Asymmetric Male Mating Success in Lek-Breeding Rhinella arenarum
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
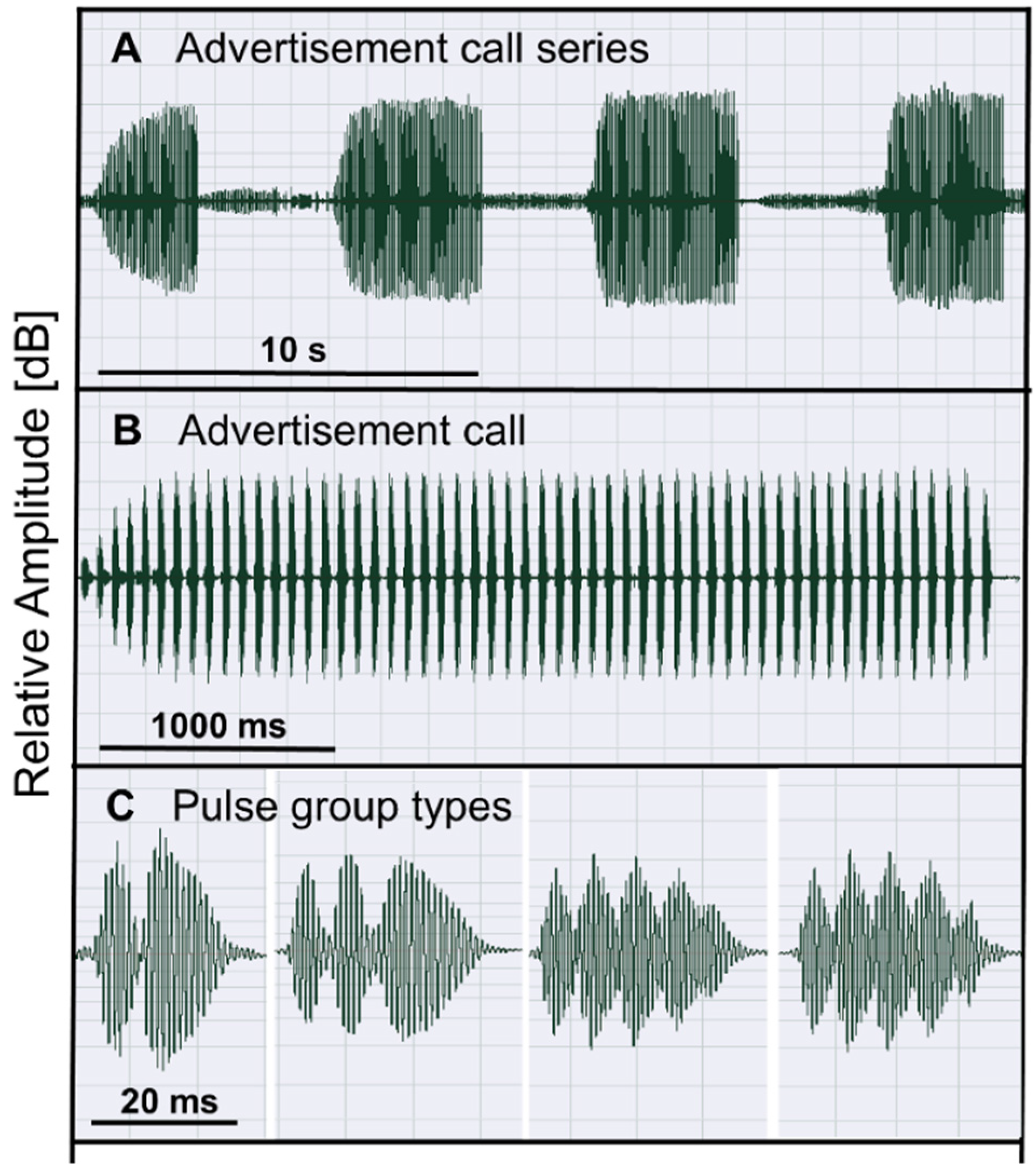
2.1. Study Species
2.2. Study Site
2.3. Male Traits
2.4. Female Response to Male Calls
2.5. Statistical Analyses
3. Results
3.1. Reproductive Behavior at the Study Pond
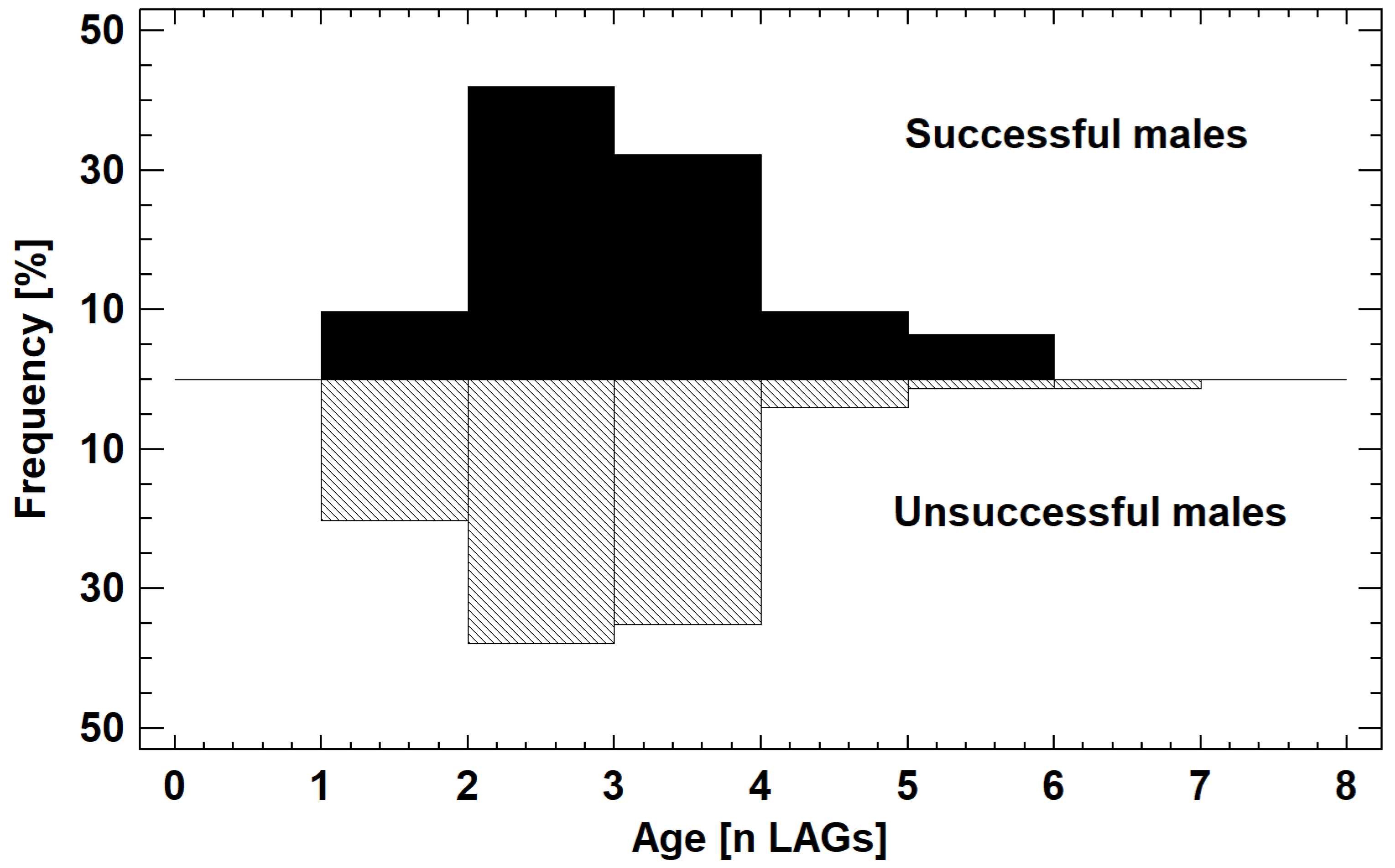
3.2. Traits of Males with and without Mating Success
3.3. Female Response to Male Calls
4. Discussion
4.1. Prediction 1: Mating Success Differs among Males and Is Related to Phenotypic Traits
4.2. Prediction 2: Heterozygous Males Enjoy Mating Success More Frequently Than Homozygous Ones
4.3. Prediction 3: Female Choice Is Based on Acoustic Signaling of Males
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kempenaers, B. Mate Choice and Genetic Quality: A Review of the Heterozygosity Theory. In Advances in the Study of Behavior; Academic Press: Cambridge, MA, USA, 2007; Volume 37, pp. 189–278. [Google Scholar] [CrossRef]
- Ahnesjö, I.; Gonçalves, I.B. Mate Choice in Males and Females. In Encyclopedia of Animal Behavior, 2nd ed.; Choe, J.C., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 432–440. [Google Scholar] [CrossRef]
- Wong, B.B.M.; Candolin, U. How is female mate choice affected by male competition? Biol. Rev. 2005, 80, 559–571. [Google Scholar] [CrossRef]
- Edward, D.A.; Chapman, T. The evolution and significance of male mate choice. Trends. Ecol. Evol. 2011, 26, 647–654. [Google Scholar] [CrossRef]
- Kokko, H.; Lindström, J.; Alatalo, R.V.; Rintamäki, P.T. Queuing for territory positions in the lekking black grouse (Tetrao tetrix). Behav. Ecol. 1998, 9, 376–383. [Google Scholar] [CrossRef][Green Version]
- Llusia, D.; Marquez, R.; Francisco Beltran, J.; Moreira, C.; do Amaral, J.P. Environmental and social determinants of anuran lekking behavior: Intraspecific variation in populations at thermal extremes. Behav. Ecol. Sociobiol. 2013, 67, 493–511. [Google Scholar] [CrossRef]
- Höglund, J.; Alatalo, R.V. Leks; Princeton University Press: Princeton, NJ, USA, 1995; p. 264. [Google Scholar]
