Gill Oxidative Stress Protection through the Use of Phytogenics and Galactomannan Oligosaccharides as Functional Additives in Practical Diets for European Sea Bass (Dicentrarchus labrax) Juveniles
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Diets
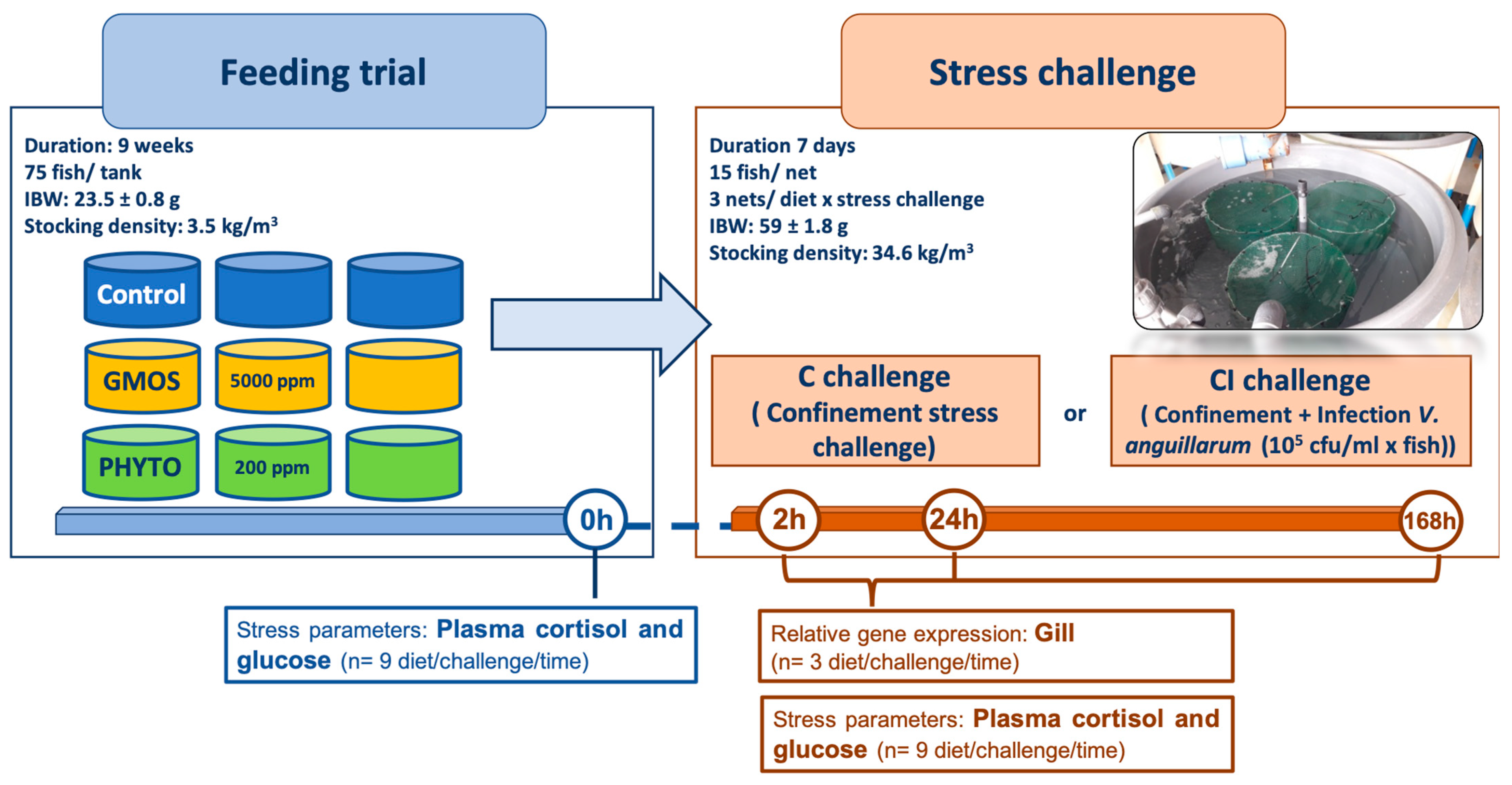
2.2. Feeding Trial
2.3. Stress Challenge
2.4. Sampling Methodology
2.5. RNA Extraction and Real-Time PCR Analysis
2.6. Statistical Analyses
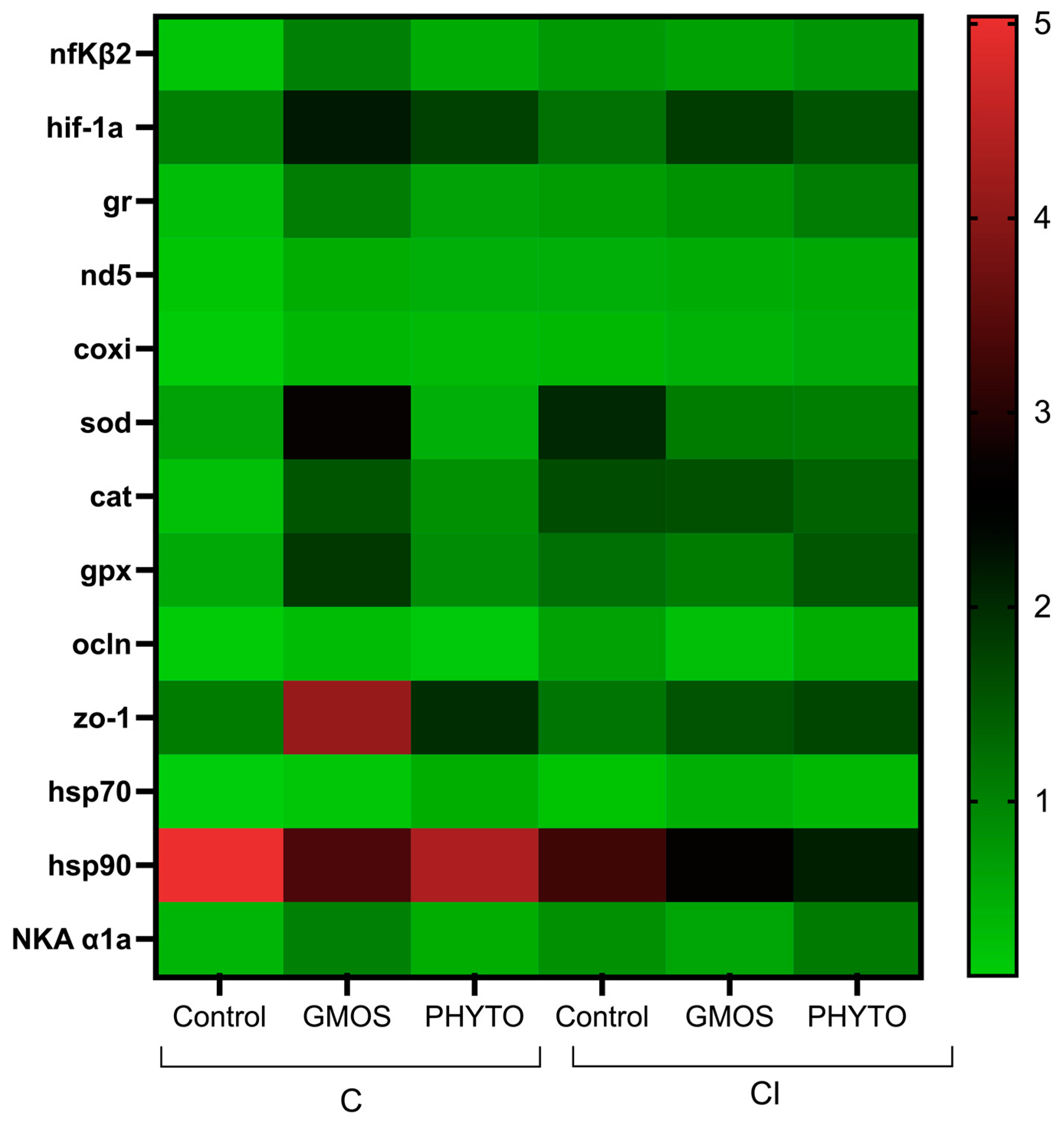
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ATP | (adenosine triphosphate) |
| C | challenge (confinement stress challenge) |
| cat | (catalase) |
| CI challenge | (confinement combined with infection stress challenge) |
| coxi | (cytochrome oxidase c) |
| ETC | (electron-transport chain) |
| FM | (fishmeal) |
| FO | (fish oil) |
| Fw | (forward primer sequence) |
| GIALT | (gill-associated lymphoid tissue) |
| GMOS | (galactomannan–oligosaccharides) |
| gpx | (glutathione peroxidase) |
| gr | (glucocorticoid receptor) |
| hif-1α | (hypoxia inducible factor 1 α ) |
| hsp70 | (heat-shock protein 70) |
| hsp90 | (heat-shock protein 90) |
| MAPKs | (mitogen-activated protein kinases) |
| MRCs | (mitochondria-rich cells) |
| nd5 | (NADH dehydrogenase subunit 5) |
| nfkb2 | (nuclear factor kappa beta) |
| NKA α 1a | (Na+/K+ ATPase) |
| ocln | (occludin) |
| PAMPs | (pathogen-associated molecular pattern) |
| PFAs | (phyoteginc feed additives) |
| PHYTO | (phytogenic) |
| ROS | (reactive oxygen species) |
| Rv | (reverse primer sequence) |
| sod | (superoxide dismutase) |
| zo-1 | (zonula occludens) |
References
- Ashley, P.J. Fish welfare: Current issues in aquaculture. Appl. Anim. Behav. Sci. 2007, 104, 199–235. [Google Scholar] [CrossRef]
- Montero, D.; Izquierdo, M. Welfare and health of fish fed vegetable oils as alternative lipid sources to fish oil. In Fish Oil Replacement and Alternative Lipid Sources in Aquaculture Feeds; CRC Press: Boca Raton, FL, USA, 2010. [Google Scholar]
- Oliva-Teles, A. Nutrition and health of aquaculture fish. J. Fish Dis. 2012, 35, 83–108. [Google Scholar] [CrossRef] [PubMed]
- Krogdahl, Å.; Bakke-McKellep, A.M.; Baeverfjord, G. Effects of graded levels of standard soybean meal on intestinal structure, mucosal enzyme activities, and pancreatic response in Atlantic salmon (Salmo salar L.). Aquac. Nutr. 2003, 9, 361–371. [Google Scholar] [CrossRef]
- Tort, L. Stress and immune modulation in fish. Dev. Comp. Immunol. 2011, 35, 1366–1375. [Google Scholar] [CrossRef]
- Schreck, C.B.; Tort, L. The concept of stress in fish. In Fish Physiology; Academic Press: Cambridge, MA, USA, 2016; Volume 35, pp. 1–34. [Google Scholar]
- Torrecillas, S.; Mompel, D.; Caballero, M.J.; Montero, D.; Merrifield, D.; Rodiles, A.; Robaina, L.; Zamorano, M.J.; Karalazos, V.; Kaushik, S.; et al. Effect of fishmeal and fish oil replacement by vegetable meals and oils on gut health of European sea bass (Dicentrarchus labrax). Aquaculture 2017, 468, 386–398. [Google Scholar] [CrossRef]
- Torrecillas, S.; Caballero, M.J.; Mompel, D.; Montero, D.; Zamorano, M.J.; Robaina, L.; Rivero-Ramírez, F.; Karalazos, V.; Kaushik, S.; Izquierdo, M. Disease resistance and response against Vibrio anguillarum intestinal infection in European seabass (Dicentrarchus labrax) fed low fish meal and fish oil diets. Fish Shellfish Immunol. 2017, 67, 302–311. [Google Scholar] [CrossRef]
- Hwang, P.P.; Lee, T.H.; Lin, L.Y. Ion regulation in fish gills: Recent progress in the cellular and molecular mechanisms. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2011, 301, R28–R47. [Google Scholar] [CrossRef]
- Li, M.; Zhu, X.; Tian, J.; Liu, M.; Wang, G. Dietary flavonoids from Allium mongolicum Regel promotes growth, improves immune, antioxidant status, immune-related signaling molecules and disease resistance in juvenile northern snakehead fish (Channa argus). Aquaculture 2019, 501, 473–481. [Google Scholar] [CrossRef]
