Implications of Salinity and Acidic Environments on Fitness and Oxidative Stress Parameters in Early Developing Seahorses Hippocampus reidi
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Bioethics
2.2. Live Prey Culture
2.3. Seahorse Breeding
2.4. Experiments
2.4.1. Trial 1: Effect of pH and Salinity on Seahorse Juveniles
2.4.2. Trial 2: Effect of Acidification on Seahorse Juveniles Reared in SW at pH 8.0
2.5. Biochemical Analyses
2.6. Treatment of Data
- -
- Survival (S, %): (final number of fishes/initial number of fishes) × 100, accounting for sampled juveniles
- -
- Specific growth rate (SGR, % day−1): Ln wf–Ln wi/t × 100, where wf and wi are the final and initial mean weight, and t is the experimental time in days.
- -
- Fulton’s Factor Condition Index: K = W/L3 × 10, where W and L are mean weight and length, respectively.
2.7. Statistical Analysis
3. Results
3.1. Trial 1: Effect of pH and Salinity on Juveniles
3.1.1. Biochemical Oxidative Stress Indices
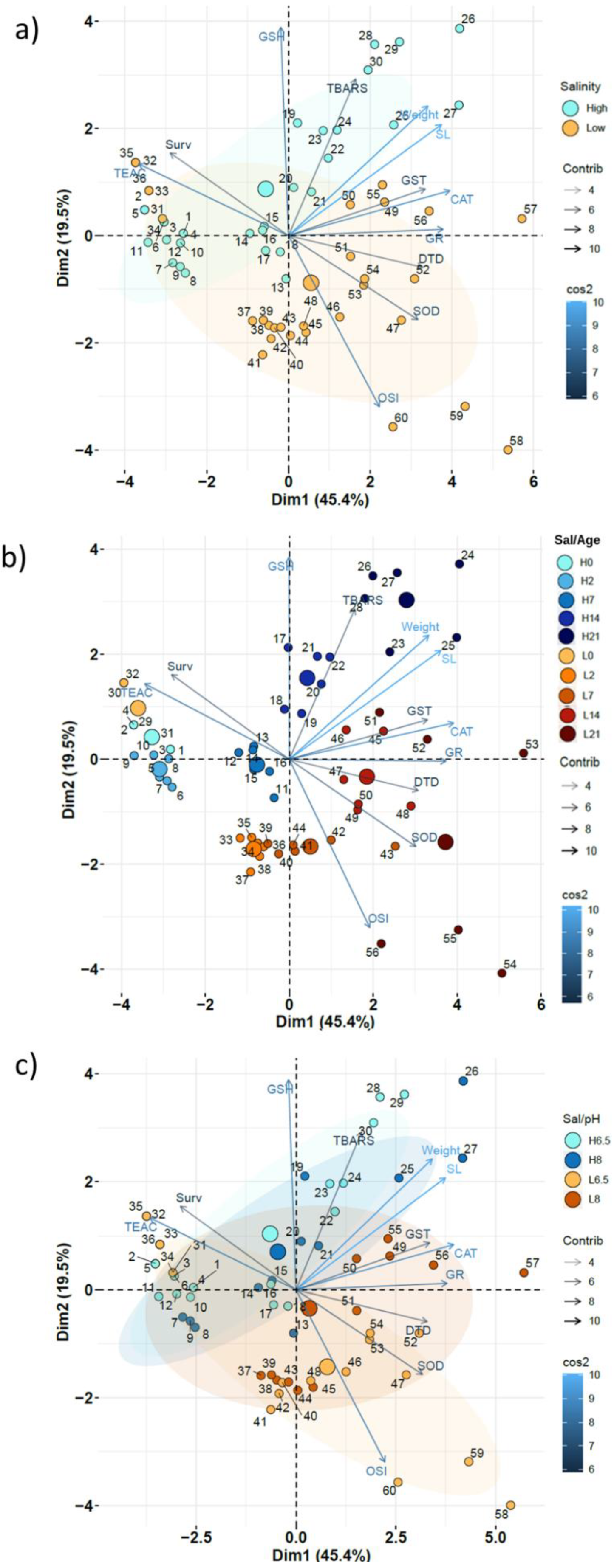
3.1.2. Global Assessment: SW and BW
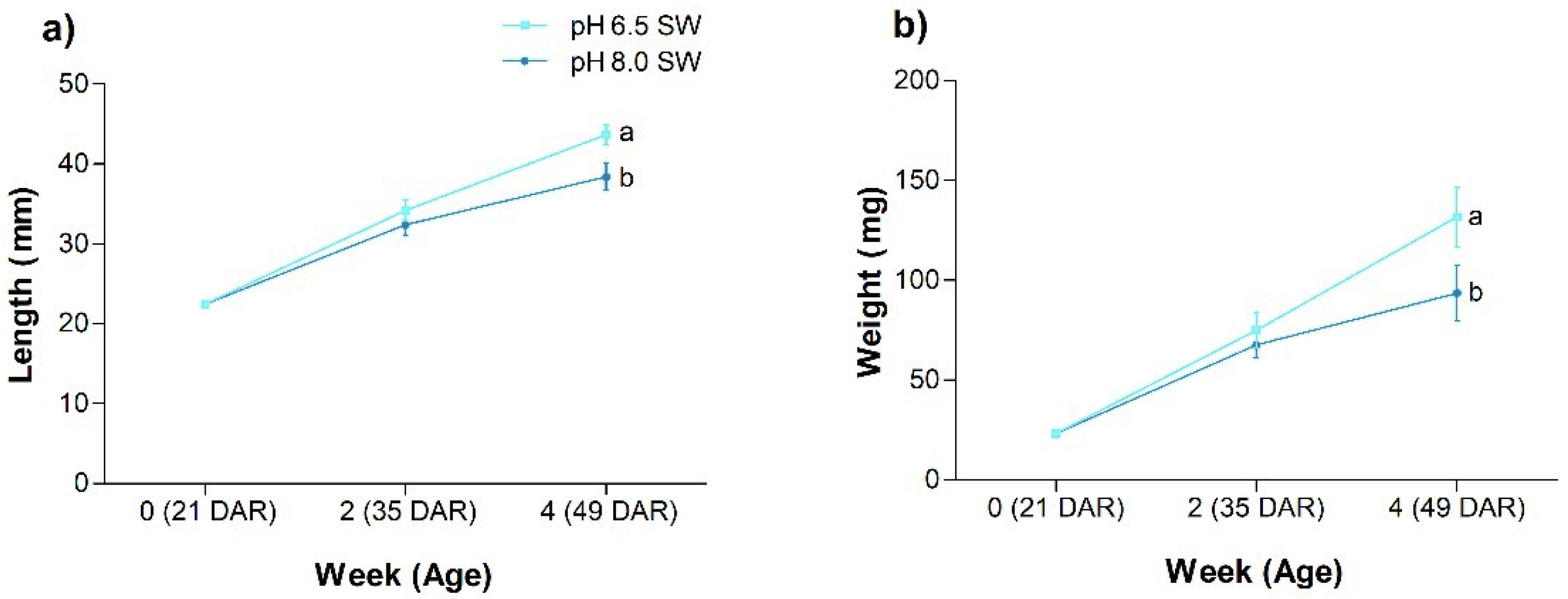
3.2. Trial 2: Effect of Acidification on Juveniles
4. Discussion
4.1. Combined Effects of Salinity and pH on Early-Developing Juveniles
4.2. Biochemical Indices: Seawater–SW (S33) and Brackish Water–BW (S11)
4.3. Global Assessment (PCA)
4.4. Trial 2: Effect of pH on the Growth of Seahorse Juveniles
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abreu, P.C.; Robaldo, R.B.; Sampaio, L.A.; Bianchini, A.; Odebrecht, C. Recurrent Amyloodiniosis on Broodstock of the Brazilian Flounder Paralichthys orbignyanus: Dinospore Monitoring and Prophylactic Measures. J. World Aquac. Soc. 2007, 36, 42–50. [Google Scholar] [CrossRef]
- Cohen, F.P.; Planas, M.; Valenti, W.C.; Lillebø, A.; Calado, R. Optimizing packing of live seahorses for shipping. Aquaculture 2018, 482, 57–64. [Google Scholar] [CrossRef]
- Huang, J.; Qin, G.; Zhang, B.; Tan, S.; Sun, J.; Lin, Q. Effects of food, salinity, and ammonia-nitrogen on the physiology of juvenile seahorse (Hippocampus erectus) in two typical culture models in China. Aquaculture 2020, 520, 734965. [Google Scholar] [CrossRef]
- Meira-Filho, M.R.C.; Ramirez, J.R.B.; Vianna, R.T.; Júnior, J.P. Efficacy of glacial acetic acid in the control of Trichodina sp. and Apiosoma sp. associated with Mugil liza. Aquaculture 2017, 479, 7–12. [Google Scholar] [CrossRef]
- Heuer, R.M.; Grosell, M. Physiological impacts of elevated carbon dioxide and ocean acidification on fish. Am. J. Physiol. Integr. Comp. Physiol. 2014, 307, R1061–R1084. [Google Scholar] [CrossRef]
- Ishimatsu, A.; Kikkawa, T.; Hayashi, M.; Lee, K.-S.; Kita, J. Effects of CO2 on Marine Fish: Larvae and Adults. J. Oceanogr. 2004, 60, 731–741. [Google Scholar] [CrossRef]
- Ishimatsu, A.; Hayashi, M.; Kikkawa, T. Fishes in high-CO2, acidified oceans. Mar. Ecol. Prog. Ser. 2008, 373, 295–302. [Google Scholar] [CrossRef]
- Lee, C.; Kwon, B.-O.; Hong, S.; Noh, J.; Lee, J.; Ryu, J.; Kang, S.-G.; Khim, J.S. Sub-lethal and lethal toxicities of elevated CO2 on embryonic, juvenile, and adult stages of marine medaka Oryzias melastigma. Environ. Pollut. 2018, 241, 586–595. [Google Scholar] [CrossRef]
- Calado, R. Location. In Marine Ornamental Species Aquaculture; Calado, R., Olivotto, I., Planas, M., Holt, G.J., Eds.; Wiley Blackwell: West Sussex, UK, 2017; pp. 75–79. [Google Scholar]
- Tlusty, M. The benefits and risks of aquacultural production for the aquarium trade. Aquaculture 2002, 205, 203–219. [Google Scholar] [CrossRef]
- Timmons, M.B.; Ebeling, J.M. Recirculating Aquaculture, 2nd ed.; Northeastern Regional Aquaculture Center: College Park, MD, USA, 2010. [Google Scholar]
- Park, M.S.; Shin, H.S.; Choi, C.Y.; Na Kim, N.; Park, D.-W.; Kil, G.-S.; Lee, J. Effect of hypoosmotic and thermal stress on gene expression and the activity of antioxidant enzymes in the cinnamon clownfish, Amphiprion melanopus. Anim. Cells Syst. Seoul 2011, 15, 219–225. [Google Scholar] [CrossRef]
- Copatti, C.E.; Baldisserotto, B.; de Freitas Souza, C.; Monserrat, J.M.; Garcia, L. Water pH and hardness alter ATPases and oxidative stress in the gills and kidney of pacu (Piaractus mesopotamicus). Neotrop. Ichthyol. 2019, 17, e190032. [Google Scholar] [CrossRef]