- Andersson, M. Sexual Selection; Princeton University Press: Princeton, NJ, USA, 1994; p. 624. [Google Scholar]
- Andersson, M.; Simmons, L.W. Sexual selection and mate choice. Trends. Ecol. Evolut. 2006, 21, 296–302. [Google Scholar] [CrossRef]
- Kokko, H.; Brooks, R.; Jennions, M.D.; Morley, J. The evolution of mate choice and mating biases. Proc. R. Soc. B 2003, 270, 653–664. [Google Scholar] [CrossRef] [PubMed]
- Neff, B.D.; Pitcher, T.E. Genetic quality and sexual selection: An integrated framework for good genes and compatible genes. Mol. Ecol. 2005, 14, 19–38. [Google Scholar] [CrossRef]
- Mitton, J.; Schuster, W.; Cothran, E.; De Fries, J. Correlation between the individual heterozygosity of parents and their offspring. Heredity 1993, 71, 59–63. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.L. A theory of mate choice based on heterozygosity. Behav. Ecol. 1997, 8, 60–65. [Google Scholar] [CrossRef]
- Sardell, R.J.; Kempenaers, B.; DuVal, E.H. Female mating preferences and offspring survival: Testing hypotheses on the genetic basis of mate choice in a wild lekking bird. Mol. Ecol. 2014, 23, 933–946. [Google Scholar] [CrossRef]
- Weatherhead, P.J.; Dufour, K.W.; Lougheed, S.C.; Eckert, C.G. A test of the good-genes-as-heterozygosity hypothesis using red-winged blackbirds. Behav. Ecol. 1999, 10, 619–625. [Google Scholar] [CrossRef][Green Version]
- Landry, C.; Garant, D.; Duchesne, P.; Bernatchez, L. ‘Good genes as heterozygosity’: The major histocompatibility complex and mate choice in Atlantic salmon (Salmo salar). Proc. R. Soc. B 2001, 268, 1279–1285. [Google Scholar] [CrossRef]
- Jaquiery, J.; Broquet, T.; Aguilar, C.; Evanno, G.; Perrin, N. Good genes drive female choice for mating partners in the lek-breeding european treefrog. Evolution 2010, 64, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Botto, V.; Castellano, S. Attendance, but not performance, predicts good genes in a lek-breeding treefrog. Behavl. Ecol. 2016, 27, 1141–1148. [Google Scholar] [CrossRef]
