakova, D.; Komrskova, K.; et al. Boar Sperm Cryopreservation Improvement Using Semen Extender Modification by Dextran and Pentaisomaltose. Animals 2022, 12, 868. [Google Scholar] [CrossRef]
- Deplazes, E.; Poger, D.; Cornell, B.; Cranfield, C.G. The Effect of Hydronium Ions on the Structure of Phospholipid Membranes. Phys. Chem. Chem. Phys. 2017, 20, 357–366. [Google Scholar] [CrossRef]
- Veselský, L.; Pěknicová, J.; Cechová, D.; Kraus, M.; Geussová, G.; Jonáková, V. Characterization of Boar Spermadhesins by Monoclonal and Polyclonal Antibodies and Their Role in Binding to Oocytes. Am. J. Reprod. Immunol. 1999, 42, 187–197. [Google Scholar] [CrossRef]
- Gloria, A.; Toscani, T.; Robbe, D.; Parrillo, S.; De Amicis, I.; Contri, A. Cryopreservation of Turkey Spermatozoa Without Permeant Cryoprotectants. Anim. Reprod. Sci. 2019, 211, 106218. [Google Scholar] [CrossRef]
- Schäfer-Somi, S.; Aurich, C. Use of a new computer-assisted sperm analyzer for the assessment of motility and viability of dog spermatozoa and evaluation of four different semen extenders for predilution. Anim. Reprod. Sci. 2007, 102, 1–13. [Google Scholar] [CrossRef]
- Rodenas, C.; Parrilla, I.; Roca, J.; Martinez, E.A.; Lucas, X. Effects of Rapid Cooling Prior to Freezing on the Quality of Canine Cryopreserved Spermatozoa. J. Reprod. Dev. 2014, 60, 355–361. [Google Scholar] [CrossRef] [PubMed]
- Fernández, J.L.; Muriel, L.; Rivero, M.T.; Goyanes, V.; Vazquez, R.; Alvarez, J.G. The Sperm Chromatin Dispersion Test: A Simple Method for the Determination of Sperm DNA Fragmentation. J. Androl. 2003, 24, 59–66. [Google Scholar] [PubMed]
- Westfalewicz, B.; Słowińska, M.; Judycka, S.; Ciereszko, A.; Dietrich, M.A. Comparative Proteomic Analysis of Young and Adult Bull (Bos taurus) Cryopreserved Semen. Animals 2021, 11, 2013. [Google Scholar] [CrossRef] [PubMed]
- Long, J.A.; Bongalhardo, D.C.; Pelaéz, J.; Saxena, S.; Settar, P.; O’Sullivan, N.P.; Fulton, J.E. Rooster Semen Cryopreservation: Effect of Pedigree Line and Male Age on Postthaw Sperm Function. Poult. Sci. 2010, 89, 966–973. [Google Scholar] [CrossRef]
