Effects of Protein-Chelated Zinc Combined with Mannan-Rich Fraction to Replace High-Dose Zinc Oxide on Growth Performance, Nutrient Digestibility, and Intestinal Health in Weaned Piglets
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Experimental Design
2.2. Sample Collection
2.3. Analytical Methods
2.4. Statistical Analysis
3. Results
3.1. Performance and Diarrhea
3.2. Fecal Zinc Concentration and Nutrient Digestibility
3.3. Serum Growth-Related Hormone
3.4. Intestinal Morphology
3.5. Intestinal Volatile Fatty Acid Concentration
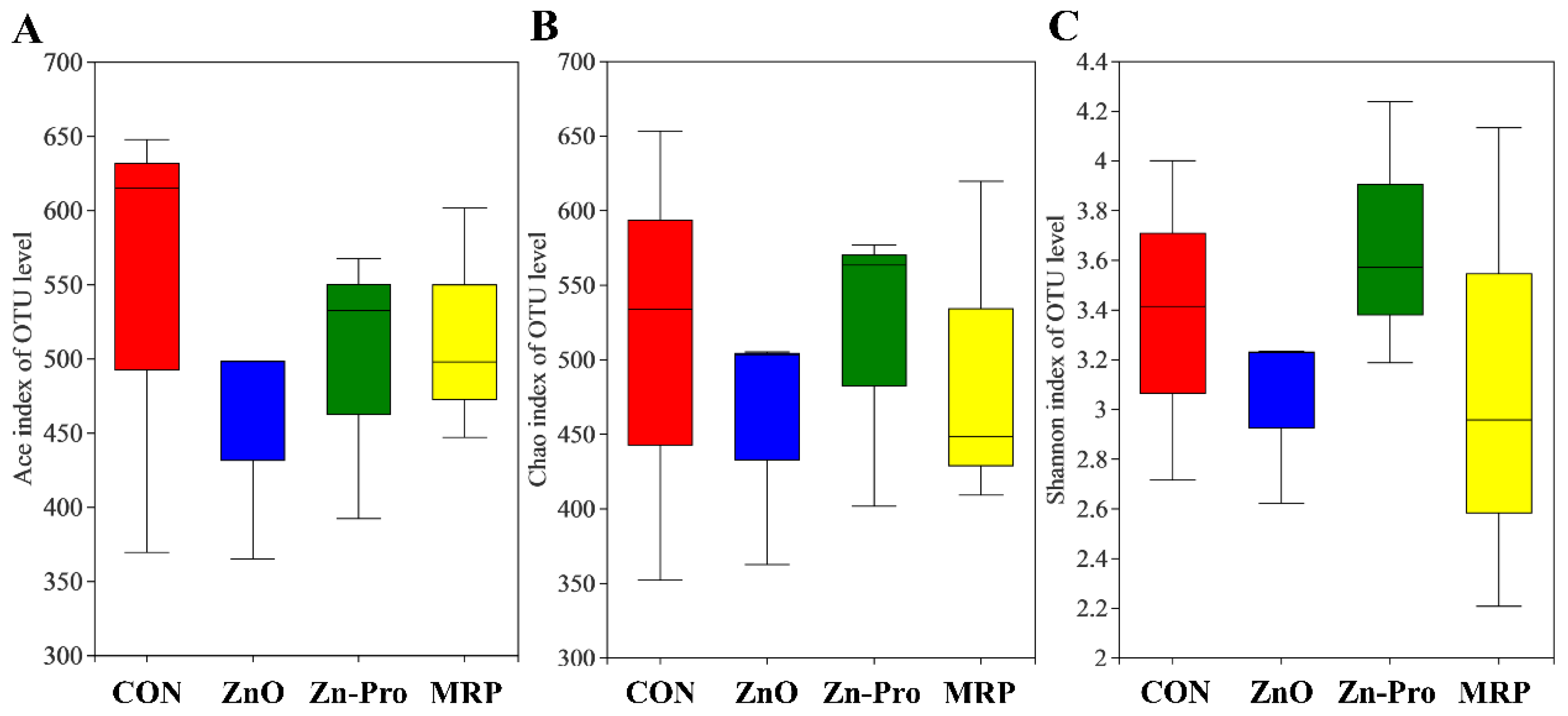
3.6. Microbiota Community
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tang, M.; Laarveld, B.; Van Kessel, A.G.; Hamilton, D.L.; Estrada, A.; Patience, J.F. Effect of segregated early weaning on postweaning small intestinal development in pigs. J. Anim. Sci. 1999, 77, 3191–3200. [Google Scholar] [CrossRef] [PubMed]
- Campbell, J.M.; Crenshaw, J.D.; Polo, J. The biological stress of early weaned piglets. J. Anim. Sci. Biotechnol. 2013, 4, 19. [Google Scholar] [CrossRef]
- Milani, N.C.; Sbardella, M.; Ikeda, N.Y.; Arnoc, A.; Mascarenhasd, B.C.; Miyadaa, V.S. Dietary zinc oxide nanoparticles as growth promoter for weanling pigs. Anim. Feed Sci. Technol. 2017, 227, 13–23. [Google Scholar] [CrossRef]
- Lei, X.J.; Liu, Z.Z.; Park, J.H.; Kim, I.H. Novel zinc sources as antimicrobial growth promoters for monogastric animals: A review. J. Anim. Sci. Technol. 2022, 64, 187–196. [Google Scholar] [CrossRef]
- Vahjen, W.; Pietruszyńska, D.; Starke, I.C.; Zentek, J. High dietary zinc supplementation increases the occurrence of tetracycline and sulfonamide resistance genes in the intestine of weaned pigs. Gut. Pathog. 2015, 7, 23. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Xia, T.; Zhao, J.; Liu, L.; He, P.; Zhang, S.; Zhang, L. Moderate tetrabasic zinc chloride supplementation improves growth performance and reduces diarrhea incidence in weaned pigs. Asian Austral. J. Anim. Sci. 2019, 33, 264–276. [Google Scholar] [CrossRef]
- Meyer, T.A.; Lindemann, M.D.; Cromwell, G.L.; Monegue, H.J.; Inocencio, N. Effects of pharmacological levels of zinc as zinc oxide on fecal zinc and mineral excretion in weanling pigs. Prof. Anim. Sci. 2002, 18, 162–168. [Google Scholar] [CrossRef]
- Ministry of Agriculture of the People’s Republic of China. Announcement of the Ministry of Agriculture of the People’s Republic of China; Ministry of Agriculture of the People’s Republic of China: Beijing, China, 2017.
- European Medicines Agency. European Medicines Agency-News and Events. In Proceedings of the Committee for Medicinal Products for Veterinary Use (CVMP) Meeting, London, UK, 17–19 May 2016. [Google Scholar]