- O´Brien, D.M.; Keogh, J.S.; Silla, A.J.; Byrne, P.G. Female choice for related males in wild red-backed toadlets (Pseudophryne coriacea). Behav. Ecol. 2019, 30, 928–937. [Google Scholar] [CrossRef]
- Cheng, Y.-C.; Chen, Y.-H.; Chang, C.; Chuang, M.-F.; Hsu, Y. Endurance rivalry and female choice jointly influence male mating success in the emerald treefrog (Zhangixalus prasinatus), a lek-chorusing anuran. BMC Zool. 2022, 7, 17. [Google Scholar] [CrossRef]
- Wells, K.D. The courtship of frogs. In The Reproductive Biology of Amphibians; Taylor, D.H., Guttman, S.I., Eds.; Plenum Corporation: Oxford, OH, USA, 1977; pp. 233–262. [Google Scholar]
- Zamudio, K.R.; Chan, L.M. Alternative reproductive tactics in amphibians. In Alternative Reproductive Tactics: An Integrative Approach; Brockmann, H.J., Taborsky, M., Oliveira, R.F., Eds.; Cambridge University Press: Cambridge, UK, 2008; pp. 300–331. [Google Scholar] [CrossRef]
- Godwin, G.J.; Roble, S.M. Mating Success in Male Treefrogs, Hyla chrysoscelis (Anura: Hylidae). Herpetologica 1983, 39, 141–146. [Google Scholar] [CrossRef]
- Brede, E.G.; Beebee, T.J.C. Large variations in the ratio of effective breeding and census population sizes between two species of pond-breeding anurans. Biol. J. Linn. Soc. 2006, 89, 365–372. [Google Scholar] [CrossRef]
- Rowe, G.; Beebee, T.J.C. Reconciling genetic and demographic estimators of effective population size in the anuran amphibian Bufo calamita. Conserv. Genet. 2004, 5, 287–298. [Google Scholar] [CrossRef]
- Gross, M.R. Alternative reproductive strategies and tactics: Diversity within sexes. Trends. Ecol. Evol. 1996, 11, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Arak, A. Male-male competition and mate choice in anuran amphibians. In Mate Choice; Bateson, P., Ed.; Cambridge University Press: Cambridge, UK, 1983; pp. 181–210. [Google Scholar]
- Bourne, G.R. Lekking behavior in the neotropical frog Ololygon rubra. Behav. Ecol. Sociobiol. 1992, 31, 173–180. [Google Scholar] [CrossRef]
- Bourne, G.R. Proximate costs and benefits of mate acquisition at leks of the frog Ololygon rubra. Anim. Behav. 1993, 45, 1051–1059. [Google Scholar] [CrossRef]
