Composition of Fatty Acids and Localization of SREBP1 and ELOVL2 Genes in Cauda Epididymides of Hu Sheep with Different Fertility
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Animals and Sample Collection
2.3. Determination of Crude Fat Content and Fatty Acid Profiles
2.4. Immunohistochemistry
2.5. RNA Extraction and cDNA Synthesis
2.6. Quantitative Real-Time PCR Analysis
2.7. Statistical Analyses
3. Results
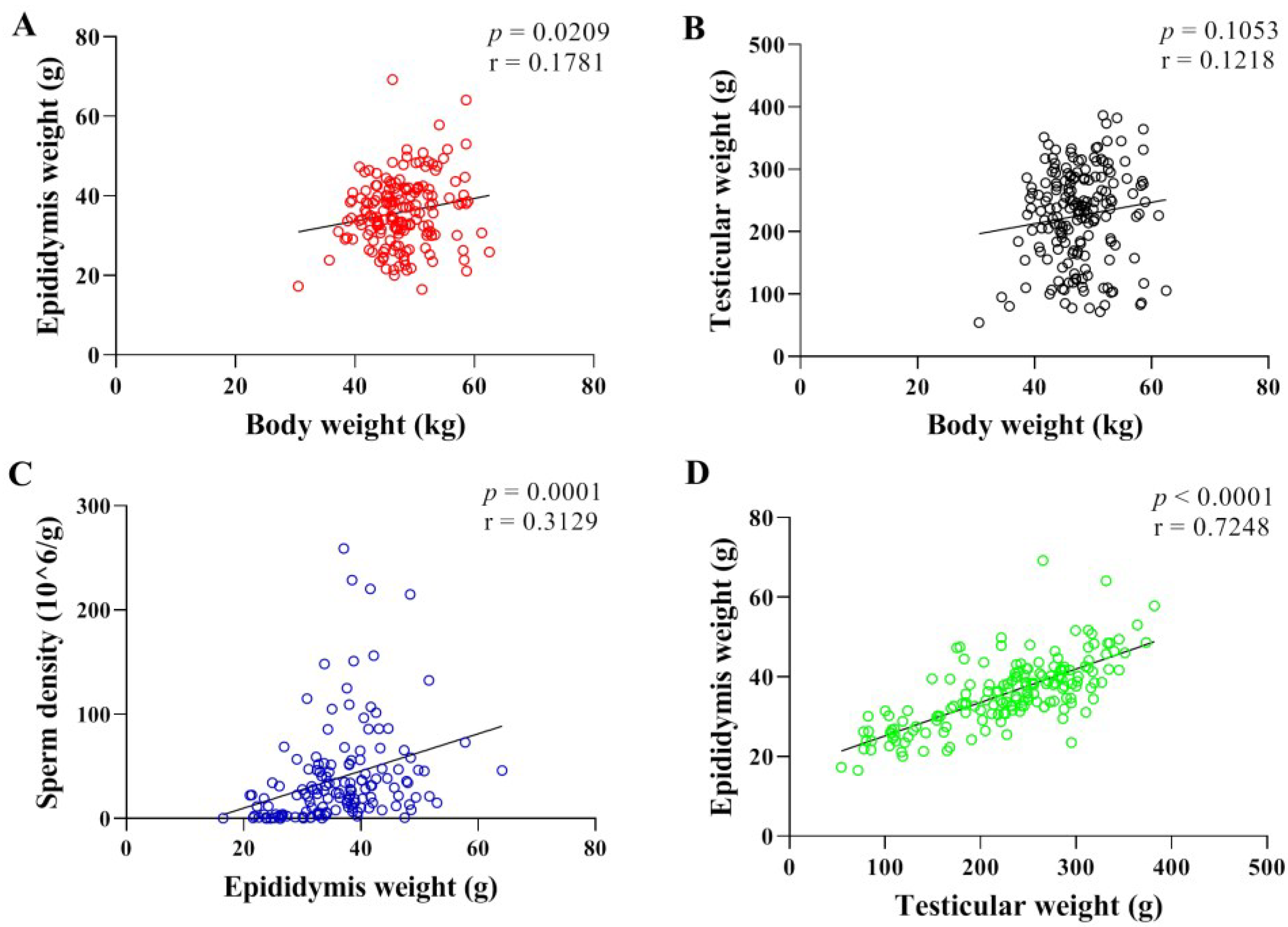
3.1. Correlation Analysis of Body Weight, Sperm Density, Testicular and Epididymal Weight in Hu Sheep
3.2. Crude Fat Content
3.3. Fatty Acid Content
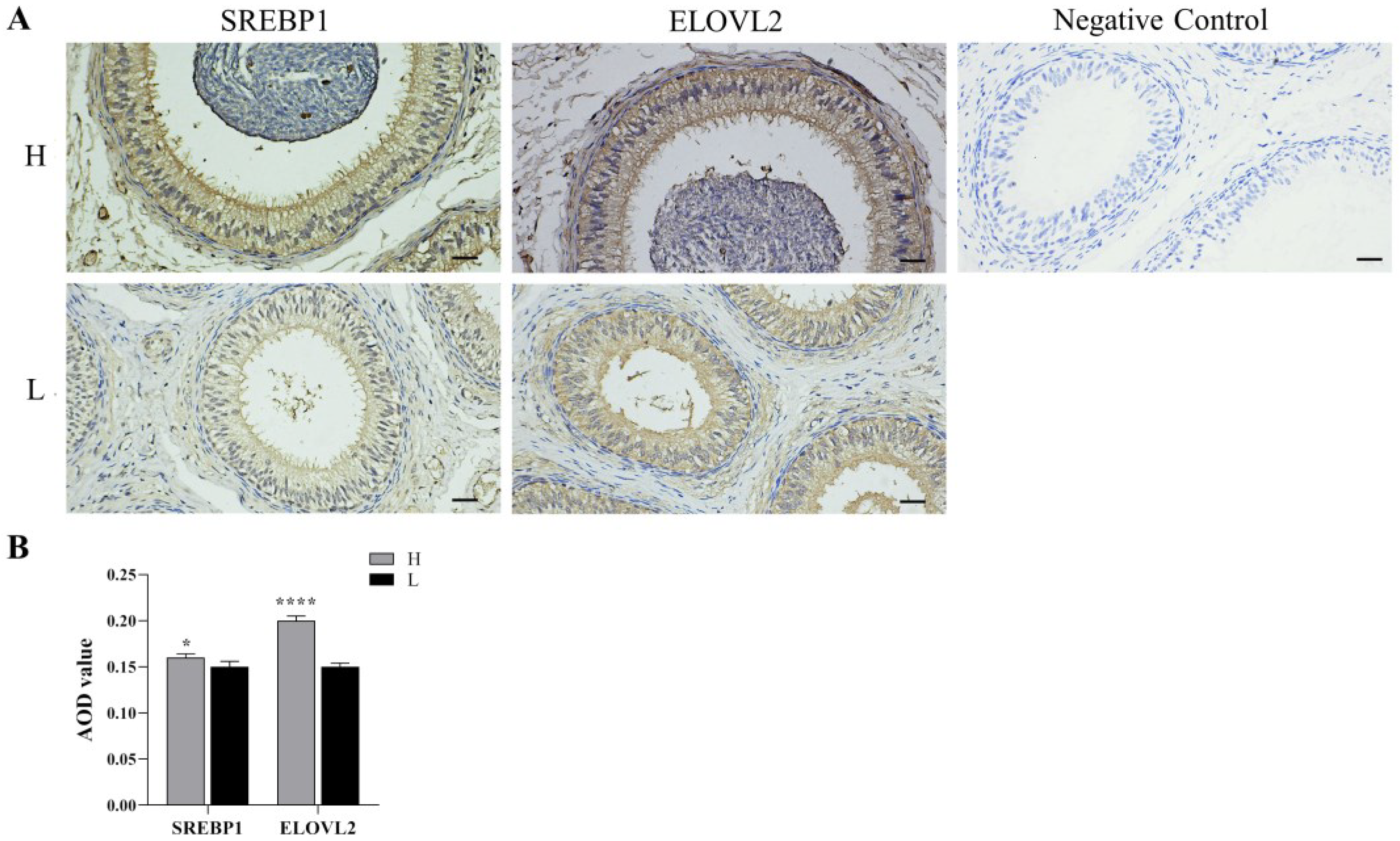
3.4. Immunohistochemistry
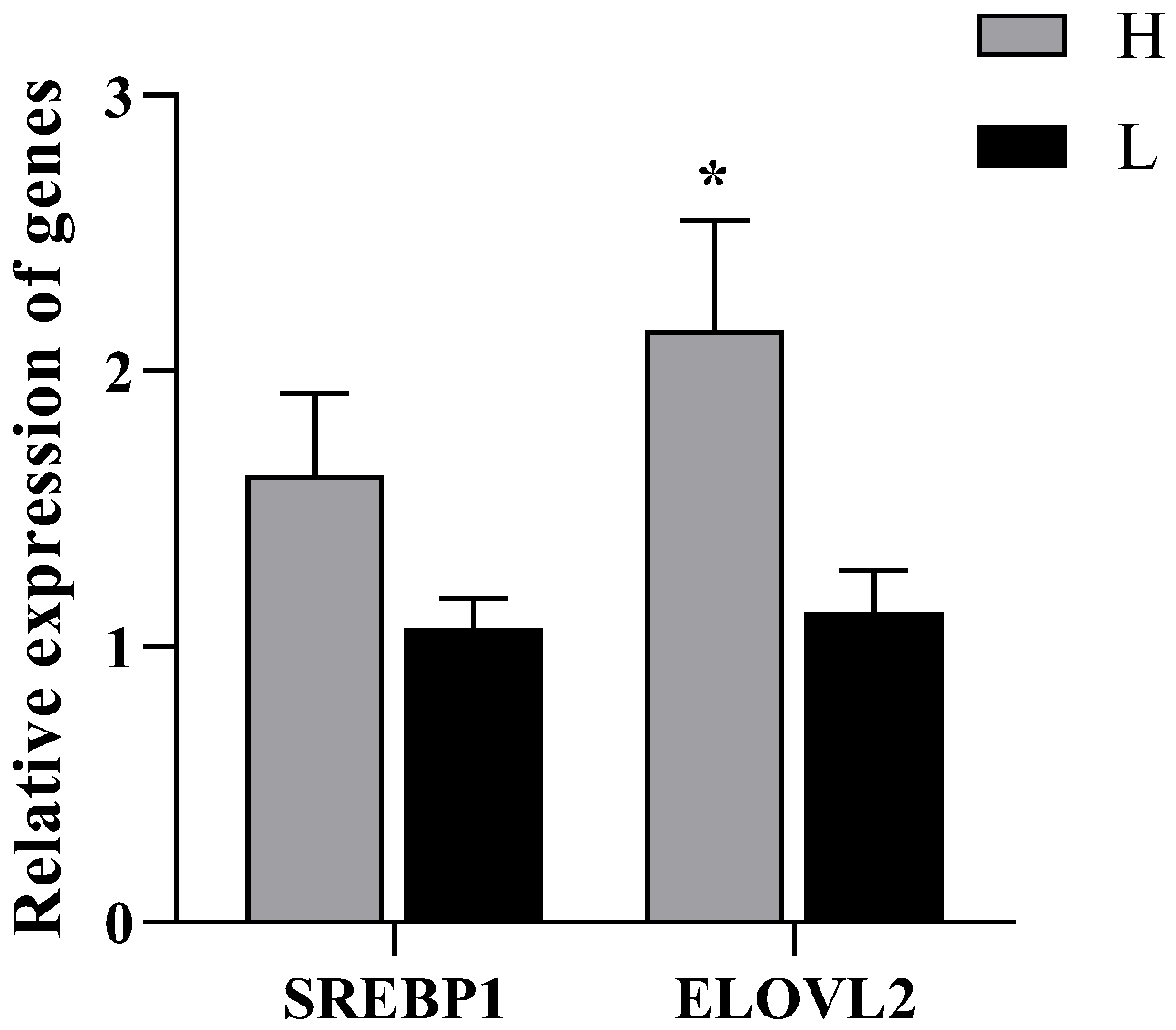
3.5. Relative Expression of SREBP1and ELOVL2 Genes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Simon, C.; Greening, D.W.; Bolumar, D.; Balaguer, N.; Salamonsen, L.A.; Vilella, F. Extracellular Vesicles in Human Reproduction in Health and Disease. Endocr. Rev. 2018, 39, 292–332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Samanta, L.; Parida, R.; Dias, T.R.; Agarwal, A. The enigmatic seminal plasma: A proteomics insight from ejaculation to fertilization. Reprod. Biol. Endocrinol. 2018, 16, 41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haidl, G.; Opper, C. Changes in lipids and membrane anisotropy in human spermatozoa during epididymal maturation. Hum. Reprod. 1997, 12, 2720–2723. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martínez-Soto, J.C.; Landeras, J.; Gadea, J. Spermatozoa and seminal plasma fatty acids as predictors of cryopreservation success. Andrology 2013, 1, 365–375. [Google Scholar] [CrossRef] [Green Version]
- Björkgren, I.; Gylling, H.; Turunen, H.; Huhtaniemi, I.; Strauss, L.; Poutanen, M.; Sipilä, P. Imbalanced lipid homeostasis in the conditional Dicer1 knockout mouse epididymis causes instability of the sperm membrane. FASEB J. 2015, 29, 433–442. [Google Scholar] [CrossRef] [PubMed]