- Pellegrin, L.; Nitz, L.F.; Maltez, L.C.; Copatti, C.E.; Garcia, L. Alkaline water improves the growth and antioxidant responses of pacu juveniles (Piaractus mesopotamicus). Aquaculture 2019, 519, 734713. [Google Scholar] [CrossRef]
- Kim, J.-H.; Park, H.-J.; Kim, K.-W.; Hwang, I.-K.; Kim, D.-H.; Oh, C.W.; Lee, J.S.; Kang, J.-C. Growth performance, oxidative stress, and non-specific immune responses in juvenile sablefish, Anoplopoma fimbria, by changes of water temperature and salinity. Fish Physiol. Biochem. 2017, 43, 1421–1431. [Google Scholar] [CrossRef]
- Lemos, C.H.D.P.; Ribeiro, C.V.D.M.; de Oliveira, C.P.B.; Couto, R.D.; Copatti, C.E. Effects of interaction between pH and stocking density on the growth, haematological and biochemical responses of Nile tilapia juveniles. Aquaculture 2018, 495, 62–67. [Google Scholar] [CrossRef]
- Ebeling, J.M.; Timmons, M.B.; Bisogni, J. Engineering analysis of the stoichiometry of photoautotrophic, autotrophic, and heterotrophic removal of ammonia–nitrogen in aquaculture systems. Aquaculture 2006, 257, 346–358. [Google Scholar] [CrossRef]
- Cohen, F.P.A.; Valenti, W.C.; Calado, R. Traceability Issues in the Trade of Marine Ornamental Species. Rev. Fish. Sci. 2013, 21, 98–111. [Google Scholar] [CrossRef]
- Foster, S.; Wiswedel, S.; Vincent, A. Opportunities and challenges for analysis of wildlife trade using CITES data—Seahorses as a case study. Aquat. Conserv. Mar. Freshw. Ecosyst. 2014, 26, 154–172. [Google Scholar] [CrossRef]
- Olivotto, I.; Planas, M.; Simoes, N.; Holt, G.J.; Avella, M.A.; Calado, R. Advances in Breeding and Rearing Marine Ornamentals. J. World Aquac. Soc. 2011, 42, 135–166. [Google Scholar] [CrossRef]
- Koldewey, H.J.; Martin-Smith, K.M. A global review of seahorse aquaculture. Aquaculture 2010, 302, 131–152. [Google Scholar] [CrossRef]
- Kuo, T.-C.; Vincent, A. Assessing the changes in international trade of marine fishes under CITES regulations—A case study of seahorses. Mar. Policy 2018, 88, 48–57. [Google Scholar] [CrossRef]
- Foster, S.J.; Kuo, T.-C.; Wan, A.K.Y.; Vincent, A.C. Global seahorse trade defies export bans under CITES action and national legislation. Mar. Policy 2019, 103, 33–41. [Google Scholar] [CrossRef]
- Hora, M.D.S.C.D.; Joyeux, J.-C. Closing the reproductive cycle: Growth of the seahorse Hippocampus reidi (Teleostei, Syngnathidae) from birth to adulthood under experimental conditions. Aquaculture 2009, 292, 37–41. [Google Scholar] [CrossRef]
- Olivotto, I.; Avella, M.; Sampaolesi, G.; Piccinetti, C.; Ruiz, P.N.; Carnevali, O. Breeding and rearing the longsnout seahorse Hippocampus reidi: Rearing and feeding studies. Aquaculture 2008, 283, 92–96. [Google Scholar] [CrossRef]
- Fonseca, T.; David, F.S.; Ribeiro, F.A.S.; A Wainberg, A.; Valenti, W.C. Technical and economic feasibility of integrating seahorse culture in shrimp/oyster farms. Aquac. Res. 2015, 48, 655–664. [Google Scholar] [CrossRef]
- Cohen, F.; Valenti, W.C.; Planas, M.; Calado, R. Seahorse Aquaculture, Biology and Conservation: Knowledge Gaps and Research Opportunities. Rev. Fish. Sci. Aquac. 2016, 25, 100–111. [Google Scholar] [CrossRef]
- Oliver, M.P.; Burhans, R.; Simões, N. Seahorses and Pipefish. In Marine Ornamental Species Aquaculture; Calado, R., Olivotto, I., Planas, M., Holt, G.J., Eds.; Wiley Blackwell: West Sussex, UK, 2017; pp. 299–326. [Google Scholar]
- Hora, M.d.S.C.d.; Joyeux, J.-C.; Rodrigues, R.V.; Sousa-Santos, L.P.d.; Gomes, L.C.; Tsuzuki, M.Y. Tolerance and growth of the longsnout seahorse Hippocampus reidi at different salinities. Aquaculture 2016, 463, 188–193. [Google Scholar] [CrossRef]
- Martinez-Cardenas, L.; Purser, J.G. Effect of direct transfer to different salinities on early juvenile Pot-bellied seahorse, Hippocampus abdominalis, survival in culture conditions. J. World Aquac. Soc. 2016, 47, 201–206. [Google Scholar] [CrossRef]
- Dong, X.; Duan, X.; Sun, Z.; Zhang, X.; Li, C.; Yang, S.; Ren, B.; Zheng, S.; Dionysiou, D.D. Natural illite-based ultrafine cobalt oxide with abundant oxygen-vacancies for highly efficient Fenton-like catalysis. Appl. Catal. B Environ. 2019, 261, 118214. [Google Scholar] [CrossRef]