- Oh, H.J.; Kim, M.H.; Song, M.H.; Lee, J.H.; Kim, Y.J.; Chang, S.Y.; An, J.W.; Go, Y.B.; Song, D.C.; Cho, H.A.; et al. Effects of replacing medical zinc oxide with different ratios of inorganic: Organic zinc or reducing crude protein diet with mixed feed additives in weaned piglet diets. Animals 2021, 11, 3132. [Google Scholar] [CrossRef]
- Fouhse, J.M.; Dawson, K.; Graugnard, D.; Dyck, M.; Willing, B.P. Dietary supplementation of weaned piglets with a yeast-derived mannan-rich fraction modulates cecal microbial profiles, jejunal morphology and gene expression. Animal 2019, 13, 1591–1598. [Google Scholar] [CrossRef]
- Zhang, G.; Zhao, J.B.; Dong, W.X.; Song, X.M.; Lin, G.; Li, D.F.; Zhang, S. Yeast-derived mannan-rich fraction as an alternative for zinc oxide to alleviate diarrhea incidence and improve growth performance in weaned pigs. Anim. Feed Sci. Technol. 2021, 281, 115111. [Google Scholar] [CrossRef]
- Spring, P.; Wenk, C.; Connolly, A.; Kiers, A. A review of 733 published trials on Bio-Mos®, a mannan oligosaccharide, and Actigen®, a second generation mannose rich fraction, on farm and companion animals. J. Appl. Anim. Nutr. 2015, 3, 1–11. [Google Scholar] [CrossRef]
- Davis, M.E.; Brown, D.C.; Maxwell, C.V.; Johnson, Z.B.; Kegley, E.B.; Dvorak, R.A. Effect of phosphorylated mannans and pharmacological additions of zinc oxide on growth and immunocompetence of weanling pigs. J. Anim. Sci. 2004, 82, 581–587. [Google Scholar] [CrossRef]
- Case, C.L.; Carlson, M.S. Effect of feeding organic and inorganic sources of additional zinc on growth performance and zinc balance in nursery pigs. J. Anim. Sci. 2002, 80, 1917–1924. [Google Scholar] [CrossRef] [PubMed]
- Edwards, M.V.; Edwards, A.C.; Millard, P.; Kocher, A. Mannose rich fraction of Saccharomyces cerevisiae promotes growth and enhances carcass yield in commercially housed grower-finisher pigs. Anim. Feed Sci. Technol. 2014, 197, 227–232. [Google Scholar] [CrossRef]
- Oh, H.-J.; Park, Y.-J.; Cho, J.H.; Song, M.-H.; Gu, B.-H.; Yun, W.; Lee, J.-H.; An, J.-S.; Kim, Y.-J.; Lee, J.-S.; et al. Changes in diarrhea score, nutrient digestibility, zinc utilization, intestinal immune profiles, and fecal microbiome in weaned piglets by different forms of zinc. Animals 2021, 11, 1356. [Google Scholar] [CrossRef]
- Wu, Y.; Ma, N.; Song, P.; He, T.; Levesque, C.; Bai, Y.; Zhang, A.; Ma, X. Grape seed proanthocyanidin affects lipid metabolism via changing gut microflora and enhancing propionate production in weaned pigs. J. Nutr. 2019, 149, 1523–1532. [Google Scholar] [CrossRef]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Pluske, J.R. Invited review: Aspects of gastrointestinal tract growth and maturation in the pre- and postweaning period of pigs. J. Anim. Sci. 2016, 94, 399–411. [Google Scholar] [CrossRef]