- Drögemüller, C.; Karlsson, E.K.; Hytönen, M.K.; Perloski, M.; Dolf, G.; Sainio, K.; Lohi, H.; Lindblad-Toh, K.; Leeb, T.A. Mutation in Hairless Dogs Implicates FOXI3 in Ectodermal Development. Science 2008, 321, 1462. [Google Scholar] [CrossRef]
- Golson, M.L.; Kaestner, K.H. Fox Transcription Factors: From Development to Disease. Development 2016, 143, 4558–4570. [Google Scholar] [CrossRef]
- Sanese, P.; Forte, G.; Disciglio, V.; Grossi, V.; Simone, C. FOXO3 on the Road to Longevity: Lessons from SNPs and Chromatin Hubs. Comput. Struct. Biotechnol. J. 2019, 17, 737–745. [Google Scholar] [CrossRef]
- Gloria, A.; Zambelli, D.; Carluccio, A.; Cunto, M.; Ponzio, P.; Contri, A. Is the Protective Effect of Egg Yolk Against Osmotic and Cryogenic Damage on Dog Spermatozoa Dose-Dependent? Anim. Reprod. Sci. 2020, 213, 106259. [Google Scholar] [CrossRef]
- Bergeron, A.; Manjunath, P. New Insights Towards Understanding the Mechanisms of Sperm Protection by Egg Yolk and Milk. Mol. Reprod. Dev. 2006, 73, 1338–1344. [Google Scholar] [CrossRef]
- Petrunkina, A.M.; Gröpper, B.; Günzel-Apel, A.R.; Töpfer-Petersen, E. Functional Significance of the Cell Volume for Detecting Sperm Membrane Changes and Predicting Freezability in Dog Semen. Reproduction 2004, 128, 829–842. [Google Scholar] [CrossRef] [PubMed]
- Oldenhof, H.; Gojowsky, M.; Wang, S.; Henke, S.; Yu, C.; Rohn, K.; Wolkers, W.F.; Sieme, H. Osmotic Stress and Membrane Phase Changes During Freezing of Stallion Sperm: Mode of Action of Cryoprotective Agents. Biol. Reprod. 2013, 88, 68. [Google Scholar] [CrossRef] [PubMed]
- Sieme, H.; Oldenhof, H.; Wolkers, W.F. Mode of Action of Cryoprotectants for Sperm Preservation. Anim. Reprod. Sci. 2016, 169, 2–5. [Google Scholar] [CrossRef] [PubMed]
- Fujita, Y.; Nishimura, M.; Komori, N.; Sawamoto, O.; Kaneda, S. Protein-Free Solution Containing Trehalose and Dextran 40 for Cryopreservation of Human Adipose Tissue-Derived Mesenchymal Stromal Cells. Cryobiology 2021, 100, 46–57. [Google Scholar] [CrossRef]