- Furimsky, A.; Vuong, N.; Xu, H.; Kumarathasan, P.; Xu, M.; Weerachatyanukul, W.; Bou Khalil, M.; Kates, M.; Tanphaichitr, N. Percoll gradient-centrifuged capacitated mouse sperm have increased fertilizing ability and higher contents of sulfogalactosylglycerolipid and docosahexaenoic acid-containing phosphatidylcholine compared to washed capacitated mouse sperm. Biol. Reprod. 2005, 72, 574–583. [Google Scholar] [CrossRef]
- Shan, S.W.; Xu, F.Z.; Hirschfeld, M.; Brenig, B. Sperm Lipid Markers of Male Fertility in Mammals. Int. J. Mol. Sci. 2021, 22, 8767. [Google Scholar] [CrossRef]
- Wathes, D.C.; Abayasekara, D.R.E.; Aitken, R.J. Polyunsaturated fatty acids in male and female reproduction. Biol. Reprod. 2007, 77, 190–201. [Google Scholar] [CrossRef]
- Isidori, A.; Conte, D.; Laguzzi, G.; Giovenco, P.; Dondero, F. Role of seminal prostaglandins in male-fertility. I. Relationship of prostaglandin-e and 19-OH prostaglandin-E with seminal parameters. J. Endocrinol. Investig. 1980, 3, 1–4. [Google Scholar] [CrossRef]
- Shankar, U.; Benjamin, B.R.; Agarwal, S.K. Effect of prostaglandin-F2-alpha (PGF2-alpha) on reaction-time and semen characteristics of buffalo-bulls. Indian J. Anim. Sci. 1984, 54, 38–40. [Google Scholar]
- Collodel, G.; Castellini, C.; Lee, J.C.Y.; Signorini, C. Relevance of Fatty Acids to Sperm Maturation and Quality. Oxid. Med. Cell. Longev. 2020, 2020, 7038124. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Yao, T.; Weng, X.; Yao, R.; Li, W.; Xie, L.; Yue, X.; Li, F. Antioxidant properties and transcriptome of cauda epididymis with different levels of fertility in Hu lambs. Theriogenology 2022, 182, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Li, W.H.; Weng, X.X.; Yuan, L.F.; Li, F.; Yue, X.P.; Li, F.D. Effect of feeding linseed diet on testis development, antioxidant capacity, and epididymal cauda sperm concentration in Chinese Hu lamb. Theriogenology 2021, 159, 69–76. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, C.; Kong, Y.; Li, F.; Yue, X. Effects of intramuscular fat on meat quality and its regulation mechanism in Tan sheep. Front. Nutr. 2022, 9, 908355. [Google Scholar] [CrossRef] [PubMed]
- O’Fallon, J.V.; Busboom, J.R.; Nelson, M.L.; Gaskins, C.T. A direct method for fatty acid methyl ester synthesis: Application to wet meat tissues, oils, and feedstuffs. J. Anim. Sci. 2007, 85, 1511–1521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramaswamy, S.; Plant, T.M.; Marshall, G.R. Pulsatile stimulation with recombinant single chain human luteinizing hormone elicits precocious Sertoli cell proliferation in the juvenile male rhesus monkey (Macaca mulatta). Biol. Reprod. 2000, 63, 82–88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Waldner, C.L.; Kennedy, R.I.; Palmer, C.W. A description of the findings from bull breeding soundness evaluations and their association with pregnancy outcomes in a study of western Canadian beef herds. Theriogenology 2010, 74, 871–883. [Google Scholar] [CrossRef]
- Bercovitch, F.B.; Rodriguez, J.F. Testis size, epididymis weight, and sperm competition in rhesus macaques. Am. J. Primatol. 1993, 30, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Mourvaki, E.; Cardinali, R.; Dal Bosco, A.; Corazzi, L.; Castellini, C. Effects of flaxseed dietary supplementation on sperm quality and on lipid composition of sperm subfractions and prostatic granules in rabbit. Theriogenology 2010, 73, 629–637. [Google Scholar] [CrossRef] [PubMed]
- Strzezek, J.; Fraser, L.; Kuklinska, M.; Dziekonska, A.; Lecewicz, M. Effects of dietary supplementation with polyunsaturated fatty acids and antioxidants on biochemical characteristics of boar semen. Reprod. Biol. 2004, 4, 271–287. [Google Scholar] [PubMed]