- Jiao, W.; Han, Q.; Xu, Y.; Jiang, H.; Xing, H.; Teng, X. Impaired immune function and structural integrity in the gills of common carp (Cyprinus carpio L.) caused by chlorpyrifos exposure: Through oxidative stress and apoptosis. Fish Shellfish Immunol. 2019, 86, 239–245. [Google Scholar] [CrossRef]
- Rodnick, K.J.; Planas, J.V. The stress and stress mitigation effects of exercise: Cardiovascular, metabolic, and skeletal muscle adjustments. In Fish Physiology; Academic Press: Cambridge, MA, USA, 2016; Volume 35, pp. 251–294. [Google Scholar]
- Balasch, J.C.; Tort, L. Netting the stress responses in fish. Front. Endocrinol. 2019, 10, 62. [Google Scholar] [CrossRef]
- Lang, B.J.; Guerrero, M.E.; Prince, T.L.; Okusha, Y.; Bonorino, C.; Calderwood, S.K. The functions and regulation of heat shock proteins; key orchestrators of proteostasis and the heat shock response. Arch. Toxicol. 2021, 95, 1943–1970. [Google Scholar] [PubMed]
- Lingappan, K. NF-κB in oxidative stress. Curr. Opin. Toxicol. 2018, 7, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Korbecki, J.; Simińska, D.; Gąssowska-Dobrowolska, M.; Listos, J.; Gutowska, I.; Chlubek, D.; Baranowska-Bosiacka, I. Chronic and cycling hypoxia: Drivers of cancer chronic inflammation through HIF-1 and NF-κB activation: A review of the molecular mechanisms. Int. J. Mol. Sci. 2021, 22, 10701. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Liu, M.; Guo, H.; Zhu, K.; Liu, B.; Liu, B.; Zhang, N.; Zhang, D. ROS Induced by Streptococcus agalactiae Activate Inflammatory Responses via the TNF-α/NF-κB Signaling Pathway in Golden Pompano Trachinotus ovatus (Linnaeus, 1758). Antioxidants 2022, 11, 1809. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Feng, Y.; Zhang, L.; Jia, Y.; Cai, D.; Qian, S.-B.; Du, M.; Zhao, R. GR-mediated FTO transactivation induces lipid accumulation in hepatocytes via demethylation of m6A on lipogenic mRNAs. RNA Biol. 2020, 17, 930–942. [Google Scholar] [CrossRef] [PubMed]
- Ali, F.; Sultana, S. Repeated short-term stress synergizes the ROS signalling through up regulation of NFkB and iNOS expression induced due to combined exposure of trichloroethylene and UVB rays. Mol. Cell. Biochem. 2012, 360, 133–145. [Google Scholar] [CrossRef]
- Spiers, J.G.; Chen, H.J.C.; Sernia, C.; Lavidis, N.A. Activation of the hypothalamic-pituitary-adrenal stress axis induces cellular oxidative stress. Front. Neurosci. 2015, 8, 456. [Google Scholar] [CrossRef]
- Orrenius, S.; Gogvadze, V.; Zhivotovsky, B. Mitochondrial oxidative stress: Implications for cell death. Annu. Rev. Pharmacol. Toxicol. 2007, 47, 143–183. [Google Scholar] [CrossRef]
- Slaninova, A.; Smutna, M.; Modra, H.; Svobodova, Z. A Review: Oxidative Stress in Fish Induced by Pesticides. Neuro Endocrinol. Lett. 2009, 30 (Suppl. 1), 2–12. [Google Scholar]
- Agrahari, S.; Gopal, K. Inhibition of Na+-K+-ATPase in different tissues of freshwater fish Channa punctatus (Bloch) exposed to monocrotophos. Pestic. Biochem. Physiol. 2008, 92, 57–60. [Google Scholar] [CrossRef]
- Lee, J.W.; Choi, H.; Hwang, U.K.; Kang, J.C.; Kang, Y.J.; Kim, K.I.; Kim, J.H. Toxic effects of lead exposure on bioaccumulation, oxidative stress, neurotoxicity, and immune responses in fish: A review. Environ. Toxicol. Pharmacol. 2019, 68, 101–108. [Google Scholar] [CrossRef]
- Yılmaz, S.; Ergün, S. Effects of garlic and ginger oils on hematological and biochemical variables of sea bass Dicentrarchus labrax. J. Aquat. Anim. Health 2012, 24, 219–224. [Google Scholar] [CrossRef]
- Torrecillas, S.; Montero, D.; Izquierdo, M. Improved health and growth of fish fed mannan oligosaccharides: Potential mode of action. Fish Shellfish Immunol. 2014, 36, 525–544. [Google Scholar] [CrossRef]
- Huang, C.M.; Lee, T.T. Immunomodulatory effects of phytogenics in chickens and pigs—A review. Asian Australas. J. Anim. Sci. 2018, 31, 617–627. [Google Scholar] [CrossRef]