- Satessa, G.D.; Kjeldsen, N.J.; Mansouryar, M.; Hansen, H.H.; Bache, J.K.; Nielsen, M.O. Effects of alternative feed additives to medicinal zinc oxide on productivity, diarrhoea incidence and gut development in weaned piglets. Animal 2020, 14, 1638–1646. [Google Scholar] [CrossRef]
- Yin, J.; Li, X.; Li, D.; Yue, T.; Fang, Q.; Ni, J.; Zhou, X.; Wu, G. Dietary supplementation with zinc oxide stimulates ghrelin secretion from the stomach of young pigs. J. Nutr. Biochem. 2009, 20, 783–790. [Google Scholar] [CrossRef]
- Christensen, B.; Zhu, C.; Mohammadigheisar, M.; Schulze, H.; Huber, L.A.; Kiarie, E.G. Growth performance, immune status, gastrointestinal tract ecology, and function in nursery pigs fed enzymatically treated yeast without or with pharmacological levels of zinc. J. Anim. Sci. 2022, 100, skac094. [Google Scholar] [CrossRef] [PubMed]
- Halas, V.; Nochta, I. Mannan oligosaccharides in nursery pig nutrition and their potential mode of action. Animals 2012, 2, 261–274. [Google Scholar] [CrossRef] [PubMed]
- Castillo, M.; Martín-Orúe, S.M.; Taylor-Pickard, J.A.; Pérez, J.F.; Gasa, J. Use of mannan-oligosaccharides and zinc chelate as growth promoters and diarrhea preventative in weaning pigs: Effects on microbiota and gut function. J. Anim. Sci. 2008, 86, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Long, S.; He, T.; Kim, S.W.; Shang, Q.; Kiros, T.; Mahfuz, S.U.; Wang, C.; Piao, X. Live yeast or live yeast combined with zinc oxide enhanced growth performance, antioxidative capacity, immunoglobulins and gut health in nursery pigs. Animals 2021, 11, 1626. [Google Scholar] [CrossRef] [PubMed]
- Lei, X.J.; Kim, I.H. Low dose of coated zinc oxide is as effective as pharmacological zinc oxide in promoting growth performance, reducing fecal scores, and improving nutrient digestibility and intestinal morphology in weaned pigs. Anim. Feed Sci. Technol. 2018, 245, 117–125. [Google Scholar] [CrossRef]
- Zhang, G.; Hu, G.; Yang, Z.; Zhao, J.B. Effects of tetrabasic zinc chloride on growth performance, nutrient digestibility and fecal microbial community in weaned piglets. Front. Vet. Sci. 2022, 9, 905242. [Google Scholar] [CrossRef]
- Hansen, S.V.; Nørskov, N.P.; Nørgaard, J.V.; Woyengo, T.A.; Poulsen, H.D.; Nielsen, T.S. Determination of the optimal level of dietary zinc for newly weaned pigs: A dose-response study. Animals 2022, 12, 1552. [Google Scholar] [CrossRef]