- Faleiro, F.; Baptista, M.; Santos, C.; Aurélio, M.L.; Pimentel, M.; Pegado, M.R.; Paula, J.R.; Calado, R.; Repolho, T.; Rosa, R. Seahorses under a changing ocean: The impact of warming and acidification on the behaviour and physiology of a poor-swimming bony-armoured fish. Conserv. Physiol. 2015, 3, cov009. [Google Scholar] [CrossRef]
- Simčič, T.; Jesenšek, D.; Brancelj, A. Metabolic potential, respiration rate and their relationship in offspring of different sizes of marble trout (Salmo marmoratus Cuvier). Turk. J. Fish Aquat Sci 2015, 15, 39–48. [Google Scholar] [CrossRef]
- Sokolova, I.M. Energy-limited tolerance to stress as a conceptual framework to integrate the effects of multiple stressors. Integr Comp. Biol 2013, 53, 597–608. [Google Scholar] [CrossRef]
- Sokolova, I.M.; Frederich, M.; Bagwe, R.; Lannig, G.; Sukhotin, A. Energy homeostasis as an integrative tool for assessing limits of environmental stress tolerance in aquatic invertebrates. Mar. Environ. Res. 2012, 79, R713–R715. [Google Scholar] [CrossRef]
- Lushchak, V.I. Contaminant-induced oxidative stress in fish: A mechanistic approach. Fish Physiol. Biochem. 2016, 42, 711–747. [Google Scholar] [CrossRef]
- Shrivastava, J.; Ndugwa, M.; Caneos, W.; De Boeck, G. Physiological trade-offs, acid-base balance and ion-osmoregulatory plasticity in European sea bass (Dicentrarchus labrax) juveniles under complex scenarios of salinity variation, ocean acidification and high ammonia challenge. Aquat. Toxicol. 2019, 212, 54–69. [Google Scholar] [CrossRef]
- Sampaio, E.; Lopes, A.R.; Francisco, S.; Paula, J.R.; Pimentel, M.; Maulvault, A.L.; Repolho, T.; Grilo, T.F.; Pousão-Ferreira, P.; Marques, A.; et al. Ocean acidification dampens physiological stress response to warming and contamination in a commercially-important fish (Argyrosomus regius). Sci Total Environ. 2018, 618, 388–398. [Google Scholar] [CrossRef]
- Tseng, C.; Chien, J.H.; Chu, T.; Cheng, A.; Shiu, Y.; Han, T.; Liu, C. Effects of food type, temperature and salinity on the growth performance and antioxidant status of the longsnout seahorse, Hippocampus reidi. Aquac. Res. 2020, 51, 4793–4804. [Google Scholar] [CrossRef]
- Lushchak, V.I. Environmentally induced oxidative stress in aquatic animals. Aquat. Toxicol. 2011, 101, 13–30. [Google Scholar] [CrossRef]
- Stoliar, O.B.; Lushchak, V.I. Environmental pollution and oxidative stress in fish. In Oxidative stress—Environmental Induction and Dietary Antioxidants; Lushchak, V., Ed.; InTech: Rang-Du-Fliers, France, 2012; pp. 131–166. [Google Scholar]
- Carneiro, M.D.; García-Mesa, S.; Sampaio, L.; Planas, M. Primary, secondary, and tertiary stress responses of juvenile seahorse Hippocampus reidi exposed to acute acid stress in brackish and seawater. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2021, 255, 110592. [Google Scholar] [CrossRef]
- Planas, M.; Silva, C.; Quintas, P.; Chamorro, A.; Piñero, S. Ongrowing and enhancement of n-3 HUFA profile in adult Artemia: Short- vs long-time enrichment. J. Appl. Phycol. 2017, 29, 1409–1420. [Google Scholar] [CrossRef]
- Randazzo, B.; Rolla, L.; Ofelio, C.; Planas, M.; Gioacchini, G.; Vargas, A.; Giorgini, E.; Olivotto, I. The influence of diet on the early development of two seahorse species (H. guttulatus and H. reidi): Traditional and innovative approaches. Aquaculture 2018, 490, 75–90. [Google Scholar] [CrossRef]
- Planas, M.; Chamorro, A.; Quintas, P.; Vilar, A. Establishment and maintenance of threatened long-snouted seahorse, Hippocampus guttulatus, broodstock in captivity. Aquaculture 2008, 283, 19–28. [Google Scholar] [CrossRef]
- Blanco, A.; Chamorro, A.; Planas, M. Implications of physical key factors in the early rearing of the long-snouted seahorse Hippocampus guttulatus. Aquaculture 2014, 433, 214–222. [Google Scholar] [CrossRef]
- Kikkawa, T.; Kita, J.; Ishimatsu, A. Comparison of the lethal effect of CO 2 and acidification on red sea bream (Pagrus major) during the early developmental stages. Mar. Pollut. Bull. 2004, 48, 108–110. [Google Scholar] [CrossRef]
- Eaton, A.D.; Clesceri, L.S.; Rice, E.W.; Greenberg, A.E. Standard Methods for the Examination of Water and Wastewater, 21st ed.; American Public Health Association: Washington, DC, USA, 2005. [Google Scholar]
- Solórzano, L. Determination of ammonia in natural waters by the phenolhypochlorite. Method Limnol. Oceanogr. 1969, 14, 799–801. [Google Scholar] [CrossRef]
