Case Report of Puffinosis in a Manx Shearwater (Puffinus puffinus) Suggesting Environmental Aetiology
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Necropsy Including Histology, Bacteriology, and Parasitology
2.3. Nucleic Acid Extraction, Library Preparation and Sequencing
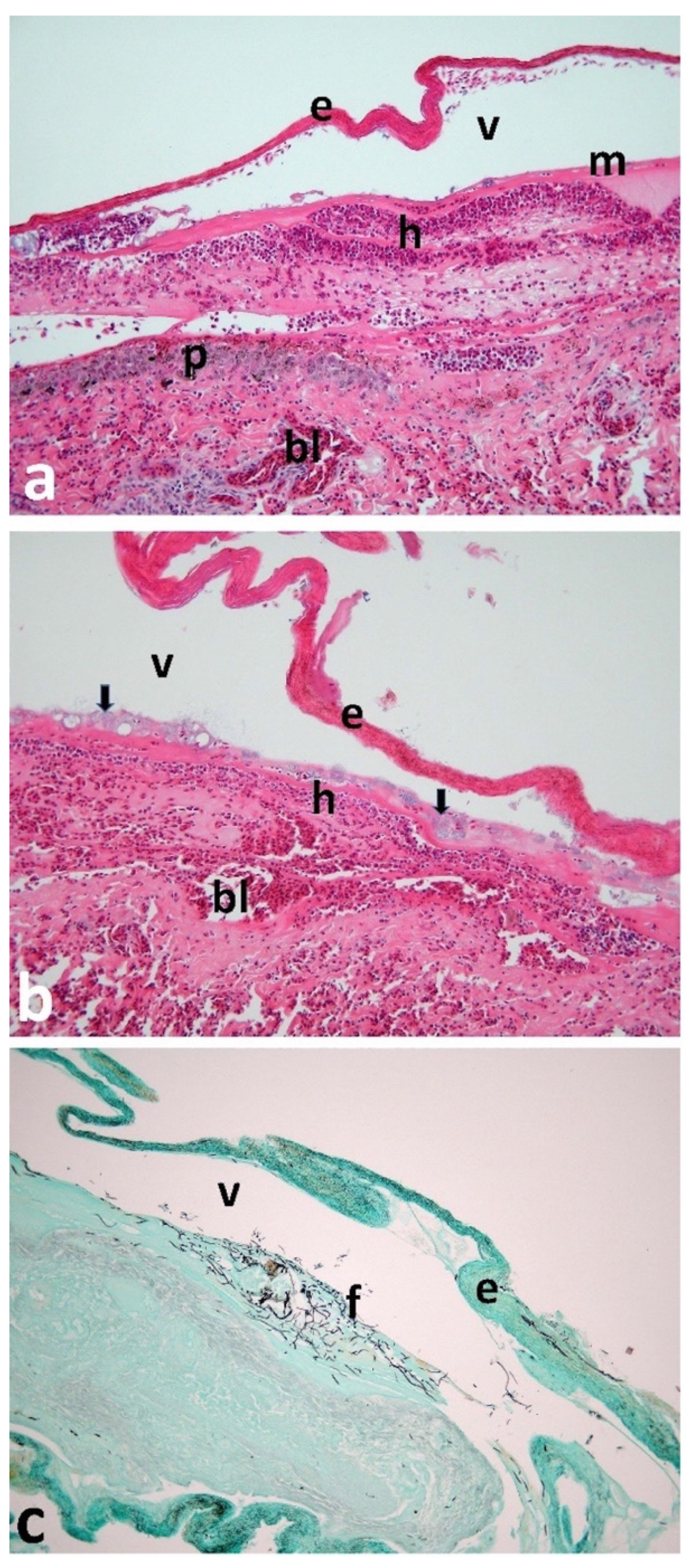
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guilford, T.; Meade, J.; Willis, J.; Phillips, R.A.; Boyle, D.; Roberts, S.; Collett, M.; Freeman, R.; Perrins, C.M. Migration and Stopover in a Small Pelagic Seabird, the Manx Shearwater Puffinus puffinus: Insights from Machine Learning. Proc. R. Soc. Lond. B Biol. Sci. 2009, 276, 1215–1223. [Google Scholar]
- Wood, M.J.; Canonne, C.; Besnard, A.; Lachish, S.; Fairhurst, S.M.; Liedvogel, M.; Boyle, D.; Patrick, S.C.; Josey, S.; Kirk, H.; et al. Demographic Profiles and Environmental Drivers of Variation Relate to Individual Breeding State in a Long-Lived Trans-Oceanic Migratory Seabird, the Manx Shearwater. PLoS ONE 2021, 16, e0260812. [Google Scholar] [CrossRef]
- Cardoso, M.D.; de Moura, J.F.; Tavares, D.C.; Gonçalves, R.A.; Colabuono, F.I.; Roges, E.M.; de Souza, R.L.; Rodrigues, D.D.P.; Montone, R.C.; Siciliano, S. The Manx Shearwater (Puffinus puffinus) as a Candidate Sentinel of Atlantic Ocean Health. Aquat. Biosyst. 2014, 10, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nuttall, P.A.; Brooke, M.D.L.; Perrins, C.M. Poxvirus Infection of the Manx Shearwater (Puffinus puffinus). J. Wildl. Dis. 1985, 21, 120–124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brooke, M. The Manx Shearwater; A&C Black: London, UK, 2010. [Google Scholar]
- Vanstreels, R.E.T.; de Angeli Dutra, D.; Santos, A.P.; Hurtado, R.; Egert, L.; Braga, É.M. First Report of Avian Malaria in a Manx Shearwater. Parasitol. Int. 2020, 78, 102148. [Google Scholar] [CrossRef]
- Garamszegi, L.Z. Climate Change Increases the Risk of Malaria in Birds. Glob. Chang. Biol. 2011, 17, 1751–1759. [Google Scholar] [CrossRef]
- Dane, D.S. A Disease of Manx Shearwaters (Puffinus puffinus). J. Anim. Ecol. 1948, 158–164. [Google Scholar] [CrossRef]
- Miles, J.A.R.; Stoker, M.G.P. Puffinosis, a Virus Epizootic of the Manx Shearwater (Puffinus puffinus). Nature 1948, 161, 1016–1017. [Google Scholar] [CrossRef]
- Dane, D.S.; Miles, J.A.R.; Stoker, M.G.P. A Disease of Manx Shearwaters: Further Observations in the Field. J. Anim. Ecol. 1953, 22, 123–133. [Google Scholar] [CrossRef]
- Harris, M.P. Puffinosis among Manx Shearwaters on Skokholm. Br. Birds 1965, 58, 426–433. [Google Scholar]
- Macdonald, J.W.; McMartin, D.A.; Walker, K.C.; Carins, M.; Dennis, R.H. Puffinosis in Fulmars in Orkney and Shetland. Br. Birds 1967, 60, 356–360. [Google Scholar]