- Carlson, M.S.; Boren, C.A.; Wu, C.; Huntington, C.E.; Bollinger, D.W.; Veum, T.L. Evaluation of various inclusion rates of organic zinc either as polysaccharide or proteinate complex on the growth performance, plasma, and excretion of nursery pigs. J. Anim. Sci. 2004, 82, 1359–1366. [Google Scholar] [CrossRef]
- Buff, C.E.; Bollinger, D.W.; Ellersieck, M.R.; Brommelsiek, W.A.; Veum, T.L. Comparison of growth performance and zinc absorption, retention, and excretion in weanling pigs fed diets supplemented with zinc-polysaccharide or zinc oxide1. J. Anim. Sci. 2005, 83, 2380–2386. [Google Scholar] [CrossRef]
- Bonetti, A.; Tugnoli, B.; Piva, A.; Grilli, E. Towards zero zinc oxide: Feeding strategies to manage post-weaning diarrhea in piglets. Animals 2021, 11, 642. [Google Scholar] [CrossRef]
- Duan, X.; Tian, G.; Chen, D.; Huang, L.; Zhang, D.; Zheng, P.; Mao, X.; Yu, J.; He, J.; Huang, Z.; et al. Mannan oligosaccharide supplementation in diets of sow and (or) their offspring improved immunity and regulated intestinal bacteria in piglet. J. Anim. Sci. 2019, 97, 4548–4556. [Google Scholar] [CrossRef] [PubMed]
- Bai, M.M.; Liu, H.N.; Xu, K.; Wen, C.Y.; Yu, R.; Deng, J.P.; Yin, Y.L. Use of coated nano zinc oxide as an additive to improve the zinc excretion and intestinal morphology of growing pigs. J. Anim. Sci. 2019, 97, 1772–1783. [Google Scholar] [CrossRef] [PubMed]
- Agazzi, A.; Perricone, V.; Omodei Zorini, F.; Sandrini, S.; Mariani, E.; Jiang, X.R.; Ferrari, A.; Crestani, M.; Nguyen, T.X.; Bontempo, V.; et al. Dietary mannan oligosaccharides modulate gut inflammatory response and improve duodenal villi height in post-weaning piglets improving feed efficiency. Animals 2020, 10, 1283. [Google Scholar] [CrossRef]
- Yu, E.; Chen, D.; Yu, B.; Huang, Z.; Mao, X.; Zheng, P.; Luo, Y.; Yin, H.; Yu, J.; Luo, J.; et al. Manno-oligosaccharide attenuates inflammation and intestinal epithelium injury in weaned pigs upon enterotoxigenic Escherichia coli K88 challenge. Br. J. Nutr. 2021, 126, 993–1002. [Google Scholar] [CrossRef]
- Carlson, D.; Poulsen, H.D.; Vestergaard, M. Additional dietary zinc for weaning piglets is associated with elevated concentrations of serum IGF-I. J. Anim. Physiol. Anim. Nutr. 2004, 88, 332–339. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Guo, Y. The growth-promoting effect of tetrabasic zinc chloride is associated with elevated concentration of growth hormone and ghrelin. Asian Austral. J. Anim. Sci. 2008, 21, 1473–1478. [Google Scholar] [CrossRef]