- Green, D.M. Rarity of Size-Assortative Mating in Animals: Assessing the Evidence with Anuran Amphibians. Am. Nat. 2019, 193, 279–295. [Google Scholar] [CrossRef] [PubMed]
- Caldwell, J.P.; Shepard, D.B. Calling site fidelity and call structure of a neotropical toad, Rhinella ocellata (Anura: Bufonidae). J. Herpetol. 2007, 41, 611–621. [Google Scholar] [CrossRef]
- Yasumiba, K.; Alford, R.A.; Schwarzkopf, L. Why do male and female cane toads, Rhinella marina, respond differently to advertisement calls? Anim. Behav. 2015, 109, 141–147. [Google Scholar] [CrossRef]
- Stynoski, J.L.; Trama, F.A.; Patron, F.L.R.; Tapia, E.; Hoke, K.L. Reproductive Ecology of the Peruvian Earless Toad Rhinella yunga (Amphibia, Bufonidae) with Descriptions of Calls, Tadpole, and Female Competition. S. Am. J. Herpetol. 2020, 15, 85–96. [Google Scholar] [CrossRef]
- Costa, F.R.; Moura, P.H.A.G.; Nunes, I. On the courtship, breeding behaviour and vocalisation of Rhinella ornata (Spix, 1824) (Anura, Bufonidae): A well-marked escalated behaviour in a lek-like system. Acta Ethol. 2020, 23, 69–77. [Google Scholar] [CrossRef]
- Bionda, C.D.; Lajmanovich, R.C.; Salas, N.E.; Martino, A.L.; di Tada, I.E. Reproductive Ecology of the Common South American Toad Rhinella arenarum (Anura: Bufonidae): Reproductive Effort, Clutch Size, Fecundity, and Mate Selection. J. Herpetol. 2011, 45, 261–264. [Google Scholar] [CrossRef]
- Cei, J.M. Amphibians of Argentina. Monit. Zool. Ital. N. S. Monogr. 1980, 2, 609. [Google Scholar]
- di Tada, I.E.; Zavatieri, M.V.; Bridarolli, M.E.; Salas, N.E.; Martino, A.L. Anfibios anuros de la provincia de Córdoba. In Biodiversidad de la Provincia de Córdoba; di Tada, I.E.F., Bucher, E.H., Eds.; Ediciones Universidad Nacional de Río Cuarto: Río Cuarto, Argentina, 1996; Volume 1, pp. 191–213. [Google Scholar]
- Salas, N.E.; Zavattieri, M.V.; di Tada, I.E.; Martino, A.L.; Bridarolli, M.E. Bioacoustical and etho-ecological features in amphibian communities of southern Córdona province (Argentina). Cuad. Herpetol. 1998, 12, 37–46. [Google Scholar]