- Gürler, H.; Calisici, O.; Calisici, D.; Bollwein, H. Effects of feeding omega-3-fatty acids on fatty acid composition and quality of bovine sperm and on antioxidative capacity of bovine seminal plasma. Anim. Reprod. Sci. 2015, 160, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Soto, J.C.; Domingo, J.C.; Cordobilla, B.; Nicolás, M.; Fernández, L.; Albero, P.; Gadea, J.; Landeras, J. Dietary supplementation with docosahexaenoic acid (DHA) improves seminal antioxidant status and decreases sperm DNA fragmentation. Syst. Biol. Reprod. Med. 2016, 62, 387–395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Q.; Zhou, Y.F.; Duan, R.J.; Wei, H.K.; Peng, J.; Jiang, S.W. Dietary n-6:n-3 ratio and Vitamin E improve motility characteristics in association with membrane properties of boar spermatozoa. Asian J. Androl. 2017, 19, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Bionaz, M.; Loor, J.J. Gene networks driving bovine milk fat synthesis during the lactation cycle. BMC Genom. 2008, 9, 366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, H.F.; Luo, J.; Wang, H.P.; Wang, H.; Zhang, T.Y.; Tian, H.B.; Yao, D.W.; Loor, J.J. Sterol regulatory element binding protein-1 (SREBP-1)c promoter: Characterization and transcriptional regulation by mature SREBP-1 and liver X receptor α in goat mammary epithelial cells. J. Dairy Sci. 2016, 99, 1595–1604. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hannah, V.C.; Ou, J.; Luong, A.; Goldstein, J.L.; Brown, M.S. Unsaturated fatty acids down-regulate SREBP isoforms 1a and 1c by two mechanisms in HEK-293 cells. J. Biol. Chem. 2001, 276, 4365–4372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Botolin, D.; Wang, Y.; Christian, B.; Jump, D.B. Docosahexaneoic acid (22:6,n-3) regulates rat hepatocyte SREBP-1 nuclear abundance by Erk- and 26S proteasome-dependent pathways. J. Lipid Res. 2006, 47, 181–192. [Google Scholar] [CrossRef] [Green Version]