- Matteri, R.L.; Dyer, C.J.; Touchette, K.J.; Carroll, J.A.; Allee, G.L. Effects of weaning on somatotrophic gene expression and circulating levels of insulin-like growth factor-1 (IGF-1) and IGF-2 in pigs. Domest. Anim. Endocrinol. 2000, 19, 247–259. [Google Scholar] [CrossRef]
- Li, M.Z.; Huang, J.T.; Tsai, Y.H.; Mao, S.Y.; Fu, C.M.; Lien, T.F. Nanosize of zinc oxide and the effects on zinc digestibility, growth performances, immune response and serum parameters of weanling piglets. Anim. Sci. J. 2016, 87, 1379–1385. [Google Scholar] [CrossRef]
- Kaakoush, N.O. Insights into the role of Erysipelotrichaceae in the human host. Front. Cell. Infect. Microbiol. 2015, 5, 84, eCollection 2015. [Google Scholar] [CrossRef]
- Sun, Y.B.; Xia, T.; Wu, H.; Zhang, W.J.; Zhu, Y.H.; Xue, J.X.; He, D.T.; Zhang, L.Y. Effects of nano zinc oxide as an alternative to pharmacological dose of zinc oxide on growth performance, diarrhea, immune responses, and intestinal microflora profile in weaned piglets. Anim. Feed Sci. Technol. 2019, 258, 114312. [Google Scholar] [CrossRef]
- Starke, I.C.; Pieper, R.; Neumann, K.; Zentek, J.; Vahjen, W. The impact of high dietary zinc oxide on the development of the intestinal microbiota in weaned piglets. FEMS Microbiol. Ecol. 2014, 87, 416–427. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.; Zhu, C.; Chen, S.; Gao, L.; Lv, H.; Feng, R.; Zhu, Q.; Xu, J.; Chen, Z.; Jiang, Z. Dietary high zinc oxide modulates the microbiome of ileum and colon in weaned piglets. Front. Microbiol. 2017, 8, 825. [Google Scholar] [CrossRef] [PubMed]
- Shang, Q.; Liu, S.; Liu, H.; Mahfuz, S.; Piao, X. Impact of sugar beet pulp and wheat bran on serum biochemical profile, inflammatory responses and gut microbiota in sows during late gestation and lactation. J. Anim. Sci. Biotechnol. 2021, 12, 54. [Google Scholar] [CrossRef] [PubMed]
- Xia, T.; Lai, W.; Han, M.; Han, M.; Ma, X.; Zhang, L. Dietary ZnO nanoparticles alters intestinal microbiota and inflammation response in weaned piglets. Oncotarget 2017, 8, 64878–64891. [Google Scholar] [CrossRef] [PubMed]
- Benítez-Páez, A.; Kjølbæk, L.; Gómez Del Pulgar, E.M.; Brahe, L.K.; Astrup, A.; Matysik, S.; Schött, H.F.; Krautbauer, S.; Liebisch, G.; Boberska, J.; et al. A multi-omics approach to unraveling the microbiome-mediated effects of arabinoxylan oligosaccharides in overweight humans. mSystems 2019, 4, e00209-19. [Google Scholar] [CrossRef]
- Zou, J.; Shen, Y.; Chen, M.; Zhang, Z.; Xiao, S.; Liu, C.; Wan, Y.; Yang, L.; Jiang, S.; Shang, E.; et al. Lizhong decoction ameliorates ulcerative colitis in mice via modulating gut microbiota and its metabolites. Appl. Microbiol. Biotechnol. 2020, 104, 5999–6012. [Google Scholar] [CrossRef]