- Fernández-Montero, Á.; Torrecillas, S.; Acosta, F.; Kalinowski, T.; Bravo, J.; Sweetman, J.; Roo, J.; Makol, A.; Docando, J.; Carvalho, M.; et al. Improving greater amberjack (Seriola dumerili) defenses against monogenean parasite Neobenedenia girellae infection through functional dietary additives. Aquaculture 2021, 534, 736317. [Google Scholar] [CrossRef]
- Torrecillas, S.; Makol, A.; Caballero, M.J.; Montero, D.; Dhanasiri, A.K.S.; Sweetman, J.; Izquierdo, M. Effects on mortality and stress response in European sea bass, Dicentrarchus labrax (L.), fed mannan oligosaccharides (MOS) after Vibrio anguillarum exposure. J. Fish Dis. 2012, 35, 591–602. [Google Scholar] [CrossRef]
- Serradell, A.; Torrecillas Burriel, S.; Makol, A.; Acosta, F.; Valdenegro, V.; Montero, D. Functional additives in low fish meal and fish oil based diets for European sea bass (Dicentrarchus labrax): Effects on immune response, stress and disease resistance. Fish Shellfish Immunol. 2020, 91, 464–465. [Google Scholar] [CrossRef]
- Dawood, M.A.; El Basuini, M.F.; Zaineldin, A.I.; Yilmaz, S.; Hasan, M.T.; Ahmadifar, E.; El Asely, A.M.; Abdel-Latif, H.M.R.; Alagawany, M.; Abu-Elala, N.M.; et al. Antiparasitic and antibacterial functionality of essential oils: An alternative approach for sustainable aquaculture. Pathogens 2021, 10, 185. [Google Scholar] [CrossRef]
- Irkin, L.C.; Yigit, M.; Yilmaz, S.; Maita, M. Toxicological Evaluation of Dietary Garlic (Allium sativum) Powder in European Sea Bass Dicentrarchuslabrax Juveniles. Food Nutr. Sci. 2014, 5, 46429. [Google Scholar]
- Yonar, M.E.; Yonar, S.M.; İspir, Ü.; Ural, M.Ş. Effects of curcumin on haematological values, immunity, antioxidant status and resistance of rainbow trout (Oncorhynchus mykiss) against Aeromonas salmonicida subsp. achromogenes. Fish Shellfish Immunol. 2019, 89, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Hoseinifar, S.H.; Yousefi, S.; Van Doan, H.; Ashouri, G.; Gioacchini, G.; Maradonna, F.; Carnevali, O. Oxidative stress and antioxidant defense in fish: The implications of probiotic, prebiotic, and synbiotics. Rev. Fish. Sci. Aquac. 2020, 29, 198–217. [Google Scholar] [CrossRef]
- Fanouraki, E.; Mylonas, C.C.; Papandroulakis, N.; Pavlidis, M. Species specificity in the magnitude and duration of the acute stress response in Mediterranean marine fish in culture. Gen. Comp. Endocrinol. 2011, 173, 313–322. [Google Scholar] [CrossRef]
- Montero, D.; Terova, G.; Rimoldi, S.; Tort, L.; Negrin, D.; Zamorano, M.J.; Izquierdo, M. Modulation of adrenocorticotrophin hormone (ACTH)-induced expression of stress-related genes by PUFA in inter-renal cells from European sea bass (Dicentrarchus labrax). J. Nutr. Sci. 2015, 4, e16. [Google Scholar] [CrossRef]
- Samaras, A.; Pavlidis, M.; Lika, K.; Theodoridi, A.; Papandroulakis, N. Scale matters: Performance of European sea bass, Dicentrarchus labrax, L. (1758), reared in cages of different volumes. Aquac. Res. 2017, 48, 990–1005. [Google Scholar] [CrossRef]
- Caipang, C.M.A.; Suharman, I.; Avillanosa, A.L.; Gonzales-Plasus, M.M. Influence of phytogenic feed additives on the health status in the gut and disease resistance of cultured fish. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2021; Volume 695, p. 012024. [Google Scholar]
- Bozin, B.; Mimica-Dukic, N.; Samojlik, I.; Jovin, E. Antimicrobial and antioxidant properties of rosemary and sage (Rosmarinus officinalis L. and Salvia officinalis L., Lamiaceae) essential oils. J. Agric. Food Chem. 2007, 55, 7879–7885. [Google Scholar] [CrossRef] [PubMed]
- Militz, T.A.; Southgate, P.C.; Carton, A.G.; Hutson, K.S. Dietary supplementation of garlic (Allium sativum) to prevent monogenean infection in aquaculture. Aquaculture 2013, 408, 95–99. [Google Scholar] [CrossRef]
- Torrecillas, S.; Terova, G.; Makol, A.; Serradell, A.; Valdenegro, V.; Gini, E.; Izquierdo, M.S.; Acosta, F.; Montero, D. Dietary phytogenics and galactomannan oligosaccharides in low fish meal and fish oil-based diets for European sea bass (Dicentrarchus labrax) juveniles: Effects on gut health and implications on in vivo gut bacterial translocation. PLoS ONE 2019, 14, e0222063. [Google Scholar] [CrossRef]