- Zadravec, D.; Tvrdik, P.; Guillou, H.; Haslam, R.; Kobayashi, T.; Napier, J.A.; Capecchi, M.R.; Jacobsson, A. ELOVL2 controls the level of n-6 28:5 and 30:5 fatty acids in testis, a prerequisite for male fertility and sperm maturation in mice. J. Lipid Res. 2011, 52, 245–255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Botolin, D.; Christian, B.; Busik, J.; Xu, J.; Jump, D.B. Tissue-specific, nutritional, and developmental regulation of rat fatty acid elongases. J. Lipid Res. 2005, 46, 706–715. [Google Scholar] [CrossRef] [Green Version]
- Castellini, C.; Mattioli, S.; Moretti, E.; Cotozzolo, E.; Perini, F.; Dal Bosco, A.; Signorini, C.; Noto, D.; Belmonte, G.; Lasagna, E.; et al. Expression of genes and localization of enzymes involved in polyunsaturated fatty acid synthesis in rabbit testis and epididymis. Sci. Rep. 2022, 12, 2637. [Google Scholar] [CrossRef]
- Li, W.H.; Tang, D.F.; Li, F.D.; Tian, H.Q.; Yue, X.P.; Li, F.; Weng, X.X.; Sun, W.; Wang, W.M.; Mo, F.T. Supplementation with dietary linseed oil during peri-puberty stimulates steroidogenesis and testis development in rams. Theriogenology 2017, 102, 10–15. [Google Scholar] [CrossRef] [PubMed]

| Gene (ID) | Primer Sequence (5′-3′) | Product Size (bp) |
|---|---|---|
| HPRT1 | F: CGACTGGCTCGAGATGTGAT | 197 |
| XM_015105023.2 | R: TCACCTGTTGACTGGTCGTT | |
| RPS18 | F: CACTGAGGACGAGGTGGAAC | 186 |
| XM_004018745.3 | R: CTGTGGGCCCGAATCTTCTT | |
| RPLP2 | F: AGCGCCAAGGACATCAAAAAG | 136 |
| XM_004023349.4 | R: TGGCCAGCTTGCCGATAC | |
| SREBP1 | F: ATGGCTTTGATTCTCGTGGC | 202 |
| XM_012151742.2 | R: TTTTCAGTGTCCGCAACTGG | |
| ELOVL2 | F: ACAGACCTGCTCTTTCCCTC | 114 |
| XM_015093202.1 | R: TGTAGCCTCCTTCCCAACTG |
| Traits | Mean | 95% Confidence Interval for Mean | Min | Max | SD | CV | Number | |