- Hansen, H.P.; Grasshoff, K. Automated chemical analysis. In Methods of Seawater Analysis, 2nd ed.; Grasshoff, K., Ehrdhardt, M., Kremling, K., Eds.; Verlag Chemie: Weinheim, Germany, 1983; pp. 347–395. [Google Scholar]
- Novelli, B.; Socorro, J.A.; Caballero, M.J.; Ferrer, F.J.O.; Segade-Botella, A.; Domínguez, L.M. Development of seahorse (Hippocampus reidi, Ginsburg 1933): Histological and histochemical study. Fish Physiol. Biochem. 2015, 41, 1233–1251. [Google Scholar] [CrossRef]
- Suarez-Bregua, P.; Rosendo, S.; Comesaña, P.; Sánchez-Ruiloba, L.; Morán, P.; Planas, M.; Rotllant, J. Dynamic changes in DNA methylation during seahorse (Hippocampus reidi) postnatal development and settlement. Front. Zool. 2021, 18, 52. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Mccord, J.M.; Fridovich, I. Superoxide dismutase: An enzymic function for erythrocuprein (hemocuprein). J. Biol. Chem. 1969, 244, 6049–6055. [Google Scholar] [CrossRef]
- Sturve, J.; Stephensen, E.; Förlin, L. Effects of redox cycling on DT diaphorase activity in the liver of rainbow trout (Oncorhynchus mykiss). Comp. Hepatol. 2005, 4, 4. [Google Scholar] [CrossRef]
- Aebi, H. Catalase in vitro, Methods Enzymol. 1984, 105, 121–126. 105. [CrossRef]
- Lupiañez, J.A.; Adroher, F.J.; Vargas, A.M.; Osuna, A. Differential behaviour of glucose 6-phosphate dehydrogenase in two morphological forms of Trypanosoma cruzi. Int. J. Biochem. 1987, 19, 1085–1089. [Google Scholar] [CrossRef]
- Barroso, J.B.; García-Salguero, L.; Peragón, J.; de la Higuera, M.; Lupiáñez, J. The influence of dietary protein on the kinetics of NADPH production systems in various tissues of rainbow trout (Oncorhynchus mykiss). Aquaculture 1994, 124, 47–59. [Google Scholar] [CrossRef]
- Flohé, L.; Günzler, W.A. Assays of glutathione peroxidase. Methods Enzymol. 1984, 105, 114–120. [Google Scholar] [CrossRef]
- Carlberg, I.; Mannervik, B. Purification and characterization of the flavoenzyme glutathione reductase from rat liver. J. Biol. Chem. 1975, 250, 5475–5480. [Google Scholar] [CrossRef]
- Habig, W.H.; Pabst, M.J.; Jakoby, W.B. Glutathione S-transferases: The first enzymatic step in mercapturic acid formation. J. Biol. Chem. 1974, 249, 7130–7139. [Google Scholar] [CrossRef]
- Anderson, M.E. Determination of glutathione and glutathione disulfide in biological samples. Methods Enzymol. 1985, 113, 548–555. [Google Scholar] [CrossRef]
- Baker, M.A.; Cerniglia, G.J.; Zaman, A. Microtiter plate assay for the measurement of glutathione and glutathione disulfide in large numbers of biological samples. Anal. Biochem. 1990, 190, 360–365. [Google Scholar] [CrossRef]
- Vandeputte, C.; Guizon, I.; Genestie-Denis, I.; Vannier, B.; Lorenzon, G. A microtiter plate assay for total glutathione and glutathione disulfide contents in cultured/isolated cells: Performance study of a new miniaturized protocol. Cell Biol. Toxicol. 1994, 10, 415–421. [Google Scholar] [CrossRef]
- Erel, O. A novel automated direct measurement method for total antioxidant capacity using a new generation, more stable ABTS radical cation. Clin. Biochem. 2004, 37, 277–285. [Google Scholar] [CrossRef]
- Buege, J.A.; Aust, S.D. Microsomal lipid peroxidation. Methods Enzymol. 1978, 52, 302–310. [Google Scholar] [CrossRef]
- Lourie, S.A.; Foster, S.J.; Cooper, E.W.T.; Vincent, A.C.J. A Guide to the Identification of Seahorses; University of British Columbia and World Wildlife Fund: Washington, DC, USA, 2004. [Google Scholar]
- Friedman, M. The Use of Ranks to Avoid the Assumption of Normality Implicit in the Analysis of Variance. J. Am. Stat. Assoc. 1937, 32, 675–701. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing. Available online: https://www.R-project.org/ (accessed on 23 February 2018).
- Husson, F.; Josse, J.; Le, S.; Mazet, J. FactoMineR: Multivariate Exploratory Data Analysis and Data Mining. Available online: https://CRAN.R-project.org/package=FactoMineR (accessed on 20 March 2020).
- Kassambara, A. Factoextra: Extract and Visualize the Results of Multivariate Data Analyses. Available online: https://CRAN.R-project.org/package=factoextra (accessed on 23 February 2020).
- Wei, T.; Simko, V.; Levy, M.; Xie, Y.; Jin, Y.; Zemla, J. Corrplot: Visualization of a Correlation Matrix. Available online: https://CRAN.R-project.org/package=corrplot (accessed on 6 March 2020).