- Torrecillas, S.; Terova, G.; Makol, A.; Serradell, A.; Valdenegro-Vega, V.; Izquierdo, M.; Acosta, F.; Montero, D. Dietary phytogenics and galactomannan oligosaccharides in low fish meal and fish oil-based diets for European sea bass (Dicentrarchus labrax) juveniles: Effects on gill structure and health and implications on oxidative stress status. Front. Immunol. 2021, 12, 663106. [Google Scholar] [CrossRef] [PubMed]
- Valenzuela-Gutiérrez, R.; Lago-Lestón, A.; Vargas-Albores, F.; Cicala, F.; Martínez-Porchas, M. Exploring the garlic (Allium sativum) properties for fish aquaculture. Fish Physiol. Biochem. 2021, 47, 1179–1198. [Google Scholar] [CrossRef]
- Guerreiro, I.; Serra, C.R.; Enes, P.; Couto, A.; Salvador, A.; Costas, B.; Oliva-Teles, A. Effect of short chain fructooligosaccharides (scFOS) on immunological status and gut microbiota of gilt- head sea bream (Sparus aurata) reared at two temperatures. Fish Shellfish Immunol. 2016, 49, 122–131. [Google Scholar] [CrossRef]
- Guardiola, F.A.; Bahi, A.; Messina, C.M.; Mahdhi, A.; Santulli, A.; Arena, R.; Bakhrouf, A.; Esteban, M.A. Quality and antioxidant response of gilthead seabream (Sparus aurata L.) to dietary supplements of fenugreek (Trigonella foenum graecum) alone or combined with probiotic strains. Fish Shellfish Immunol. 2017, 63, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Guerreiro, I.; Oliva-Teles, A.; Enes, P. Prebiotics as functional ingredients: Focus on Mediterranean fish aquaculture. Rev. Aquac. 2018, 10, 800–832. [Google Scholar] [CrossRef]
- Rimoldi, S.; Torrecillas, S.; Montero, D.; Gini, E.; Makol, A.; Valdenegro, V.V.; Izquierdo, M.S.; Terova, G. Assessment of dietary supplementation with galactomannan oligosaccharides and phytogenics on gut microbiota of European sea bass (Dicentrarchus labrax) fed low fishmeal and fish oil based diet. PLoS ONE 2020, 15, e0231494. [Google Scholar] [CrossRef] [PubMed]
- Lupatsch, I.; Santos, G.A.; Schrama, J.W.; Verreth, J.A.J. Effect of stocking density and feeding level on energy expenditure and stress responsiveness in European sea bass Dicentrarchus labrax. Aquaculture 2010, 298, 245–250. [Google Scholar] [CrossRef]
- Torrecillas, S.; Makol, A.; Caballero, M.J.; Montero, D.; Robaina, L.; Real, F.; Sweetman, J.; Tort, L.; Izquierdo, M.S. Immune stimulation and improved infection resistance in European sea bass (Dicentrarchus labrax) fed mannan oligosaccharides. Fish Shellfish Immunol. 2007, 23, 969–981. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Kokkinopoulou, I.; Moutsatsou, P. Mitochondrial Glucocorticoid Receptors and Their Actions. Int. J. Mol. Sci. 2021, 22, 6054. [Google Scholar] [CrossRef]
- Vijayan, M.M.; Raptis, S.; Sathiyaa, R. Cortisol treatment affects glucocorticoid receptor and glucocorticoid-responsive genes in the liver of rainbow trout. Gen. Comp. Endocrinol. 2003, 132, 256–263. [Google Scholar] [CrossRef]
- Eisner, V.; Picard, M.; Hajnóczky, G. Mitochondrial dynamics in adaptive and maladaptive cellular stress responses. Nat. Cell Biol. 2018, 20, 755–765. [Google Scholar] [CrossRef]
- Brunetti, C.; Di Ferdinando, M.; Fini, A.; Pollastri, S.; Tattini, M. Flavonoids as antioxidants and developmental regulators: Relative significance in plants and humans. Int. J. Mol. Sci. 2013, 14, 3540–3555. [Google Scholar] [CrossRef]
- Reyes-Cerpa, S.; Vallejos-Vidal, E.; Gonzalez-Bown, M.J.; Morales-Reyes, J.; Pérez-Stuardo, D.; Vargas, D.; Imarai, M.; Cifuente, V.; Pencer, E.; Sandino, A.M.; et al. Effect of yeast (Xanthophyllomyces dendrorhous) and plant (Saint John’s wort, lemon balm, and rosemary) extract based functional diets on antioxidant and immune status of Atlantic salmon (Salmo salar) subjected to crowding stress. Fish Shellfish Immunol. 2018, 74, 250–259. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.Z.; Jiang, S.; Zhang, L.; Yu, Z.B. Mitochondrial electron transport chain, ROS generation and uncoupling. Int. J. Mol. Med. 2019, 44, 3–15. [Google Scholar] [CrossRef]