|---|---|---|---|---|---|---|---|---|
| Lower | Upper | |||||||
| Body weight (kg) | 47.69 | 46.89 | 48.49 | 30.50 | 62.50 | 5.42 | 0.11 | 178 |
| Left testicular weight (g) | 113.30 | 107.62 | 118.98 | 29.00 | 193.90 | 38.41 | 0.34 | 178 |
| Right testicular weight (g) | 112.70 | 107.05 | 118.35 | 25.60 | 193.70 | 38.22 | 0.34 | 178 |
| Left testicular index (g/kg) | 2.39 | 2.27 | 2.51 | 0.70 | 4.00 | 0.82 | 0.34 | 178 |
| Right testicular index (g/kg) | 2.38 | 2.26 | 2.50 | 0.68 | 4.47 | 0.81 | 0.34 | 178 |
| Left epididymal weight (g) | 18.03 | 17.33 | 18.73 | 9.00 | 39.00 | 4.65 | 0.26 | 171 |
| Right epididymal weight (g) | 17.72 | 17.07 | 18.38 | 6.30 | 30.10 | 4.36 | 0.25 | 174 |
| Left epididymal index (g/kg) | 0.38 | 0.37 | 0.39 | 0.17 | 0.84 | 0.10 | 0.25 | 171 |
| Right epididymal index (g/kg) | 0.37 | 0.36 | 0.39 | 0.12 | 0.65 | 0.09 | 0.24 | 174 |
| Sperm density (106/g) | 38.26 | 30.65 | 45.87 | 0.06 | 259.19 | 46.69 | 1.22 | 147 |
| Fatty Acid Content (mg/g) | H | L | p-Value |
|---|---|---|---|
| C14:0 | 0.813 ± 0.078 | 0.632 ± 0.057 | 0.078 |
| C15:0 | 0.050 ± 0.004 | 0.048 ± 0.003 | 0.731 |
| C16:0 | 4.851 ± 0.229 | 4.315 ± 0.131 | 0.059 |
| C16:1 | 0.094 ± 0.005 | 0.091 ± 0.007 | 0.740 |
| C17:0 | 0.148 ± 0.008 | 0.131 ± 0.005 | 0.099 |
| C18:0 | 3.747 ± 0.153 | 3.693 ± 0.134 | 0.795 |
| C18:1n9t | 0.032 ± 0.002 | 0.050 ± 0.008 | 0.060 |
| C18:1n9c | 2.747 ± 0.124 | 2.709 ± 0.109 | 0.822 |
| C18:2n6t | 0.005 ± 0.001 | 0.005 ± 0.000 | 0.331 |
| C18:2n6c | 1.376 ± 0.088 | 1.366 ± 0.055 | 0.923 |
| C20:0 | 0.124 ± 0.007 | 0.133 ± 0.006 | 0.330 |
| C18:3n6 | 0.019 ± 0.002 | 0.025 ± 0.003 | 0.074 |
| C20:1 | 0.114 ± 0.007 | 0.110 ± 0.006 | 0.707 |
| C18:3n3 | 0.031 ± 0.002 | 0.031 ± 0.001 | 0.776 |
| C20:2 | 0.170 ± 0.030 | 0.159 ± 0.012 | 0.750 |
| C22:0 | 0.057 ± 0.002 | 0.064 ± 0.003 | 0.050 |