- Birnie-Gauvin, K.; Costantini, D.; Cooke, S.J.; Willmore, W.G. A comparative and evolutionary approach to oxidative stress in fish: A review. Fish Fish. 2017, 18, 928–942. [Google Scholar] [CrossRef]
- Gressler, L.T.; Riffel, A.P.K.; Parodi, T.V.; Saccol, E.M.H.; Koakoski, G.; da Costa, S.T.; Pavanato, M.A.; Heinzmann, B.M.; Caron, B.; Schmidt, D.; et al. Silver catfish Rhamdia quelen immersion anaesthesia with essential oil of Aloysia triphylla (L’Hérit) Britton or tricaine methanesulfonate: Effect on stress response and antioxidant status. Aquac. Res. 2014, 45, 1061–1072. [Google Scholar] [CrossRef]
- Planas, M.; Olivotto, I.; González, M.J.; Laurà, R.; Angeletti, C.; Amici, A.; Zarantoniello, M. Pre-breeding Diets in the Seahorse Hippocampus reidi: How Do They Affect Fatty Acid Profiles, Energetic Status and Histological Features in Newborn? Front. Mar. Sci. 2021, 8, 688058. [Google Scholar] [CrossRef]
- Whittington, C.M.; Griffith, O.W.; Qi, W.; Thompson, M.B.; Wilson, A.B. Seahorse brood pouch transcriptome reveals common genes associated with vertebrate pregnancy. Mol. Biol. Evol. 2015, 32, 3114–3131. [Google Scholar] [CrossRef] [PubMed]
- Kang, C.-K.; Yang, W.-K.; Lin, S.-T.; Liu, C.-C.; Lin, H.-M.; Chen, H.-H.; Cheng, C.-W.; Lee, T.-H.; Hwang, P.-P. The acute and regulatory phases of time-course changes in gill mitochondrion-rich cells of seawater-acclimated medaka (Oryzias dancena) when exposed to hypoosmotic environments. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2013, 164, 181–191. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, R.V.; Pedron, J.D.S.; Romano, L.A.; Tesser, M.B.; Sampaio, L.A. Acute responses of juvenile cobia Rachycentron canadum (Linnaeus 1766) to acid stress. Aquac. Res. 2015, 46, 1241–1247. [Google Scholar] [CrossRef]
- Kwan, G.T.; Shen, S.G.; Drawbridge, M.; Checkley, D.M.; Tresguerres, M. Ion-transporting capacity and aerobic respiration of larval white seabass (Atractoscion nobilis) may be resilient to ocean acidification conditions. Sci. Total Environ. 2021, 791, 148285. [Google Scholar] [CrossRef]
- Mota, V.C.; Hop, J.; Sampaio, L.A.; Heinsbroek, L.T.N.; Verdegem, M.C.J.; Eding, E.H.; Verreth, J.A.J. The effect of low pH on physiology, stress status and growth performance of turbot (Psetta maxima L.) cultured in recirculating aquaculture systems. Aquac. Res. 2018, 49, 3456–3467. [Google Scholar] [CrossRef]
- Tresguerres, M.; Hamilton, T.J. Acid-base physiology, neurobiology and behaviour in relation to CO2-induced ocean acidification. J. Exp. Biol. 2017, 220, 2136–2148. [Google Scholar] [CrossRef]
- Sánchez-Muros, M.; García-Rejón, L.; Lupiáñez, J.; Higuera, M. Long-term nutritional effects on the primary liver and kidney metabolism in rainbow trout, Oncorhynchus mykiss (Walbaum): Adaptive response to a high-protein/non-carbohydrate diet and starvation of glucose 6-phosphate dehydrogenase activity. Aquac. Nutr. 2015, 1, 213–220. [Google Scholar] [CrossRef]
- Gallagher, E.P.; Hasspieler, B.M.; Di Glulio, R.T. Effects of buthionine sulfoximine and diethyl maleate on glutathione turnover in the channel catfish. Biochem. Pharmacol. 1992, 43, 2209–2215. [Google Scholar] [CrossRef]
- Otto, D.M.E.; Moon, T.W. Endogenous antioxidant systems of two teleost fish, the rainbow trout and the black bullhead, and the effect of age. Fish Physiol. Biochem. 1996, 15, 349–358. [Google Scholar] [CrossRef]
- Winston, G.W.; Di Giulio, R.T. Prooxidant and antioxidant mechanisms in aquatic organisms. Aquat. Toxicol. 1991, 19, 137–161. [Google Scholar] [CrossRef]
- Martínez-Álvarez, R.M.; Morales, A.E.; Sanz, A. Antioxidant Defenses in Fish: Biotic and Abiotic Factors. Rev. Fish Biol. Fish. 2005, 15, 75–88. [Google Scholar] [CrossRef]
- Fernández-Díaz, C.; Kopecka, J.; Cañavate, J.; Sarasquete, C.; Solé, M. Variations on development and stress defences in Solea senegalensis larvae fed on live and microencapsulated diets. Aquaculture 2006, 251, 573–584. [Google Scholar] [CrossRef]
- livotto, I.; Di Stefano, M.; Rosetti, S.; Cossignani, L.; Pugnaloni, A.; Giantomassi, F.; Carnevali, O. Live prey enrichment, with particular emphasis on HUFAs, as limiting factor in false percula clownfish (Amphiprion ocellaris, Pomacentridae) larval development and metamorphosis: Molecular and biochemical implications. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2011, 159, 207–218. [Google Scholar] [CrossRef]
- Costantini, D. Understanding diversity in oxidative status and oxidative stress: The opportunities and challenges ahead. J. Exp. Biol. 2019, 222, jeb194688. [Google Scholar] [CrossRef]