- Ma, J.; Piao, X.; Mahfuz, S.; Long, S.; Wang, J. The interaction among gut microbes, the intestinal barrier and short chain fatty acids. Anim. Nutr. 2021, 9, 159–174. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, H.; Zhu, W.; Yu, K.F. Cecal infusion of sodium propionate promotes intestinal development and jejunal barrier function in growing pigs. Animals 2019, 9, 284. [Google Scholar] [CrossRef]
- Yang, Y.; Iji, P.A.; Choct, M. Effects of different dietary levels of mannanoligosaccharide on growth performance and gut development of broiler chickens. Asian Austral. J. Anim. Sci. 2007, 20, 1084–1091. [Google Scholar] [CrossRef]
| Items | Content |
|---|---|
| Ingredients | |
| Corn | 60.54 |
| Soybean meal | 10.00 |
| Extruded soybean | 9.00 |
| Whey power | 5.00 |
| Soy protein concentrate | 4.00 |
| Fish meal | 3.00 |
| Soybean oil | 2.50 |
| Glucose | 2.00 |
| Dicalcium phosphate | 1.14 |
| Limestone | 0.87 |
| Salt | 0.15 |
| L-Lys HCl, 78% | 0.62 |
| DL-Met, 98% | 0.11 |
| L-Trp, 98% | 0.05 |
| L-Thr, 98% | 0.22 |
| Chromic oxide | 0.30 |
| Premix 1 | 0.50 |
| Total | 100.00 |
| Nutrient levels 2 | |
| Metabolizable energy, kcal/kg | 3410 |
| Total Ca | 0.80 |
| Total P | 0.59 |
| Available P | 0.40 |
| SID Trp | 0.22 |
| SID Met | 0.40 |
| SID Thr | 0.79 |
| SID Lys | 1.35 |
| Analyzed composition | |
| Dry matter | 88.72 |
| Crude protein | 18.21 |
| Neutral detergent fiber | 86.70 |
| Acid detergent fiber | 32.60 |
| Ash | 51.40 |
| Item | Groups | SEM | p-Value | |||
|---|---|---|---|---|---|---|
| CON | ZnO | Zn-Pro | MRP | |||
| Initial weight/kg (d 28 of birth) | 7.71 | 7.70 | 7.70 | 7.69 | 0.01 | 0.88 |
| The weight on d 14 (d 42 of birth) | 11.62 | 11.89 | 11.69 | 11.90 | 0.20 | 0.71 |
| Final weight/kg (d 56 of birth) | 17.38y | 18.19 xy | 17.65 xy | 18.53 x | 0.31 | 0.07 |
| d 1 to 14 of experiment | ||||||
| ADG/g | 279 | 299 | 295 | 310 | 11.92 | 0.37 |
| ADFI/g | 440 | 457 | 451 | 459 | 12.68 | 0.71 |
| Feed/Gain | 1.58 | 1.53 | 1.53 | 1.49 | 0.03 | 0.29 |
| Diarrhea rate/% | 17.01 a | 5.95 b | 9.52 b | 8.67 b | 1.47 | <0.01 |
| d 15 to 28 of experiment | ||||||
| ADG/g | 412 b | 450 ab | 420 b | 476 a | 12.45 | <0.01 |
| ADFI/g | 704 y | 760 x | 714 y | 760 x | 19.17 | 0.10 |
| Feed/Gain | 1.72 | 1.68 | 1.70 | 1.60 | 0.04 | 0.16 |
| Diarrhea rate/% | 7.14 a | 2.72 b | 4.42 b | 1.36 b | 0.85 | <0.01 |
| d 1 to 28 of experiment | ||||||
| ADG/g | 346 b | 374 ab | 358 b | 393 a | 9.73 | 0.02 |
| ADFI/g | 572 | 608 | 582 | 609 | 12.87 | 0.10 |
| Feed/Gain | 1.66 | 1.62 | 1.63 | 1.55 | 0.03 | 0.11 |
| Diarrhea rate/% | 12.07 a | 4.34 b | 6.97 b | 5.02 b | 0.93 | <0.01 |
| Item, % | Groups | SEM | p-Value | |||