- di Tada, I.E.; Martino, A.; Sinsch, U. Release vocalizations in neotropical toads (Bufo): Ecological constraints and phylogenetic implications. J. Zool. Syst. Evolut. Res. 2001, 39, 13–23. [Google Scholar] [CrossRef]
- Nöller, H.G. Eine einfache Technik der Blutentnahme beim Frosch. Pflügers Arch. Physiol. 1959, 269, 98–100. [Google Scholar] [CrossRef]
- Sinsch, U. Review: Skeletochronological assessment of demographic life-history traits in amphibians. Herpetol. J. 2015, 25, 5–13. [Google Scholar]
- Bionda, C.D.; Kost, S.; Salas, N.E.; Lajmanovich, R.C.; Sinsch, U.; Martino, A.L. Age structure, growth and longevity in the common toad, Rhinella arenarum, from Argentina. Acta Herpetol. 2015, 10, 55–62. [Google Scholar] [CrossRef]
- Hebert, P.D.N.; Beaton, M.J. Methodologies for Allozyme analysis Using Cellulose Acetate Electrophoresis, 2nd ed.; Helena Laboratories: Beaumont, TX, USA, 1993; p. 32. [Google Scholar]
- Gray, H.; MacKenzie, T. Tactics used by cane toads, Rhinella marina (Linnaeus 1758) (Anura: Bufonidae), to disrupt amplectant pairs and to avoid persistent satellite males. Herpetol. Notes 2016, 9, 233–235. [Google Scholar]
- Vargas-Salinas, F. Breeding Behavior of the Cane Toad Bufo marinus (Bufonidae): A Successfully Invasive Species. Herpetol. Rev. 2007, 38, 12–17. [Google Scholar]
- Bowcock, H.; Brown, G.P.; Shine, R. Sexual selection in cane toads Rhinella marina: A male’s body size affects his success and his tactics. Curr. Zool. 2013, 59, 747–753. [Google Scholar] [CrossRef][Green Version]
- Pereyra, M.; Blotto, B.L.; Baldo, D.; Chaparro, J.C.; Ron, S.; Elias-Costa, A.; Iglesias, P.; Venegas, P.; Thomé, M.T.; Ospina-Sarria, J.; et al. Evolution in the Genus Rhinella: A Total Evidence Phylogenetic Analysis of Neotropical True Toads (Anura: Bufonidae). Bull. Am. Mus. Nat. Hist. 2021, 447, 1–156. [Google Scholar] [CrossRef]
- Emlen, S.T. Lek organization and mating strategies in the bullfrog. Behav. Ecol. Sociobiol. 1976, 1, 283–313. [Google Scholar] [CrossRef]