- Ray, P.D.; Huang, B.-W.; Tsuji, Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell. Signal. 2012, 24, 981–990. [Google Scholar] [CrossRef]
- Wang, Y.; Branicky, R.; Noë, A.; Hekimi, S. Superoxide dismutases: Dual roles in controlling ROS damage and regulating ROS signaling. J. Cell Biol. 2018, 217, 1915–1928. [Google Scholar] [CrossRef]
- Kamler, E.; Keckei, H.; Bauer-Nemeschkal, E. Temperature-induced changes of survival, development and yolk partitioning in Chondrostoma nasus. J. Fish Biol. 1998, 53, 658–682. [Google Scholar] [CrossRef]
- Toth, L.G.; Szabo, M.; Webb, D.J. Adaptation of the tetrazolium reduction test for the measurement of the electron transport system (ETS) activity during embryonic development of medaka. J. Fish Biol. 1995, 46, 835–844. [Google Scholar] [CrossRef]
- Boveris, A.; Chance, B. The mitochondrial generation of hydrogen peroxide. General properties and effect of hyperbaric oxygen. Biochem. J. 1973, 134, 707–716. [Google Scholar] [CrossRef] [PubMed]
- Biller, J.D.; Takahashi, L.S. Oxidative stress and fish immune system: Phagocytosis and leukocyte respiratory burst activity. An. Acad. Bras. Cienc. 2018, 90, 3403–3414. [Google Scholar] [CrossRef] [PubMed]
- Lin, G.; Zheng, M.; Gao, D.; Li, S.; Fang, W.; Huang, J.; Xie, J.; Liu, J.; Liu, Y.; Li, Z.; et al. Hypoosmotic stress induced tissue-specific immune responses of yellowfin seabream (Acanthopagrus latus) revealed by transcriptomic analysis. Fish Shellfish Immunol. 2020, 99, 473–482. [Google Scholar] [CrossRef] [PubMed]
- Lin, G.; Zheng, M.; Li, S.; Xie, J.; Fang, W.; Gao, D.; Huang, J.; Lu, J. Response of gut microbiota and immune function to hypoosmotic stress in the yellowfin seabream (Acanthopagrus latus). Sci. Total. Environ. 2020, 745, 140976. [Google Scholar] [CrossRef] [PubMed]
- Cui, W.; Cao, L.; Liu, J.; Ren, Z.; Zhao, B.; Dou, S. Effects of seawater acidification and cadmium on the antioxidant defense of flounder Paralichthys olivaceus larvae. Sci. Total Environ. 2020, 718, 137234. [Google Scholar] [CrossRef]
- Moreira, A.; Figueira, E.; Soares, A.M.V.M.; Freitas, R. The effects of arsenic and seawater acidification on antioxidant and biomineralization responses in two closely related Crassostrea species. Sci. Total Environ. 2016, 545–546, 569–581. [Google Scholar] [CrossRef]
- Díaz, M.E.; Furné, M.; Trenzado, C.E.; García-Gallego, M.; Domezain, A.; Sanz, A. Antioxidant defences in the first life phases of the sturgeon Acipenser naccarii. Aquaculture 2010, 307, 123–129. [Google Scholar] [CrossRef]
- Robergs, R.A. Competitive cation binding computations of proton balance for reactions of the phosphagen and glycolytic energy systems within skeletal muscle. PLoS ONE 2017, 12, e0189822. [Google Scholar] [CrossRef]
- Robergs, R.A. Invited review: Quantifying proton exchange from chemical reactions—Implications for the biochemistry of metabolic acidosis. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2019, 235, 29–45. [Google Scholar] [CrossRef]
- Matsuyama, S.; Reed, J.C. Mitochondria-dependent apoptosis and cellular pH regulation. Cell Death Differ. 2000, 7, 1155–1165. [Google Scholar] [CrossRef]
- Carcupino, M.; Baldacci, A.; Mazzini, M.; Franzoi, P. Functional significance of the male brood pouch in the reproductive strategies of pipefishes and seahorses: A morphological and ultrastructural comparative study on three anatomically different pouches. J. Fish Biol. 2002, 61, 1465–1480. [Google Scholar] [CrossRef]
- Sommer, S.; Whittington, C.M.; Wilson, A.B. Standardised classification of pre-release development in male-brooding pipefish, seahorses, and seadragons (Family Syngnathidae). BMC Dev. Biol. 2012, 12, 39. [Google Scholar] [CrossRef]
- Hermes-Lima, M.; Moreira, D.C.; Rivera-Ingraham, G.A.; Giraud-Billoud, M.; Genaro-Mattos, T.C.; Campos, É.G. Preparation for oxidative stress under hypoxia and metabolic depression: Revisiting the proposal two decades later. Free Radic. Biol. Med. 2015, 89, 1122–1143. [Google Scholar] [CrossRef]
- Maulvault, A.L.; Barbosa, V.; Alves, R.; Anacleto, P.; Camacho, C.; Cunha, S.; Fernandes, J.O.; Ferreira, P.P.; Rosa, R.; Marques, A.; et al. Integrated multi-biomarker responses of juvenile seabass to diclofenac, warming and acidification co-exposure. Aquat. Toxicol. 2018, 202, 65–79. [Google Scholar] [CrossRef]
- Silva, C.S.E.; Novais, S.C.; Lemos, M.F.L.; Mendes, S.; Oliveira, A.P.; Gonçalves, E.J.; Faria, A. M Effects of ocean acidification on the swimming ability, development and biochemical responses of sand smelt larvae. Sci. Total Environ. 2016, 563–564, 89–98. [Google Scholar] [CrossRef]