|---|---|---|---|---|---|---|
| CON | ZnO | Zn-Pro | MRP | |||
| Apparent nutrient digestibility | ||||||
| Gross energy | 80.66 y | 82.01 xy | 81.98 xy | 82.82 x | 0.56 | 0.09 |
| Dry matter | 81.15 b | 82.41 ab | 82.09 ab | 83.07 a | 0.45 | 0.05 |
| Organic matter | 83.52 b | 84.90 ab | 84.72 ab | 85.49 a | 0.45 | 0.04 |
| Crude protein | 73.81 b | 74.95 ab | 75.60 ab | 76.92 a | 0.77 | 0.05 |
| Neutral detergent fiber | 52.61 | 54.40 | 55.12 | 58.37 | 1.89 | 0.22 |
| Acid detergent fiber | 49.28 | 47.83 | 51.90 | 57.70 | 2.67 | 0.10 |
| Feces | ||||||
| Zn, g/kg DM | 0.25 c | 12.51 a | 0.81 b | 0.69 b | 0.04 | <0.01 |
| Items | Groups | SEM | p-Value | |||
|---|---|---|---|---|---|---|
| CON | ZnO | Zn-Pro | MRP | |||
| d 14 of experiment | ||||||
| Growth hormone/(ng/mL) | 3.90 | 4.41 | 3.80 | 4.40 | 0.23 | 0.16 |
| Insulin-like growth factor-1/(ng/mL) | 139.30 b | 136.36 b | 128.27 b | 179.05 a | 8.22 | <0.01 |
| Ghrelin/(ng/mL) | 61.79 | 70.92 | 57.61 | 70.58 | 4.38 | 0.11 |
| d 28 of experiment | ||||||
| Growth hormone/(ng/mL) | 6.76 ab | 7.18 a | 5.38 b | 5.99 ab | 0.44 | 0.04 |
| Insulin-like growth factor-1/(ng/mL) | 246.70 | 267.30 | 260.61 | 278.24 | 9.85 | 0.18 |
| Ghrelin/(ng/mL) | 81.96 | 87.48 | 78.55 | 105.34 | 8.56 | 0.16 |
| Items | Groups | SEM | p-Value | |||
|---|---|---|---|---|---|---|
| CON | ZnO | Zn-Pro | MRP | |||
| Jejunum | ||||||
| Villus height/(μm) | 292 | 289 | 284 | 313 | 22.4 | 0.82 |
| Crypt depth/(μm) | 180 | 159 | 159 | 157 | 9.00 | 0.26 |
| Villus height/Crypt depth | 1.63 | 1.84 | 1.84 | 2.02 | 0.21 | 0.65 |
| Ileum | ||||||
| Villus height/(μm) | 220 y | 240 xy | 227 xy | 255 x | 9.36 | 0.09 |
| Crypt depth/(μm) | 180 | 159 | 154 | 158 | 8.70 | 0.21 |
| Villus height/Crypt depth | 1.24 | 1.52 | 1.49 | 1.63 | 0.12 | 0.18 |
| Items, mg/g | Groups | SEM | p-Value | |||
|---|---|---|---|---|---|---|
| CON | ZnO | Zn-Pro | MRP | |||
| Cecum | ||||||
| Lactic acid | 0.64 | 0.44 | 1.37 | 0.58 | 0.51 | 0.58 |
| Acetic acid | 4.59 c | 5.28 ab | 5.69 a | 4.94 bc | 0.18 | <0.01 |
| Propionic acid | 2.83 b | 3.37 ab | 2.99 b | 3.73 a | 0.18 | 0.01 |
| Butyric acid | 1.25 | 1.26 | 1.57 | 1.31 | 0.16 | 0.50 |
| Valeric acid | 0.07 | 0.08 | 0.11 | 0.04 | 0.03 | 0.47 |
| Total volatile fatty acids | 9.59 b | 10.47 ab | 11.77 a | 10.62 ab | 0.42 | 0.02 |
| Colon | ||||||
| Lactic acid | 0.31 | 0.21 | 0.07 | 0.02 | 0.08 | 0.13 |
| Acetic acid | 5.18 | 5.43 | 5.50 | 5.15 | 0.17 | 0.37 |
| Propionic acid | 2.92 | 3.44 | 3.11 | 3.45 | 0.27 | 0.56 |
| Butyric acid | 1.40 | 1.70 | 1.76 | 1.84 | 0.17 | 0.31 |
| Valeric acid | 0.20 | 0.21 | 0.21 | 0.13 | 0.04 | 0.54 |