- Terio, K.A.; McAloose, D.; Leger, J.S. Pathology of Wildlife and Zoo Animals; Academic Press: Cambridge, MA, USA, 2018; ISBN 0-12-809219-X. [Google Scholar]
- Nuttall, P.A.; Perrins, C.M.; Harrap, K.A. Further Studies on Puffinosis, a Disease of the Manx Shearwater (Puffinus puffinus). Can. J. Zool. 1982, 60, 3462–3465. [Google Scholar] [CrossRef]
- CBO. Annual Report for 1991; Copeland Bird Observatory: County Down, UK, 1991. [Google Scholar]
- CBO. Annual Report for 1990; Copeland Bird Observatory: County Down, UK, 1990. [Google Scholar]
- Wynn, J.; Padget, O.; Mouritsen, H.; Perrins, C.; Guilford, T. Natal Imprinting to the Earth’s Magnetic Field in a Pelagic Seabird. Curr. Biol. 2020, 30, 2869–2873.e2. [Google Scholar] [CrossRef] [PubMed]
- Botman, J.; Gourlay, P. Suspected Cases of Puffinosis in Manx Shearwaters (Puffinus puffinus) Admitted to Wildlife Rescue Centres in France in 2017 and 2018 Late Summers. Vet. Rec. Case Rep. 2021, 9, e119. [Google Scholar] [CrossRef]
- Stoker, M.G.P.; Miles, J.A.R. Studies on the Causative Agent of an Epizootic amongst Manx Shearwaters (Puffinus puffinus). Epidemiol. Infect. 1953, 51, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Răpuntean, S.; Răpuntean, G. Puffinosis on Manx Shearwaters (Puffinus puffinus). Rom. J. Vet. Med. Pharmacol. 2019, 4, 166–171. [Google Scholar]
- Nuttall, P.A.; Harrap, K.A. Isolation of a Coronavirus during Studies on Puffinosis, a Disease of the Manx Shearwater (Puffinus puffinus). Arch. Virol. 1982, 73, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Stewart, J.R.; Leonard, K. Survey of the Manx Shearwater Breeding Populations on Lighthouse Island and Big Copeland Island in 2007; Report to the Environment and Heritage Service; Department of the Environment for Northern Ireland: County Down, UK, 2007. [Google Scholar]
- Lloyd, H.G.; McCowan, D. Some Observations on the Breeding Burrows of the Wild Rabbit Oryctolagus cuniculus on the Island of Skokholm. J. Zool. 1968, 156, 540–549. [Google Scholar] [CrossRef]
- Bancroft, J.D.; Gamble, M. Theory and Practice of Histological Techniques; Elsevier Health Sciences: Amsterdam, The Netherlands, 2008. [Google Scholar]
- Urquhart, G.M.; Armour, J.; Duncan, J.L.; Dunn, A.M.; Jennings, F.W. Veterinary Parasitiology, 1st ed.; Longman Scientific and Technical; Bath Press: England, UK, 1987. [Google Scholar]
- Meyer, F.; Paarmann, D.; D’Souza, M.; Olson, R.; Glass, E.M. The Metagenomics RAST Server–a Public Resource for the Automatic Phylogenetic and Functional Analysis of Metagenomes. Bmcbioinformatics. BMC Bioinform. 2008, 9, 386. [Google Scholar] [CrossRef] [Green Version]