- Diler, O.; Gormez, O.; Diler, I.; Metin, S. Effect of oregano (Origanum onites L.) essential oil on growth, lysozyme and antioxidant activity and resistance against Lactococcus garvieae in rainbow trout, Oncorhynchus mykiss (Walbaum). Aquac. Nutr. 2017, 23, 844–851. [Google Scholar] [CrossRef]
- Hoseini, S.M.; Yousefi, M. Beneficial effects of thyme (Thymus vulgaris) extract on oxytetracycline-induced stress response, immunosuppression, oxidative stress and enzymatic changes in rainbow trout (Oncorhynchus mykiss). Aquac. Nutr. 2019, 25, 298–309. [Google Scholar] [CrossRef]
- Mansour, A.T.; Espinosa, C.; García-Beltrán, J.M.; Miao, L.; Ceballos Francisco, D.C.; Alsaqufi, A.S.; Esteban, M. Dietary supplementation of drumstick tree, Moringa oleifera, improves mucosal immune response in skin and gills of seabream, Sparus aurata, and attenuates the effect of hydrogen peroxide exposure. Fish Physiol. Biochem. 2020, 46, 981–996. [Google Scholar] [CrossRef] [PubMed]
- Chasiotis, H.; Kolosov, D.; Bui, P.; Kelly, S.P. Tight junctions, tight junction proteins and paracellular permeability across the gill epithelium of fishes: A review. Respir. Physiol. Neurobiol. 2012, 184, 269–281. [Google Scholar] [CrossRef] [PubMed]
- Rao, R. Oxidative stress-induced disruption of epithelial and endothelial tight junctions. Front. Biosci. 2008, 13, 7210–7226. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Chen, Y.P.; Jiang, W.D.; Liu, Y.; Jiang, J.; Wu, P.; Zhao, J.; Kuang, S.Y.; Tang, L.; Tang, W.N.; et al. Modulation of immune response, physical barrier and related signaling factors in the gills of juvenile grass carp (Ctenopharyngodon idella) fed supplemented diet with phospholipids. Fish Shellfish Immunol. 2016, 48, 79–93. [Google Scholar] [CrossRef]
- Trujillo, J.; Molina-Jijón, E.; Medina-Campos, O.N.; Rodríguez-Muñoz, R.; Reyes, J.L.; Loredo, M.L.; Barrera-Oviedo, D.; Pinzón, E.; Rodríguez-Rangel, D.S.; Pedraza-Chaverri, J. Curcumin prevents cisplatin-induced decrease in the tight and adherens junctions: Relation to oxidative stress. Food Funct. 2016, 7, 279–293. [Google Scholar] [CrossRef]
- Basuroy, S.; Seth, A.; Elias, B.; Naren, A.P.; Rao, R. MAPK interacts with occludin and mediates EGF-induced prevention of tight junction disruption by hydrogen peroxide. Biochem. J. 2006, 393, 69–77. [Google Scholar] [CrossRef]
- Aguilar-Cazares, D.; Chavez-Dominguez, R.; Carlos-Reyes, A.; Lopez-Camarillo, C.; de la Cruz, O.N.H.; Lopez-Gonzalez, J.S. Contribution of angiogenesis to inflammation and cancer. Front. Oncol. 2019, 9, 1399. [Google Scholar] [CrossRef] [PubMed]
- Rességuier, J.; Dalum, A.S.; Du Pasquier, L.; Zhang, Y.; Koppang, E.O.; Boudinot, P.; Wiegertjes, G.F. Lymphoid tissue in teleost gills: Variations on a theme. Biology 2020, 9, 127. [Google Scholar] [CrossRef] [PubMed]
- Alagawany, M.; Farag, M.R.; Salah, A.S.; Mahmoud, M.A. The role of oregano herb and its derivatives as immunomodulators in fish. Rev. Aquac. 2020, 12, 2481–2492. [Google Scholar] [CrossRef]
- Alagawany, M.; Farag, M.R.; Abdelnour, S.A.; Elnesr, S.S. A review on the beneficial effect of thymol on health and production of fish. Rev. Aquac. 2021, 13, 632–641. [Google Scholar] [CrossRef]
- Alagawany, M.; Farag, M.R.; Abdelnour, S.A.; Dawood, M.A.; Elnesr, S.S.; Dhama, K. Curcumin and its different forms: A review on fish nutrition. Aquaculture 2021, 532, 736030. [Google Scholar] [CrossRef]
- Hoseinifar, S.H.; Mirvaghefi, A.; Amoozegar, M.A.; Sharifian, M.; Esteban, M.Á. Modulation of innate immune response, mucosal parameters and disease resistance in rainbow trout (Oncorhynchus mykiss) upon symbiotic feeding. Fish Shellfish Immunol. 2015, 45, 27–32. [Google Scholar] [CrossRef]