- Quan, G.B.; Han, Y.; Liu, M.X.; Fang, L.; Du, W.; Ren, S.P.; Wang, J.X.; Wang, Y. Addition of Oligosaccharide Decreases the Freezing Lesions on Human Red Blood Cell Membrane in the Presence of Dextran and Glucose. Cryobiology 2011, 62, 135–144. [Google Scholar] [CrossRef]
- Kundu, C.N.; Chakrabarty, J.; Dutta, P.; Bhattacharyya, D.; Ghosh, A.; Majumder, G.C. Effect of Gextrans on Cryopreservation of Goat Cauda Epididymal Spermatozoa Using a Chemically Defined Medium. Reproduction 2002, 123, 907–913. [Google Scholar] [CrossRef]
- Viudes-de-Castro, M.P.; Talaván, A.G.; Vicente, J.S. Evaluation of Dextran for Rabbit Sperm Cryopreservation: Effect on Frozen-Thawed Rabbit Sperm Quality Variables and Reproductive Performance. Anim. Reprod. Sci. 2021, 226, 106714. [Google Scholar] [CrossRef]
- Soeiro, V.C.; Melo, K.R.T.; Alves, M.G.C.F.; Medeiros, M.J.C.; Grilo, M.L.P.M.; Almeida-Lima, J.; Pontes, D.L.; Costa, L.S.; Rocha, H.A.O. Dextran: Influence of Molecular Weight in Antioxidant Properties and Immunomodulatory Potential. Int. J. Mol. Sci. 2016, 17, 1340. [Google Scholar] [CrossRef]


| Age Group | Group 1 | Group 2 | Group 3 |
|---|---|---|---|
| Semen volume, mL | 0.99 ± 0.23 a | 1.66 ± 0.27 a | 2.87 ± 0.88 b |
| Sperm concentration, ×106 cell/mL | 523.3 ± 120.4 a | 274.4 ± 21.86 b | 73.89 ± 32.86 c |
| Total sperm count, ×106 cell | 498.4 ± 78.45 a | 453.0 ± 69.93 a | 201.4 ± 77.75 b |
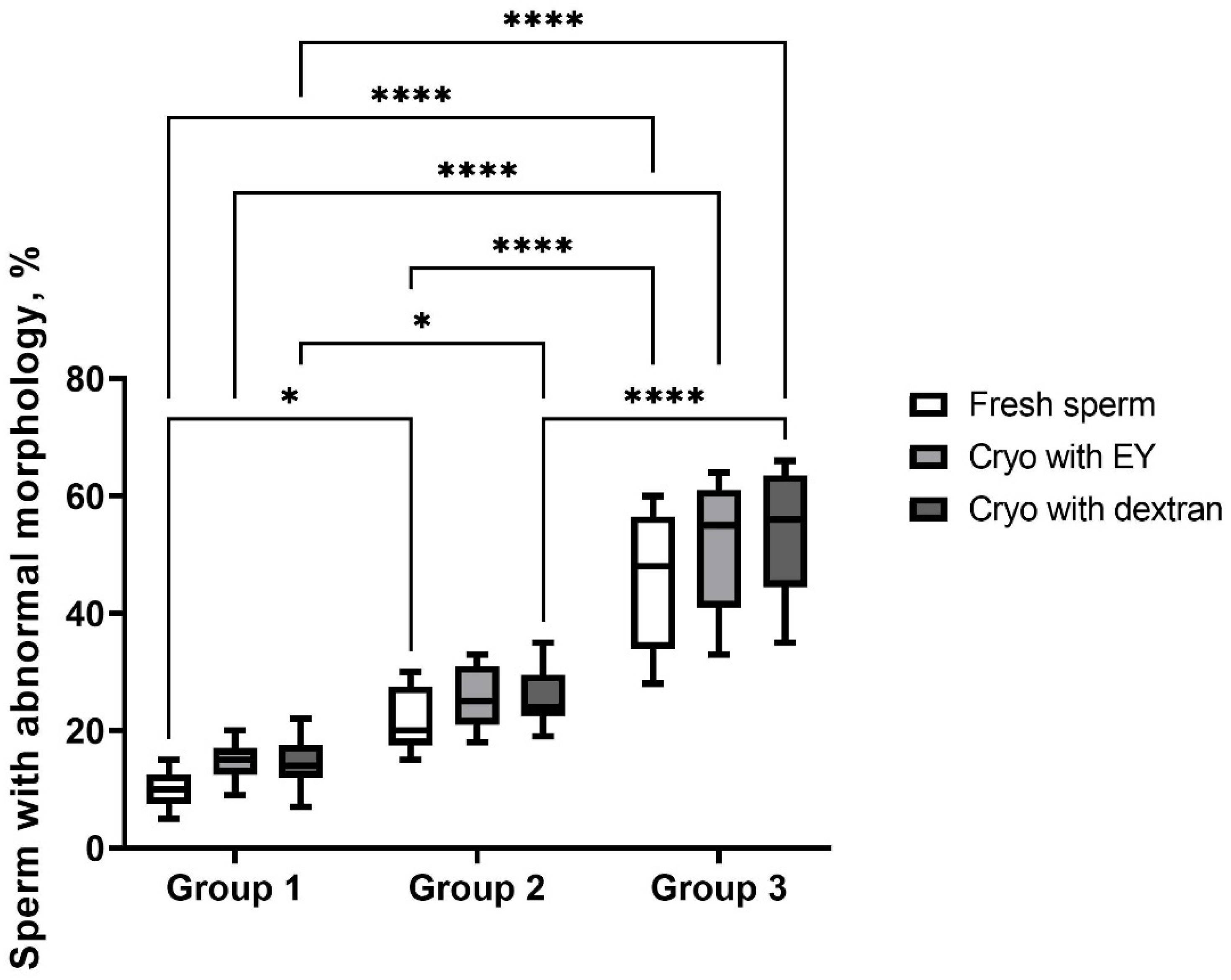
| Group | Morphology Abnormalities | ||||
|---|---|---|---|---|---|
| Head, % | Midpiece, % | Tail, % | Multiple Abnormalities, % | ||
| Fresh | 1 | 1.7 ± 0.4 a | 1.8 ± 0.9 a | 1.5 ± 0.7 a | 4.9 ± 0.5 a |
| 2 | 3.7 ± 0.9 b | 3.3 ± 0.6 b | 3.4 ± 0.5 b | 11.11 ± 1.5 b | |
| 3 | 9.1 ± 1.1 c | 8.9 ± 0.7 c | 6.3 ± 0.7 c | 21.3 ± 2.1 cd | |
| Cryo with EY | 1 | 2.3 ± 0.3 a | 2.5 ± 0.5 ab | 3.8 ± 0.4 b | 5.9 ± 1.0 a |
| 2 | 3.9 ± 0.7 b | 4.2 ± 0.3 b | 7.4 ± 0.9 c | 10.1 ± 0.9 b | |
| 3 | 10.0 ± 1.2 c | 9.1 ± 0.5 c | 9.3 ± 1.0 c | 22.9 ± 1.9 d | |
| Cryo with dextran | 1 | 2.1 ± 0.4 a | 2.3 ± 0.4 a | 3.7 ± 0.2 b | 6.3 ± 0.7 a |
| 2 | 4.3 ± 0.4 b | 4.1 ± 0.7 b | 5.3 ± 0.4 b | 12.2 ± 1.0 b | |
| 3 | 12.2 ±1.1 c | 12.2 ± 1.2 d | 11.8 ± 1.1 c | 17.6 ± 1.5 c | |