- Cox, M.P.; Peterson, D.A.; Biggs, P.J. SolexaQA: At-a-Glance Quality Assessment of Illumina Second-Generation Sequencing Data. BMC Bioinform. 2010, 11, 485. [Google Scholar] [CrossRef] [Green Version]
- Hanson, N.W.; Konwar, K.M.; Hallam, S.J. LCA*: An Entropy-Based Measure for Taxonomic Assignment within Assembled Metagenomes. Bioinformatics 2016, 32, 3535–3542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Belli, S.I.; Smith, N.C.; Ferguson, D.J.P. The Coccidian Oocyst: A Tough Nut to Crack! Trends Parasitol. 2006, 22, 416–423. [Google Scholar] [CrossRef] [PubMed]
- Sørensen, J.; Nybroe, O. Pseudomonas in the Soil Environment. In Pseudomonas: Volume 1 Genomics, Life Style and Molecular Architecture; Ramos, J.-L., Ed.; Springer US: Boston, MA, USA, 2004; pp. 369–401. ISBN 978-1-4419-9086-0. [Google Scholar]
- Petersen, L.M.; Tisa, L.S. Friend or Foe? A Review of the Mechanisms That Drive Serratia towards Diverse Lifestyles. Can. J. Microbiol. 2013, 59, 627–640. [Google Scholar] [CrossRef] [PubMed]
- Spain, A.M.; Krumholz, L.R.; Elshahed, M.S. Abundance, Composition, Diversity and Novelty of Soil Proteobacteria. ISME J. 2009, 3, 992–1000. [Google Scholar] [CrossRef]
- Tilgar, V.; Mänd, R.; Kilgas, P.; Mägi, M. Long-Term Consequences of Early Ontogeny in Free-Living Great Tits Parus major. J. Ornithol. 2010, 151, 61–68. [Google Scholar] [CrossRef]
- Barbraud, C.; Johnson, A.R.; Bertault, G. Phenotypic Correlates of Post-fledging Dispersal in a Population of Greater Flamingos: The Importance of Body Condition. J. Anim. Ecol. 2003, 72, 246–257. [Google Scholar] [CrossRef] [Green Version]
- McCasland, J. Neuronal Control of Bird Song Production. J. Neurosci. 1987, 7, 23–39. [Google Scholar] [CrossRef] [Green Version]
- Marusak, R.A.; Guy, J.S.; Abdul-Aziz, T.A.; West, M.A.; Fletcher, O.J.; Day, J.M.; Zsak, L.; Barnes, H.J. Parvovirus-Associated Cerebellar Hypoplasia and Hydrocephalus in Day Old Broiler Chickens. Avian Dis. 2010, 54, 156–160. [Google Scholar] [CrossRef]
- Harris, M.P. Age of Return to the Colony, Age of Breeding and Adult Survival of Manx Shearwaters. Bird Study 1966, 13, 84–95. [Google Scholar] [CrossRef]
- Ashcroft, R.E. Survival Rates and Breeding Biology of Puffins on Skomer Island, Wales. Ornis Scand. 1979, 10, 100–110. [Google Scholar] [CrossRef]
- Harms, R.H.; Damron, B.L.; Simpson, C.F. Effect of Wet Litter and Supplemental Biotin and/or Whey on the Production of Foot Pad Dermatitis in Broilers. Poult. Sci. 1977, 56, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Shepherd, E.M.; Fairchild, B.D. Footpad Dermatitis in Poultry. Poult. Sci. 2010, 89, 2043–2051. [Google Scholar] [CrossRef] [PubMed]