- Yilmaz, S.; Yilmaz, E.; Dawood, M.A.; Ringø, E.; Ahmadifar, E.; Abdel-Latif, H.M. Probiotics, prebiotics, and synbiotics used to control vibriosis in fish: A review. Aquaculture 2022, 547, 737514. [Google Scholar] [CrossRef]
- Biller, J.D.; Takahashi, L.S. Oxidative stress and fish immune system: Phagocytosis and leukocyte respiratory burst activity. An. Acad. Bras. Ciênc. 2018, 90, 3403–3414. [Google Scholar] [CrossRef]
- Eltzschig, H.K.; Carmeliet, P. Hypoxia and inflammation. N. Engl. J. Med. 2011, 364, 656–665. [Google Scholar] [CrossRef]
- Basu, N.; Todgham, A.E.; Ackerman, P.A.; Bibeau, M.R.; Nakano, K.; Schulte, P.M.; Iwama, G.K. Heat shock protein genes and their functional significance in fish. Gene 2002, 295, 173–183. [Google Scholar] [CrossRef]
- Roberts, R.J.; Agius, C.; Saliba, C.; Bossier, P.; Sung, Y.Y. Heat shock proteins (chaperones) in fish and shellfish and their potential role in relation to fish health: A review. J. Fish Dis. 2010, 33, 789–801. [Google Scholar] [CrossRef] [PubMed]
- Ikwegbue, P.C.; Masamba, P.; Oyinloye, B.E.; Kappo, A.P. Roles of heat shock proteins in apoptosis, oxidative stress, human inflammatory diseases, and cancer. Pharmaceuticals 2018, 11, 2. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Tang, L.; Lu, L.; Zhang, L.; Xi, L.; Liu, H.C.; Odle, J.; Luo, X. Differential expression of heat shock transcription factors and heat shock proteins after acute and chronic heat stress in laying chickens (Gallus gallus). PLoS ONE 2014, 9, e102204. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.N.; Li, X.F.; Tian, H.Y.; Zhang, D.D.; Jiang, G.Z.; Lu, K.L.; Liu, G.X.; Liu, W.B. Effects of fructooligosaccharide on immune response, antioxidant capability and HSP70 and HSP90 expressions of blunt snout bream (Megalobrama amblycephala) under high ammonia stress. Fish Physiol. Biochem. 2015, 41, 203–217. [Google Scholar] [CrossRef]
- Evans, D.H. Teleost fish osmoregulation: What have we learned since August Krogh, Homer Smith, and Ancel Keys. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008, 295, R704–R713. [Google Scholar] [CrossRef]
- Maiti, A.K.; Saha, N.C.; Paul, G. Effect of lead on oxidative stress, Na+ K+ ATPase activity and mitochondrial electron transport chain activity of the brain of Clarias batrachus L. Bull. Environ. Contam. Toxicol. 2010, 84, 672–676. [Google Scholar] [CrossRef]
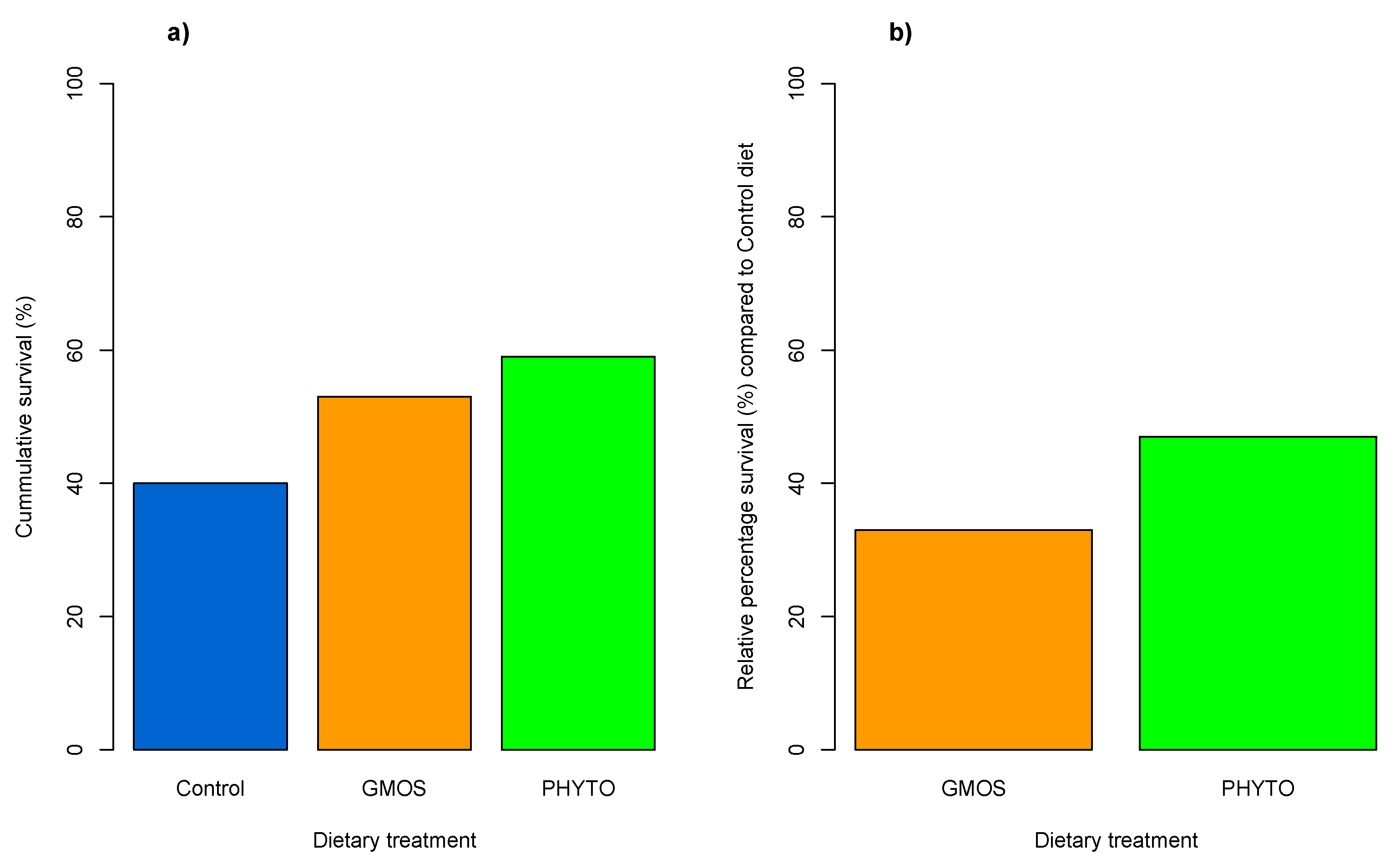


| Ingredients | Diet (%) | ||
|---|---|---|---|
| Control | GMOS | PHYTO | |
| Fishmeal 1 | 9.6 | 9.6 | 9.6 |
| Soya protein concentrate | 18.2 | 18.2 | 18.2 |
| Soya meal | 11.6 | 11.6 | 11.6 |
| Corn gluten meal | 24.1 | 24.1 | 24.1 |
| Wheat | 8.54 | 8.04 | 8.52 |
| Wheat gluten | 1.9 | 1.9 | 1.9 |
| Guar meal | 7.7 | 7.7 | 7.7 |
| Rapeseed extracted | 3.0 | 3.0 | 3.0 |
| Fish oil 2 | 6.5 | 6.5 | 6.5 |
| Rapeseed oil 3 | 5.2 | 5.2 | 5.2 |
| Vitamin and mineral premix 4 | 3.6 | 3.6 | 3.6 |
| Antioxidant 5 | 0.06 | 0.06 | 0.06 |
| Galactomannan–oligosaccharides (GMOS) 6 | 0 | 0.5 | 0 |
| Phytogenic 7 | 0 | 0 | 0.02 |
| Proximate composition (% of dry matter) | |||
| Crude lipids | 19.91 | 20.44 | 20.47 |
| Crude protein | 49.30 | 49.27 | 49.76 |
| Moisture | 5.10 | 5.01 | 5.06 |
| Ash | 7.02 | 6.41 | 6.49 |
| Gene | Accession Number | Primer | Nucleotide sequence 5′–3′ | Annealing T (°C) |
|---|---|---|---|---|
| nfΚβ2 | KM225790 | Fw | CTGGAGGAAACTGGCGGAGAAGC | 60 |
| Rv | CAGGTACAGGTGAGTCAGCGTCAC | |||
| hif-1α | DQ171936 | Fw | GACTTCAGCTGCCCTGATTC | 60 |
| Rv | GGCTGGTTTATAGCGCTGAG | |||
| gr | AY549305.1 | Fw | GTGGGCCTACAAGACCAGAA | 60 |
| Rv | CGGACGACTCTCCATACCTG | |||
| nd5 | KF857307 | Fw | CCCGATTTCTGTGCCCTACTA | 60 |
| Rv | AGGAAAGGAGTGCCTGTGA | |||
| coxi | KF857308 | Fw | ATACTTCACATCCGCAACCATAA | 60 |
| Rv | AAGCCTCCGACTGTAAATAAGAA | |||
| sod | FJ860004.1 | Fw | CATGTTGGAGACCTGGGAGA | 60 |
| Rv | TGAGCATCTTGTCCGTGATGT | |||
| cat | FJ860003.1 | Fw | TGGGACTTCTGGAGCCTGAG | 60 |