| Group | Motility Parameters | |||||||
|---|---|---|---|---|---|---|---|---|
| TMOT, % | PMOT, % | VCL, µm/s | VAP, µm/s | VSL, µm/s | ALH, µm | STR | ||
| Fresh | 1 | 84.4 ± 6.3 a | 67.9 ± 6.1 a | 175.3 ± 7.0 a | 140.5 ± 2.7 a | 112.8 ± 3.2 a | 2.2 ± 0.3 | 82.2 ± 0.8 |
| 2 | 73.3 ± 7.9 b | 55.3 ± 6.1 b | 171.8 ± 8.1 a | 136.6 ± 3.7 a | 99.7 ± 5.6 a | 2.1 ± 0.2 | 81.5 ± 1.2 | |
| 3 | 53.9 ± 9.6 c | 25.7 ± 3.9 d | 155.8 ± 6.9 b | 112.9 ±3.2 b | 91 ± 5.1 b | 2.0 ± 0.3 | 80.1 ± 0.9 | |
| Cryo with EY | 1 | 75.1 ± 5.3 b | 42.1 ± 3.7 c | 164.3 ± 5.7 a | 129.8 ± 3.2 a | 108.4 ± 4.1 a | 2.1 ± 0.4 | 79.8 ± 1.1 |
| 2 | 65.6 ± 6.2 c | 34.1 ± 5.0 d | 155.9 ± 7.2 b | 110.7 ± 3.5 b | 94.6 ± 3.2 b | 2.0 ± 0.5 | 78.9 ± 1.5 | |
| 3 | 33.9 ± 8.9 d | 18.6 ± 3.4 e | 148 ± 6.6 b | 103 ± 4.7 b | 87.9 ± 3.6 b | 1.9 ± 0.2 | 77.8 ± 0.9 | |
| Cryo with dextrann | 1 | 74.8 ± 4.5 b | 40.6 ± 4.5 c | 166.1 ±7.2 a | 127.6 ± 3.2 a | 106.9 ± 4.9 a | 2.1 ± 0.2 | 80.1 ± 0.9 |
| 2 | 64.2 ± 5.0 c | 37.0 ± 3.6 d | 151.2 ± 7.2 b | 115.6 ± 4.3 b | 92.7 ± 3.9 b | 1.9 ± 0.4 | 77.9 ± 0.7 | |
| 3 | 33.3 ± 8.03 d | 17.8 ± 4.3 e | 146.7 ± 5.3 b | 109 ± 3.2 b | 88.2 ± 3.5 b | 1.8 ± 0.3 | 79.3 ± 1.1 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yurchuk, T.; Pavlovich, O.; Petrushko, M. Using Dextran Instead of Egg Yolk in Extender for Cryopreservation of Spermatozoa of Dogs of Different Ages. Animals 2022, 12, 3480. https://doi.org/10.3390/ani12243480
Yurchuk T, Pavlovich O, Petrushko M. Using Dextran Instead of Egg Yolk in Extender for Cryopreservation of Spermatozoa of Dogs of Different Ages. Animals. 2022; 12(24):3480. https://doi.org/10.3390/ani12243480
Chicago/Turabian StyleYurchuk, Taisiia, Olena Pavlovich, and Maryna Petrushko. 2022. "Using Dextran Instead of Egg Yolk in Extender for Cryopreservation of Spermatozoa of Dogs of Different Ages" Animals 12, no. 24: 3480. https://doi.org/10.3390/ani12243480
APA StyleYurchuk, T., Pavlovich, O., & Petrushko, M. (2022). Using Dextran Instead of Egg Yolk in Extender for Cryopreservation of Spermatozoa of Dogs of Different Ages. Animals, 12(24), 3480. https://doi.org/10.3390/ani12243480