| C20:3n6 | 0.756 ± 0.057 | 0.630 ± 0.031 | 0.073 |
| C20:3n3 | 0.004 ± 0.000 | 0.003 ± 0.000 | 0.089 |
| C20:4n6 | 6.534 ± 0.185 | 5.797 ± 1.096 | 0.526 |
| C23:0 | 0.107 ± 0.005 | 0.103 ± 0.003 | 0.513 |
| C22:2 | 0.006 ± 0.001 | 0.005 ± 0.000 | 0.827 |
| C24:0 | 0.043 ± 0.004 | 0.054 ± 0.007 | 0.216 |
| C20:5n3 | 0.044 ± 0.026 | 0.030 ± 0.011 | 0.607 |
| C24:1 | 0.013 ± 0.001 | 0.014 ± 0.001 | 0.132 |
| C22:6n3 | 2.812 ± 0.215 | 1.275 ± 0.165 | <0.001 |
| Total | 34.607 ± 0.727 | 31.388 ± 1.157 | 0.032 |
| SFA | 19.852 ± 0.444 | 19.087 ± 0.286 | 0.167 |
| MUFA | 3.000 ± 0.131 | 2.975 ± 0.120 | 0.892 |
| PUFA | 11.755 ± 0.398 | 9.325 ± 1.144 | 0.073 |
| n-3 PUFA | 2.891 ± 0.217 | 1.338 ± 0.165 | <0.001 |
| n-6 PUFA | 8.689 ± 0.270 | 7.823 ± 1.080 | 0.457 |
| n-6/n-3 PUFA | 3.148 ± 0.252 | 6.158 ± 0.956 | 0.014 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Li, W.; Weng, X.; Yue, X.; Li, F. Composition of Fatty Acids and Localization of SREBP1 and ELOVL2 Genes in Cauda Epididymides of Hu Sheep with Different Fertility. Animals 2022, 12, 3302. https://doi.org/10.3390/ani12233302
Liu J, Li W, Weng X, Yue X, Li F. Composition of Fatty Acids and Localization of SREBP1 and ELOVL2 Genes in Cauda Epididymides of Hu Sheep with Different Fertility. Animals. 2022; 12(23):3302. https://doi.org/10.3390/ani12233302
Chicago/Turabian StyleLiu, Jiamei, Wanhong Li, Xiuxiu Weng, Xiangpeng Yue, and Fadi Li. 2022. "Composition of Fatty Acids and Localization of SREBP1 and ELOVL2 Genes in Cauda Epididymides of Hu Sheep with Different Fertility" Animals 12, no. 23: 3302. https://doi.org/10.3390/ani12233302
APA StyleLiu, J., Li, W., Weng, X., Yue, X., & Li, F. (2022). Composition of Fatty Acids and Localization of SREBP1 and ELOVL2 Genes in Cauda Epididymides of Hu Sheep with Different Fertility. Animals, 12(23), 3302. https://doi.org/10.3390/ani12233302