- Srikanth, K.; Pereira, E.; Duarte, A.C.; Ahmad, I. Glutathione and its dependent enzymes’ modulatory responses to toxic metals and metalloids in fish—A review. Environ. Sci. Pollut. Res. 2013, 20, 2133–2149. [Google Scholar] [CrossRef]
- Almroth, B.C.; Johansson, A.; Förlin, L.; Sturve, J. Early-age changes in oxidative stress in brown trout, Salmo trutta. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2010, 155, 442–448. [Google Scholar] [CrossRef]
- Barcellos, J.L.G.; Kreutz, L.C.; Koakoski, G.; Oliveira, T.A.; Rosa, J.G.S.d.; Fagundes, M. Fish age, instead of weight and size, as a determining factor for time course differences in cortisol response to stress. Physiol. Behav. 2012, 107, 397–400. [Google Scholar] [CrossRef]
- Halmetoja, A.; Valtonen, E.T.; Koskenniemi, E. Perch (Perca fluviatilis L.) parasites reflect ecosystem conditions: A comparison of a natural lake and two acidic reservoirs in Finland. Int. J. Parasitol. 2000, 30, 1437–1444. [Google Scholar] [CrossRef]
- Jain, S.; Nath, S. Kinetic model of ATP synthase: pH dependence of the rate of ATP synthesis. FEBS Lett. 2000, 476, 113–117. [Google Scholar] [CrossRef]
- Stanton, R.C. Glucose-6-phosphate dehydrogenase, NADPH, and cell survival. IUBMB Life 2012, 64, 362–369. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Jiang, Z.; Mao, M.; Mao, S.; Sun, Y.; Sui, Y. Effects of seawater pH on survival, growth, energy budget and oxidative stress parameters of juvenile turbot Scophthalmus maximus. Iran J. Fish. Sci. 2018, 17, 675–689. [Google Scholar] [CrossRef]
- Kuno, M.; Li, G.; Moriura, Y.; Hino, Y.; Kawawaki, J.; Sakai, H. Acid-inducible proton influx currents in the plasma membrane of murine osteoclast-like cells. Pflugers Arch. Eur. J. Physiol. 2016, 468, 837–847. [Google Scholar] [CrossRef] [PubMed]
| SW | BW | |||
|---|---|---|---|---|
| pH 6.5 | pH 8.0 | pH 6.5 | pH 8.0 | |
| Salinity (‰) | 33 ± 1 | 11 ± 1 | ||
| pH | 6.6 ± 0.01 | 8.1 ± 0.0 | 6.5 ± 0.01 | 7.8 ± 0.01 |
| Alkalinity (mg CaCO3 L−1) | 22 ± 5 b | 138 ± 5 a | 20 ± 5 b | 62 ± 5 a |
| Temperature ( °C) | 26.1 ± 0.1 | 26.1 ± 0.2 | ||
| Oxygen (mg O2 L−1) | 6.53 ± 0.03 | 6.56 ± 0.01 | ||
| TAN (mg N- NH4 + +NH3 L−1) | 0.21 ± 0.04 | 0.13 ± 0.02 | ||
| Nitrite (mg N-NO2 L−1) | 0.02 ± 0.0 | 0.05 ± 0.05 | ||
| Nitrate (mg N-NO3 L−1) | 0.13 ± 0.03 | 0.11 ± 0.01 | ||
| pH 6.5 | pH 8 | |
|---|---|---|
| Salinity (‰) | 33 ± 1 | |
| pH | 6.6 ± 0.01 | 8.1 ± 0.0 |
| Alkalinity (mg CaCO3 L−1) | 21 ± 4 b | 142 ± 7 a |
| Temperature (°C) | 26.0 ± 0.5 | |
| Oxygen (mg O2 L−1) | 6.5 ± 0.5 | |
| TAN (mg N-NH4 + +NH3 L−1) | 0.3 ± 0.1 | |
| Nitrite (mg N-NO2 L−1) | 0.1 ± 0.05 | |
| Nitrate (mg N-NO3 L−1) | 0.2 ± 0.05 | |
| SW | BW | |||
|---|---|---|---|---|
| pH 6.5 | pH 8.0 | pH 6.5 | pH 8.0 | |
| Survival (%) | 98.9 ± 0.48 a | 96.9 ± 0.96 b | 86.9 ± 2.2 b | 92.2 ± 2.2 a |
| Final length (mm) | 27.5 ± 0.86 | 26.9 ± 1.10 | 19.6 ± 0.2 b | 22.8 ± 0.7 a |
| Final weight (mg) | 37.8 ± 2.7 | 35.8 ± 3.5 | 17.6 ± 0.4 b | 27.9 ± 2.2 a |
| SGR (%) | 5.1 ± 0.2 | 5.0 ± 0.1 | 4.0 ± 0.0 b | 4.8 ± 0.1 a |
| K | 0.18 ± 0.01 | 0.18 ± 0.01 | 0.23 ± 0.03 | 0.23 ± 0.00 |
| Age (Days after Male’s Pouch Release—DAR) | ANOVA (p) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| pH | 0 | 2 | 7 | 14 | 21 | age | pH | Age × pH | |
| SOD | 6.5 | 51.5 ± 10.2 | 51.6 ± 19.9 | 45.7 ± 1.8 | 49.9 ± 3.2 | 47.4 ± 2.5 | 0.159 | 0.613 | 0.478 |
| 8.0 | 47.0 ± 0.7 | 58.1 ± 16.4 | 52.2 ± 2.8 | 75.1 ± 11.0 | |||||
| DTD | 6.5 | 3.1 ± 1.0 ab | 2.7 ± 1.0 b | 4.2 ± 0.5 ab | 4.2 ± 0.2 ab | 4.64 ± 0.3 a | 0.001 | 0.788 | 0.238 |
| 8.0 | 3.4 ± 0.2 ab | 4.3 ± 0.5 ab | 3.1 ± 0.1 ab | 4.7 ± 0.5 a | |||||
| CAT | 6.5 | 3.6 ± 0.6 cd | 2.6 ± 0.7 d | 4.5 ± 1.4 c | 7.7 ± 1.2 ab | 11.4 ± 0.5 a | <0.001 | 0.252 | 0.438 |
| 8.0 | 2.5 ± 0.0 d | 3.6 ± 0.3 cd | 5.0 ± 1.1 bc | 12.1 ± 1.5 a | |||||
| G6PDH | 6.5 | 1.0 ± 0 b | 1.22 ± 0.3 b | SNA | 2.0 ± 0.0 a | 2.7 ± 0.3 a | <0.001 | - | 0.958 |
| 8.0 | 1.1 ± 0.1 b | 1.3 ± 0.1 b | SNA | 2.6 ± 0.0 a | |||||
| Age (Days after Male’s Pouch Release—DAR) | ANOVA (p) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| pH | 0 | 2 | 7 | 14 | 21 | Age | pH | Age × pH | |
| GPx | 6.5 | 420 ± 240 ab | 398 ± 45 a | 168 ± 54 c | 227 ± 22 abc | 351 ± 36 ab | <0.001 | 0.231 | 0.934 |
| 8.0 | 344 ± 53 ab | 129 ± 52 c | 193 ± 10 bc | 342 ± 23 ab | |||||
| GR | 6.5 | 5.5 ± 0.3 c | 5.2 ± 0.8 c | 7.2 ± 0.3 bc | 8.6 ± 0.7 ab | 10.4 ± 0.8 a | <0.001 | 0.640 | 0.901 |
| 8.0 | 5.2 ± 0.2 c | 7.5 ± 0.8 bc | 7.9 ± 0.9 b | 10.0 ± 1.4 a | |||||
| GST | 6.5 | 5.0 ± 1.2 d | 6.3 ± 0.1 d | 10.6 ± 1.3 cd | 18.1 ± 3.5 b | 17.3 ± 2.3 bc | <0.001 | 0.288 | 0.087 |