| Rv | GCAAACCTCGATCGCTGAAC | |||
| gpx | FM013606.1 | Fw | AGTTCGTGCAGTTAATCCGGA | 60 |
| Rv | GCTTAGCTGTCAGGTCGTAAAAC | |||
| zo-1 | MH321323.1 | Fw | CGGCCTGCAGATGTTCCTAA | 60 |
| Rv | GCTGAGGGAATTGGCTTTGA | |||
| ocln | MH321322.1 | Fw | GGACGAAGACGACAACAACGA | 60 |
| Rv | CCATGGGAGAAAGCCTCTGA | |||
| hsp70 | AY423555.2 | Fw | GGACATCAGCCAGAACAAGAGA | 60 |
| Rv | GCTGGAGGACAGGGTTCTC | |||
| hsp90 | AY395632 | Fw | GCTTCGAGGTCCTGTACATG | 62.7 |
| Rv | GCCTTATCCTCCTCCATC | |||
| NKA α1a | KP400258 | Fw | AACCTCAGATGGCAAGGAGAAG | 60 |
| Rv | GAGACTGGTACATTCAGGCGG | |||
| α-tub (hk) | AY326429.1 | Fw | AGGCTCATTGGCCAGATTGT | 60 |
| Rv | CAACATTCAGGGCTCCATCA | |||
| eEF1α1 | XM_051391260.1 | Fw | GTTGCTGCTGGTGTTGGTGAG | 60 |
| Rv | GAAACACACTGCTGGAGGCTC | |||
| β-act | AY148350.1 | Fw | TCTTCCAGCCTTCCTTCCTC | 60 |
| Rv | GATGTCAACGTCGCACTTCA |
| Confinement (C Challenge) | Confinement + Infection (CI Challenge) | |||||
|---|---|---|---|---|---|---|
| Plasma Cortisol (ng/mL) | Control | GMOS | PHYTO | Control | GMOS | PHYTO |
| 0 h (basal) | 5.82 ± 3.45 | 5.33 ± 7.06 | 4.67 ± 8.04 | 5.82 ± 3.45 | 5.33 ± 7.06 | 4.67 ± 8.04 |
| 2 h | 321.83 a ± 171.51 | 270.86 b ± 87.28 | 307.00 b ± 53.93 | 611.29 a ± 185.62 | 254.29 b ± 121.57 | 374.50 ab ± 133.29 |
| 24 h | 71.00 ± 46.67 | 22.20 ± 10.43 | 29.43 ± 10.13 | 47.80 a ± 38.79 | 145.33 b ± 68.55 | 77.50 b ± 38.28 |
| 168 h | 16.67 ± 4.73 | 15.60 ± 11.50 | 100.43 ± 76.97 | 14.17 b ± 18.17 | 16.40 b ± 15.16 | 217.43 a ± 96.14 |
| Plasma glucose (mg/dL) | ||||||
| 0 h (basal) | 67.63 ± 16.78 | 67.43 ± 10.66 | 67.71 ± 10.95 | 67.63 ± 16.78 | 67.43 ± 10.66 | 67.71 ± 10.95 |
| 2 h | 143.33 ± 69.61 | 156.60 ± 18.61 | 194.50 ± 16.26 | 236.00 a ± 55.48 | 131.33 b ± 48.58 | 158.00 b ± 22.63 |
| 24 h | 77.20 ± 17.54 | 96.50 ± 22.02 | 95.80 ± 16.08 | 102.33 ± 28.38 | 75.33 ± 10.98 | 80.50 ± 7.78 |
| 168 h | 75.20 ± 16.08 | 93.00 ± 20.17 | 124.00 ± 54.21 | 74.25 ± 7.46 | 87.00 ± 23.39 | 148.00 ± 26.87 |
| Confinement (C Challenge) | Confinement + Infection (CI Challenge) | |||||
|---|---|---|---|---|---|---|
| Control | GMOS | PHYTO | Control | GMOS | PHYTO | |
| nfΚβ2 | 1.02 a ± 0.26 | 0.2 b ± 0.22 | 0.29 b ± 0.22 | 0.99 a ± 0.20 | 0.2 b ± 0.03 | 0.23 b ± 0.10 |
| hif-1α | 1 a ± 0.01 | 0.3 b ± 0.1 | 0.4 b ± 0.2 | 1 a ± 0.2 | 0.4 b ± 0.01 | 0.6 b ± 0.1 |
| gr | 1.01 a ± 0.17 | 0.21 b ± 0.2 | 0.18 b ± 0.13 | 0.94 a ± 0.25 | 0.21 b ± 0.02 | 0.24 b ± 0.03 |
| nd5 | 1.06 a* ± 0.50 | 0.19 b ± 0.07 | 0.30 b ± 0.19 | 0.37 ** ± 0.07 | 0.2 ± 0.02 | 0.46 ± 0.24 |
| coxi | 1 a* ± 0.14 | 0.7 b ± 0.06 | 0.1 b ± 0.1 | 0.22 ** ± 0.09 | 0.1 ± 0.03 | 0.1 ± 0.07 |
| sod | 1.08 a ± 0.58 | 0.2 b ± 0.26 | 0.22 b ± 0.21 | 0.85 a ± 0.48 | 0.3 b ± 0.13 | 0.17 b ± 0.08 |
| cat | 1 a ± 0.09 | 0.19 b ± 0.16 | 0.26 b ± 0.2 | 0.73 ± 0.31 | 0.26 ± 0.17 | 0.35 ± 0.17 |
| gpx | 1 ± 0.13 | 0.94 ± 0.89 | 0.42 ± 0.18 | 3.57 a ± 2.36 | 0.42 b ± 0.26 | 0.62 b ± 0.26 |
| ocln | 1.01 ± 0.18 | 0.82 ± 0.74 | 0.8 ± 0.29 | 1.48 ± 0.75 | 0.78 ± 0.01 | 1.2 ± 0.38 |
| zo-1 | 1.08 ± 0.56 | 2.18 ± 0.62 | 3.02 ± 0.88 | 2.64 ± 1.7 | 3.59 ± 1.32 | 2.94 ± 1.33 |
| hsp70 | 1 a ± 0.09 | 0.11 b ± 0.08 | 0.19 b ± 0.13 | 0.56 ± 0.04 | 0.28 ± 0.2 | 0.16 ± 0.05 |
| hsp90 | 1 a ± 0.01 | 0.2 b ± 0.2 | 0.2 b ± 0.2 | 1.3 a ± 0.8 | 0.2 b ± 0.01 | 0.2 b ± 0.2 |
| NKA α1a | 1 a ± 0.11 | 0.28 b ± 0.32 | 0.26 b ± 0.13 | 1.48 a ± 0.18 | 0.17 b ± 0.01 | 0.44 b ± 0.26 |
| Confinement (C Challenge) | Confinement + Infection (CI Challenge) | |||||
|---|---|---|---|---|---|---|
| Control | GMOS | PHYTO | Control | GMOS | PHYTO | |
| nfΚβ2 | 0.22 b ± 0.05 | 1.01a ± 0.04 | 0.51ab ± 0.31 | 0.73 ± 0.15 | 0.66 ± 0.24 | 0.78 ± 0.11 |
| hif-1a | 1 ± 0.3 | 2.22 ± 0.9 | 1.82 ± 0.9 | 1.2 ± 0.2 | 1.8 ± 1 | 1.6 ± 0.2 |
| gr | 0.30 ± 0.08 | 1.08 ± 0.35 | 0.64 ± 0.37 | 0.71 ± 0.19 | 0.82 ± 0.52 | 1.07 ± 0.34 |
| nd5 | 0.21 ± 0.07 | 0.5 ± 0.19 | 0.48 ± 0.39 | 0.48 ± 0.2 | 0.52 ± 0.19 | 0.57 ± 0.29 |