- Knopp, T.; Heimovirta, M.; Kokko, H.; Merilä, J. Do male moor frogs (Rana arvalis) lek with kin? Mol. Ecol. 2008, 17, 2522–2530. [Google Scholar] [CrossRef]
- Yu, T.; Green, D.M.; Deng, Y.; Han, Y. Effects of operational sex ratio and male density on size-dependent mating in Minshan’s toads, Bufo minshanicus, on the Tibetan Plateau of China. Biol. J. Linn. Soc. 2022, 136, 566–573. [Google Scholar] [CrossRef]
- Sullivan, B.K.; Wagner, W.E., Jr. Variation in advertisement and release calls, and social influences on calling behavior in the gulf coast toad (Bufo valliceps). Copeia 1988, 1014–1020. [Google Scholar] [CrossRef]
- Sinsch, U. Sex-biassed site fidelity and orientation behaviour in reproductive natterjack toads (Bufo calamita). Ethol. Ecol. Evol. 1992, 4, 15–32. [Google Scholar] [CrossRef]
- Sotelo, M.I.; Bingman, V.P.; Muzio, R.N. The Mating Call of the Terrestrial Toad, Rhinella arenarum, as a Cue for Spatial Orientation and Its Associated Brain Activity. Brain Behav. Evol. 2019, 94, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Stratman, K.D.; Oldehoeft, E.A.; Höbel, G. Woe is the loner: Female treefrogs prefer clusters of displaying males over single “hotshot” males. Evolution 2021, 75, 3026–3036. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sinsch, U.; Hecht, K.; Kost, S.; Grenat, P.R.; Martino, A.L. Asymmetric Male Mating Success in Lek-Breeding Rhinella arenarum. Animals 2022, 12, 3268. https://doi.org/10.3390/ani12233268
Sinsch U, Hecht K, Kost S, Grenat PR, Martino AL. Asymmetric Male Mating Success in Lek-Breeding Rhinella arenarum. Animals. 2022; 12(23):3268. https://doi.org/10.3390/ani12233268
Chicago/Turabian StyleSinsch, Ulrich, Katharina Hecht, Silvia Kost, Pablo R. Grenat, and Adolfo L. Martino. 2022. "Asymmetric Male Mating Success in Lek-Breeding Rhinella arenarum" Animals 12, no. 23: 3268. https://doi.org/10.3390/ani12233268
APA StyleSinsch, U., Hecht, K., Kost, S., Grenat, P. R., & Martino, A. L. (2022). Asymmetric Male Mating Success in Lek-Breeding Rhinella arenarum. Animals, 12(23), 3268. https://doi.org/10.3390/ani12233268