| Total volatile fatty acids | 10.12 | 11.19 | 10.82 | 10.42 | 0.53 | 0.54 |
| Items | Groups | SEM | p-Value | |||
|---|---|---|---|---|---|---|
| CON | ZnO | Zn-Pro | MRP | |||
| Phylum level | ||||||
| Firmicutes | 78.76 | 84.52 | 89.49 | 67.16 | 5.53 | 0.11 |
| Bacteroidetes | 19.23 | 13.59 | 6.39 | 30.10 | 5.39 | 0.11 |
| Family level | ||||||
| Ruminococcaceae | 26.72 x | 8.66 y | 17.50 xy | 16.29 xy | 4.81 | 0.09 |
| Clostridiaceae | 11.36 | 30.63 | 1.70 | 19.20 | 9.21 | 0.15 |
| Prevotellaceae | 15.28 ab | 12.04 ab | 0.85 b | 27.45 a | 5.09 | 0.05 |
| Lactobacillaceae | 1.46 y | 19.38 xy | 27.73 x | 3.30 y | 8.80 | 0.08 |
| Erysipelotrichaceae | 21.56 | 1.54 | 2.47 | 6.47 | 5.40 | 0.13 |
| Lachnospiraceae | 3.50 y | 8.27 xy | 19.78 x | 2.77 y | 3.75 | 0.09 |
| unclassified_o_Lactobacillales | 0.01 b | 0.81 ab | 2.06 a | 0.01 b | 0.60 | 0.04 |
| Genus level | ||||||
| Clostridium_sensu_stricto_1 | 11.21 | 28.51 | 1.52 | 19.00 | 8.77 | 0.15 |
| Lactobacillus | 1.46 | 19.38 | 27.73 | 3.40 | 8.80 | 0.13 |
| Prevotella_2 | 4.76 xy | 3.49 xy | 0.03 y | 13.39 x | 6.66 | 0.09 |
| Ruminococcaceae_UCG-005 | 8.30 x | 0.73 y | 0.83 y | 1.98 xy | 1.08 | 0.06 |
| Prevotella_9 | 2.95 | 2.42 | 0.27 | 4.06 | 1.33 | 0.17 |
| Prevotellaceae_NK3B31_group | 2.04 xy | 2.29 xy | 0.15 y | 3.03 x | 0.84 | 0.08 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, G.; Zhao, J.; Lin, G.; Guo, Y.; Li, D.; Wu, Y. Effects of Protein-Chelated Zinc Combined with Mannan-Rich Fraction to Replace High-Dose Zinc Oxide on Growth Performance, Nutrient Digestibility, and Intestinal Health in Weaned Piglets. Animals 2022, 12, 3407. https://doi.org/10.3390/ani12233407
Zhang G, Zhao J, Lin G, Guo Y, Li D, Wu Y. Effects of Protein-Chelated Zinc Combined with Mannan-Rich Fraction to Replace High-Dose Zinc Oxide on Growth Performance, Nutrient Digestibility, and Intestinal Health in Weaned Piglets. Animals. 2022; 12(23):3407. https://doi.org/10.3390/ani12233407
Chicago/Turabian StyleZhang, Gang, Jinbiao Zhao, Gang Lin, Yuhan Guo, Defa Li, and Yi Wu. 2022. "Effects of Protein-Chelated Zinc Combined with Mannan-Rich Fraction to Replace High-Dose Zinc Oxide on Growth Performance, Nutrient Digestibility, and Intestinal Health in Weaned Piglets" Animals 12, no. 23: 3407. https://doi.org/10.3390/ani12233407
APA StyleZhang, G., Zhao, J., Lin, G., Guo, Y., Li, D., & Wu, Y. (2022). Effects of Protein-Chelated Zinc Combined with Mannan-Rich Fraction to Replace High-Dose Zinc Oxide on Growth Performance, Nutrient Digestibility, and Intestinal Health in Weaned Piglets. Animals, 12(23), 3407. https://doi.org/10.3390/ani12233407