- De Jong, I.C.; Gunnink, H.; Van Harn, J. Wet Litter Not Only Induces Footpad Dermatitis but Also Reduces Overall Welfare, Technical Performance, and Carcass Yield in Broiler Chickens. J. Appl. Poult. Res. 2014, 23, 51–58. [Google Scholar] [CrossRef]
- Butterworth, A. Infectious Components of Broiler Lameness: A Review. World’s Poult. Sci. J. 1999, 55, 327–352. [Google Scholar] [CrossRef]
- Weaver, W.D., Jr.; Meijerhof, R. The Effect of Different Levels of Relative Humidity and Air Movement on Litter Conditions, Ammonia Levels, Growth, and Carcass Quality for Broiler Chickens. Poult. Sci. 1991, 70, 746–755. [Google Scholar] [CrossRef]
- Freeman, N.; Tuyttens, F.A.M.; Johnson, A.; Marshall, V.; Garmyn, A.; Jacobs, L. Remedying Contact Dermatitis in Broiler Chickens with Novel Flooring Treatments. Animals 2020, 10, 1761. [Google Scholar] [CrossRef]

| Phylum | Class | Order | Family | Genus | Alignment Length | % Identity | Count Abundance | % Abundance | Cumulative % Abundance |
|---|---|---|---|---|---|---|---|---|---|
| Proteobacteria | Gammaproteobacteria | Pseudomonadales | Pseudomonadaceae | Pseudomonas | 68 | 90.9 | 563 | 74.1 | 74.1 |
| Proteobacteria | Gammaproteobacteria | Enterobacteriales | Enterobacteriaceae | Escherichia | 91 | 87.6 | 35 | 4.6 | 78.7 |
| Proteobacteria | Gammaproteobacteria | Pseudomonadales | Moraxellaceae | Acinetobacter | 75 | 82.5 | 19 | 2.5 | 81.2 |
| Proteobacteria | Gammaproteobacteria | Enterobacteriales | Enterobacteriaceae | Serratia | 53 | 94.7 | 14 | 1.8 | 83.0 |
| Proteobacteria | Gammaproteobacteria | Vibrionales | Vibrionaceae | Vibrio | 36 | 74.1 | 14 | 1.8 | 84.9 |
| Firmicutes | Bacilli | Bacillales | Listeriaceae | Listeria | 29 | 75.9 | 10 | 1.3 | 86.2 |
| Proteobacteria | Gammaproteobacteria | Alteromonadales | Shewanellaceae | Shewanella | 30 | 87.7 | 9 | 1.2 | 87.4 |
| Proteobacteria | Betaproteobacteria | Burkholderiales | Burkholderia | Burkholderia | 61 | 79.1 | 8 | 1.1 | 88.4 |
| Firmicutes | Bacilli | Lactobacillales | Enterococcaceae | Enterococcus | 64 | 96.2 | 7 | 0.9 | 89.3 |
| Proteobacteria | Gammaproteobacteria | Aeromonadales | Aeromonadaceae | Aeromonas | 55 | 97.3 | 5 | 0.7 | 90.0 |
| Proteobacteria | Betaproteobacteria | Burkholderiales | Oxalobacteraceae | Janthinobacterium | 66 | 87.9 | 5 | 0.7 | 90.7 |
| Firmicutes | Clostridia | Clostridiales | Clostridiaceae | Clostridium | 63 | 74.6 | 4 | 0.5 | 91.2 |
| Proteobacteria | Alphaproteobacteria | Rhizobiales | Beijerinckiaceae | Beijerinckia | 38 | 70.5 | 3 | 0.4 | 91.6 |