| 8.0 | 5.7 ± 0.1 d | 12.1 ± 4.1 cd | 16.0 ± 0.0 bc | 24.3 ± 3.6 a | |||||
| GSH | 6.5 | 59.1 ± 2.7 ab | 48.4 ± 0.5 b | 57.6 ± 4.4 ab | 68.5 ± 11.2 a | 73.9 ± 3.5 a | <0.001 | 0.031 | 0.303 |
| 8.0 | 45.7 ± 0.9 b | 56.2 ± 3.4 ab | 58.1 ± 2.6 ab | 60.8 ± 9.6 ab | |||||
| GSSG | 6.5 | 2.6 ± 0.1 ab | 2.3 ± 0.2 b | 3.5 ± 0.3 ab | 3.4 ± 0.1 ab | 3.1 ± 0.4 ab | 0. 006 | 0.379 | 0.175 |
| 8.0 | 2.7 ± 0.0 ab | 2.9 ± 0.7 ab | 3.6 ± 0.8 ab | 3.9 ± 0.2 a | |||||
| OSI | 6.5 | 8.7 ± 0.7 | 9.7 ± 0.8 | 12.3 ± 1.9 | 10.0 ± 1.4 | 8.4 ± 1.4 | 0.379 | 0.095 | 0.171 |
| 8.0 | 11.9 ± 0.3 | 10.6 ± 3.1 | 12.5 ± 3.3 | 12.9 ± 2.7 | |||||
| TEAC | 6.5 | 207 ± 27 a | 183 ± 0.7 ab | 76 ± 43 b | 73 ± 38 b | 99 ± 25 b | <0.001 | 0.714 | 0.543 |
| 8.0 | 173 ± 32 ab | 88 ± 52 b | 121 ± 47 ab | 76 ± 24 b | |||||
| TBARS | 6.5 | 0.00 ± 0.00 c | 0.02 ± 0.02 bc | 0.06 ± 0.05 bc | 0.18 ± 0.11 ab | 0.14 ± 0.05 ab | <0.001 | 0.918 | 0.540 |
| 8.0 | 0.0 ± 0.0 c | 0.06 ± 0.03 bc | 0.17 ± 0.09 ab | 0.34 ± 0.2 a | |||||
| Age (Days after Male’s Pouch Release—DAR) | ANOVA (p) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| pH | 0 | 2 | 7 | 14 | 21 | Age | pH | Age × pH | |
| SOD | 6.5 | 39.6 ± 17.6 b | 68.5 ± 4.6 a | 100.7 ± 32.4 a | 91.3 ± 14.6 a | 89.3 ± 33.3 a | 0.012 | 0.311 | 0.331 |
| 8.0 | 68.9 ± 6.8 a | 62.9 ± 14.4 a | 71.9 ± 4.5 a | 101.6 ± 25.8 a | |||||
| DTD | 6.5 | 1.9 ± 2.1 b | 4.1 ± 0.6 a | 4.5 ± 0.3 a | 4.44 ± 0.6 a | 4.3 ± 0.1 a | 0.019 | 0.245 | 0.775 |
| 8.0 | 4.3 ± 0.9 a | 4.5 ± 0.1 a | 5.1 ± 0.5 a | 4.6 ± 0.6 a | |||||
| CAT | 6.5 | 4.7 ± 0.2 b | 5.5 ± 1.2 b | 8.2 ± 2.3 ab | 11.9 ± 1.0 a | 10.4 ± 4.7 ab | <0.001 | 0.288 | 0.374 |
| 8.0 | 5.0 ± 0.4 b | 5.3 ± 0.5 b | 8.8 ± 1.8 ab | 13.7 ± 4.1 a | |||||
| G6PDH | 6.5 | 2.0 ± 0.4 | SNA | 1.8 ± 0.3 | 1.8 ± 0.5 | 1.6 ± 0.6 | 0.147 | - | 0.110 |
| 8.0 | 1.3 ± 0.1 | 1.0 ± 0.3 | 2.2 ± 0.5 | 2.2 ± 0.4 | |||||
| Age (Days after the Male’s Pouch Release—DAR) | ANOVA (p) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| pH | 0 | 2 | 7 | 14 | 21 | Age | pH | Age × pH | |
| GPx | 6.5 | 486 ± 110 a | 271 ± 41 ab | 177 ± 2 b | 313 ± 21 ab | 226 ± 72 b | <0.001 | 0.442 | 0.485 |
| 8.0 | 256 ± 39 b | 171 ± 31 b | 323 ± 128 ab | 354 ± 59 ab | |||||
| GR | 6.5 | 7.4 ± 1.8 b | 7.4 ± 0.9 b | 10.9 ± 2.8 ab | 12.6 ± 0.9 a | 11.3 ± 3.3 a | 0.008 | 0.550 | 0.462 |
| 8.0 | 7.1 ± 0.3 b | 8.3 ± 0.6 ab | 10.7 ± 1.9 a | 13.4 ± 3.9 a | |||||
| GST | 6.5 | 13.1 ± 4.3 | 14.9 ± 4.5 | 21.0 ± 6.1 | 21.6 ± 8.2 | 14.4 ± 3.2 | 0.096 | 0.777 | 0.171 |
| 8.0 | 15.3 ± 2.1 | 12.7 ± 2.7 | 23.2 ± 6.0 | 19.7 ± 5.7 | |||||
| GSH | 6.5 | 48 ± 5.5 ab | 27.3 ± 0.1 c | 37.2 ± 5.7 c | 32.9 ± 4.3 c | 24.0 ± 0.7 c | <0.001 | <0.001 | <0.001 |
| 8.0 | 31.5 ± 3.3 c | 32.5 ± 3.8 c | 56.5 ± 7.8 a | 56.7 ± 0.3 a | |||||
| GSSG | 6.5 | 1.1 ± 1.5 d | 2.5 ± 0.5 cd | 2.6 ± 0.01 cd | 2.0 ± 0.01 d | 4.3 ± 0.4 a | <0.001 | 0.072 | 0.009 |
| 8.0 | 2.5 ± 0.2 cd | 3.2 ± 0.6 abc | 2.8 ± 0.2 bc | 3.4 ± 0.3 ab | |||||
| OSI | 6.5 | 4.7 ± 6.7 e | 18.2 ± 3.8 bc | 15.6 ± 1.4 bc | 13.6 ± 2.0 bc | 36.0 ± 2.1 a | <0.001 | 0.001 | <0.001 |
| 8.0 | 16.0 ± 0.6 bc | 19.4 ± 1.6 b | 10.1 ± 1.0 de | 11.5 ± 0.6 cde | |||||
| TEAC | 6.5 | 210 ± 15 a | 55 ± 53 abc | 40 ± 22 abcd | 48 ± 39 abcd | 2.2 ± 3.8 cd | <0.001 | 0.529 | 0.748 |
| 8.0 | 92 ± 0 ab | 67 ± 48 ab | 33 ± 23 bcd | 0 ± 0 d | |||||
| TBARS | 6.5 | 1.56 ± 1.0 | 1.21 ± 0.1 | 0.15 ± 0.2 | 0.63 ± 0.4 | 0.44 ± 0.22 | 0.081 | 0.164 | 0.300 |
| 8.0 | 1.25 ± 0.4 | 1.26 ± 0.3 | 0.38 ± 0.3 | 1.23 ± 0.94 | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carneiro, M.D.D.; García-Mesa, S.; Sampaio, L.A.; Planas, M. Implications of Salinity and Acidic Environments on Fitness and Oxidative Stress Parameters in Early Developing Seahorses Hippocampus reidi. Animals 2022, 12, 3227. https://doi.org/10.3390/ani12223227
Carneiro MDD, García-Mesa S, Sampaio LA, Planas M. Implications of Salinity and Acidic Environments on Fitness and Oxidative Stress Parameters in Early Developing Seahorses Hippocampus reidi. Animals. 2022; 12(22):3227. https://doi.org/10.3390/ani12223227
Chicago/Turabian StyleCarneiro, Mario D. D., Sergio García-Mesa, Luis A. Sampaio, and Miquel Planas. 2022. "Implications of Salinity and Acidic Environments on Fitness and Oxidative Stress Parameters in Early Developing Seahorses Hippocampus reidi" Animals 12, no. 22: 3227. https://doi.org/10.3390/ani12223227
APA StyleCarneiro, M. D. D., García-Mesa, S., Sampaio, L. A., & Planas, M. (2022). Implications of Salinity and Acidic Environments on Fitness and Oxidative Stress Parameters in Early Developing Seahorses Hippocampus reidi. Animals, 12(22), 3227. https://doi.org/10.3390/ani12223227