| coxi | 0.12 ± 0.06 | 0.36 ± 0.14 | 0.33 ± 0.29 | 0.38 ± 0.10 | 0.45 ± 0.34 | 0.54 ± 0.4 |
| sod | 0.63 b* ± 0.23 | 2.72 a* ± 0.24 | 0.48 b ± 0.15 | 2.4 ** ± 0.03 | 1.1 ** ± 0.17 | 1.06 ± 0.17 |
| cat | 0.28 ± 0.17 | 1.53 ± 0.23 | 0.84 ± 0.63 | 1.64 ± 1.34 | 1.59 ± 0.99 | 1.38 ± 1.03 |
| gpx | 0.55 b ± 0.26 | 1.85 a ± 0.6 | 0.88 ab ± 0.57 | 1.24 ± 0.09 | 1.1 ± 0.49 | 1.51 ± 0.29 |
| ocln | 0.1 b ± 0.03 | 0.18 ab ± 0.11 | 0.49 a ± 0.3 | 0.24 ± 0.1 | 0.47 ± 0.07 | 0.38 ± 0.15 |
| zo-1 | 5.04 * ± 1.69 | 3.35 * ± 0.93 | 4.33 * ± 1.04 | 3.24 ** ± 0.6 | 2.68 ** ± 1.21 | 2.14 ** ± 1.08 |
| hsp70 | 0.12 * ± 0.04 | 0.32 ± 0.01 | 0.16 ± 0.04 | 0.63 ** ± 0.2 | 0.28 ± 0.13 | 0.51 ± 0.23 |
| hsp90 | 1.1 b ± 0.5 | 4.12 a ± 1.3 | 2 ab ± 1.9 | 1.2 ± 0.4 | 1.5 ± 1.7 | 1.7 ± 0.4 |
| NKA α1a | 0.39 ± 0.24 | 1.03 ± 0.45 | 0.51 ± 0.08 | 0.85 ± 0.42 | 0.6 ± 0.22 | 1.1 ± 0.09 |
| Confinement (C Challenge) | Confinement + Infection (CI Challenge) | |||||
|---|---|---|---|---|---|---|
| Control | GMOS | PHYTO | Control | GMOS | PHYTO | |
| nfΚβ2 | 0.27 ± 0.17 | 0.47 ± 0.18 | 0.40 ± 0.08 | 0.55 ± 0.08 | 0.34 ± 0.11 | 0.43 ± 0.10 |
| hif-1a | 1 ± 0.3 | 1.3 ± 0.3 | 1 ± 0.3 | 1.1 ± 0.2 | 0.7 ± 0.3 | 0.7 ± 0.2 |
| gr | 0.24 ± 0.18 | 0.46 ± 0.09 | 0.6 ± 0.13 | 0.53 a ± 0.04 | 0.27 b ± 0.11 | 0.65 a ± 0.17 |
| nd5 | 0.25 ± 0.16 | 0.26 ± 0.11 | 0.39 ± 0.11 | 0.35 ± 0.03 | 0.19 ± 0.06 | 0.46 ± 0.21 |
| coxi | 0.17 ± 0.16 | 0.17 ± 0.13 | 0.21 ± 0.07 | 0.28 ± 0.02 | 0.11 ± 0.06 | 0.29 ± 0.1 |
| sod | 0.31 * ± 0.2 | 0.47 ± 0.19 | 0.38 ± 0.19 | 1.6 a** ± 0.56 | 0.31 b ± 0.17 | 0.54 b ± 0.13 |
| cat | 0.37 ± 0.33 | 0.54 ± 0.33 | 0.59 ± 0.12 | 1.11 ± 0.62 | 0.51 ± 0.10 | 0.68 ± 0.25 |
| gpx | 0.74 ± 0.22 | 1.17 ± 0.19 | 1.02 ± 0.11 | 0.96 b ± 0.25 | 0.46 b ± 0.03 | 1.39 a ± 0.38 |
| ocln | 0.68 a* ± 0.09 | 0.51 ab*± 0.19 | 0.20 b ± 0.02 | 0.17 b** ± 0.02 | 0.89 a** ± 0.14 | 0.43 ab ± 0.01 |
| zo-1 | 2.47 ± 0.15 | 1.44 * ± 0.64 | 1.57 ± 0.66 | 2.62 ab ± 1.31 | 3.57 a** ± 0.62 | 0.86 b ± 0.11 |
| hsp70 | 0.12 * ± 0.05 | 0.17 ± 0.06 | 0.15 ± 0.06 | 0.47 ** ± 0.01 | 0.19 ± 0.05 | 0.26 ± 0.08 |
| hsp90 | 2 ± 2.1 | 4.5 * ± 1.4 | 3.3 ± 0.7 | 1.1 ± 0.3 | 0.7 ** ± 0.4 | 1.2 ± 0.1 |
| NKA α1a | 0.2 b* ± 0.1 | 0.51 a ± 0.04 | 0.37 ab ± 0.08 | 0.61 ** ± 0.11 | 0.64 ± 0.08 | 0.49 ± 0.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Serradell, A.; Montero, D.; Fernández-Montero, Á.; Terova, G.; Makol, A.; Valdenegro, V.; Acosta, F.; Izquierdo, M.S.; Torrecillas, S. Gill Oxidative Stress Protection through the Use of Phytogenics and Galactomannan Oligosaccharides as Functional Additives in Practical Diets for European Sea Bass (Dicentrarchus labrax) Juveniles. Animals 2022, 12, 3332. https://doi.org/10.3390/ani12233332
Serradell A, Montero D, Fernández-Montero Á, Terova G, Makol A, Valdenegro V, Acosta F, Izquierdo MS, Torrecillas S. Gill Oxidative Stress Protection through the Use of Phytogenics and Galactomannan Oligosaccharides as Functional Additives in Practical Diets for European Sea Bass (Dicentrarchus labrax) Juveniles. Animals. 2022; 12(23):3332. https://doi.org/10.3390/ani12233332
Chicago/Turabian StyleSerradell, Antonio, Daniel Montero, Álvaro Fernández-Montero, Genciana Terova, Alex Makol, Victoria Valdenegro, Félix Acosta, María Soledad Izquierdo, and Silvia Torrecillas. 2022. "Gill Oxidative Stress Protection through the Use of Phytogenics and Galactomannan Oligosaccharides as Functional Additives in Practical Diets for European Sea Bass (Dicentrarchus labrax) Juveniles" Animals 12, no. 23: 3332. https://doi.org/10.3390/ani12233332
APA StyleSerradell, A., Montero, D., Fernández-Montero, Á., Terova, G., Makol, A., Valdenegro, V., Acosta, F., Izquierdo, M. S., & Torrecillas, S. (2022). Gill Oxidative Stress Protection through the Use of Phytogenics and Galactomannan Oligosaccharides as Functional Additives in Practical Diets for European Sea Bass (Dicentrarchus labrax) Juveniles. Animals, 12(23), 3332. https://doi.org/10.3390/ani12233332