| Proteobacteria | Gammaproteobacteria | Unclassified | Unclassified | Endoriftia | 54 | 71.9 | 3 | 0.4 | 92.0 |
| Proteobacteria | Betaproteobacteria | Burkholderiales | Oxalobacteraceae | Herminiimonas | 57 | 84.8 | 3 | 0.4 | 92.4 |
| Proteobacteria | Gammaproteobacteria | Enterobacteriales | Enterobacteriaceae | Pantoea | 66 | 91.4 | 3 | 0.4 | 92.8 |
| Proteobacteria | Gammaproteobacteria | Enterobacteriales | Enterobacteriaceae | Providencia | 25 | 92.0 | 3 | 0.4 | 93.2 |
| Proteobacteria | Gammaproteobacteria | Enterobacteriales | Enterobacteriaceae | Yersinia | 61 | 85.1 | 3 | 0.4 | 93.6 |
| Proteobacteria | Alphaproteobacteria | Rhizobiales | Rhizobiaceae | Agrobacterium | 98 | 79.0 | 2 | 0.3 | 93.8 |
| Proteobacteria | Gammaproteobacteria | Pseudomonadales | Pseudomonadaceae | Azotobacter | 45 | 77.2 | 2 | 0.3 | 94.1 |
| Proteobacteria | Betaproteobacteria | Burkholderiales | Alcaligenaceae | Bordetella | 62 | 90.3 | 2 | 0.3 | 94.3 |
| Proteobacteria | Betaproteobacteria | Burkholderiales | Burkholderiaceae | Cupriavidus | 66 | 89.4 | 2 | 0.3 | 94.6 |
| Proteobacteria | Gammaproteobacteria | Enterobacteriales | Enterobacteriaceae | Enterobacter | 53 | 72.0 | 2 | 0.3 | 94.9 |
| Spirochaetes | Spriochaetes | Spirochaetales | Leptospiraceae | Leptospira | 77 | 74.0 | 2 | 0.3 | 95.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Esmonde, N.P.G.; Hanna, R.E.B.; Patel, J.G.; Smyth, V.J.; Caplat, P.; Smyth, W.; Jaggers, P.; Padget, O.; Guilford, T.; Perrins, C.; et al. Case Report of Puffinosis in a Manx Shearwater (Puffinus puffinus) Suggesting Environmental Aetiology. Animals 2022, 12, 3457. https://doi.org/10.3390/ani12243457
Esmonde NPG, Hanna REB, Patel JG, Smyth VJ, Caplat P, Smyth W, Jaggers P, Padget O, Guilford T, Perrins C, et al. Case Report of Puffinosis in a Manx Shearwater (Puffinus puffinus) Suggesting Environmental Aetiology. Animals. 2022; 12(24):3457. https://doi.org/10.3390/ani12243457
Chicago/Turabian StyleEsmonde, Niamh P. G., Robert E. B. Hanna, Jignasha G. Patel, Victoria J. Smyth, Paul Caplat, Wesley Smyth, Paris Jaggers, Oliver Padget, Tim Guilford, Chris Perrins, and et al. 2022. "Case Report of Puffinosis in a Manx Shearwater (Puffinus puffinus) Suggesting Environmental Aetiology" Animals 12, no. 24: 3457. https://doi.org/10.3390/ani12243457
APA StyleEsmonde, N. P. G., Hanna, R. E. B., Patel, J. G., Smyth, V. J., Caplat, P., Smyth, W., Jaggers, P., Padget, O., Guilford, T., Perrins, C., & Reid, N. (2022). Case Report of Puffinosis in a Manx Shearwater (Puffinus puffinus) Suggesting Environmental Aetiology. Animals, 12(24), 3457. https://doi.org/10.3390/ani12243457






