Exosomes Derived from Yak Follicular Fluid Increase 2-Hydroxyestradiol Secretion by Activating Autophagy in Cumulus Cells
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Yak Ovaries and Follicular Fluid
2.2. Isolation and Identification of Exosomes
2.3. Isolation and Culture of Yak Cumulus Cells
2.4. Exosome Labeling and Co-Incubation with YCCs
2.5. Immunofluorescence
2.6. Treatment of Cells with 3-MA and RAPA
2.7. Quantitative RT-qPCR Analysis
2.8. Western Blotting (WB) Analysis
2.9. Evaluation of Fluorescent LC3 Puncta
2.10. Enzyme-Linked Immunosorbent Assay (ELISA)
2.11. Statistical Analysis
3. Results
3.1. YCC Cultures and Characterization of Yak Follicular Fluid Exosomes
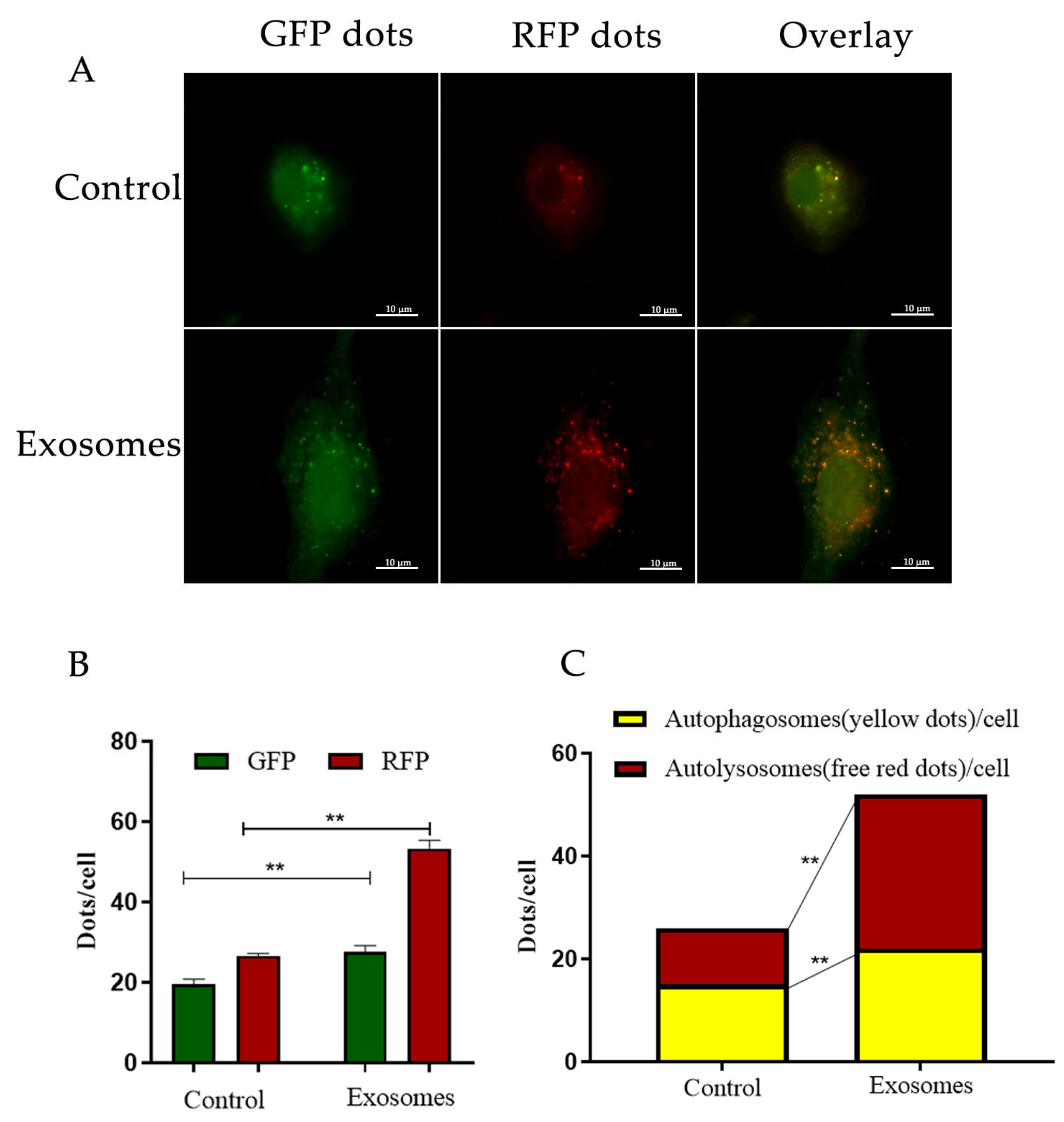
3.2. Yak Follicular Fluid Exosomes Promote Autophagy Induction by Being Taken Up by YCCs
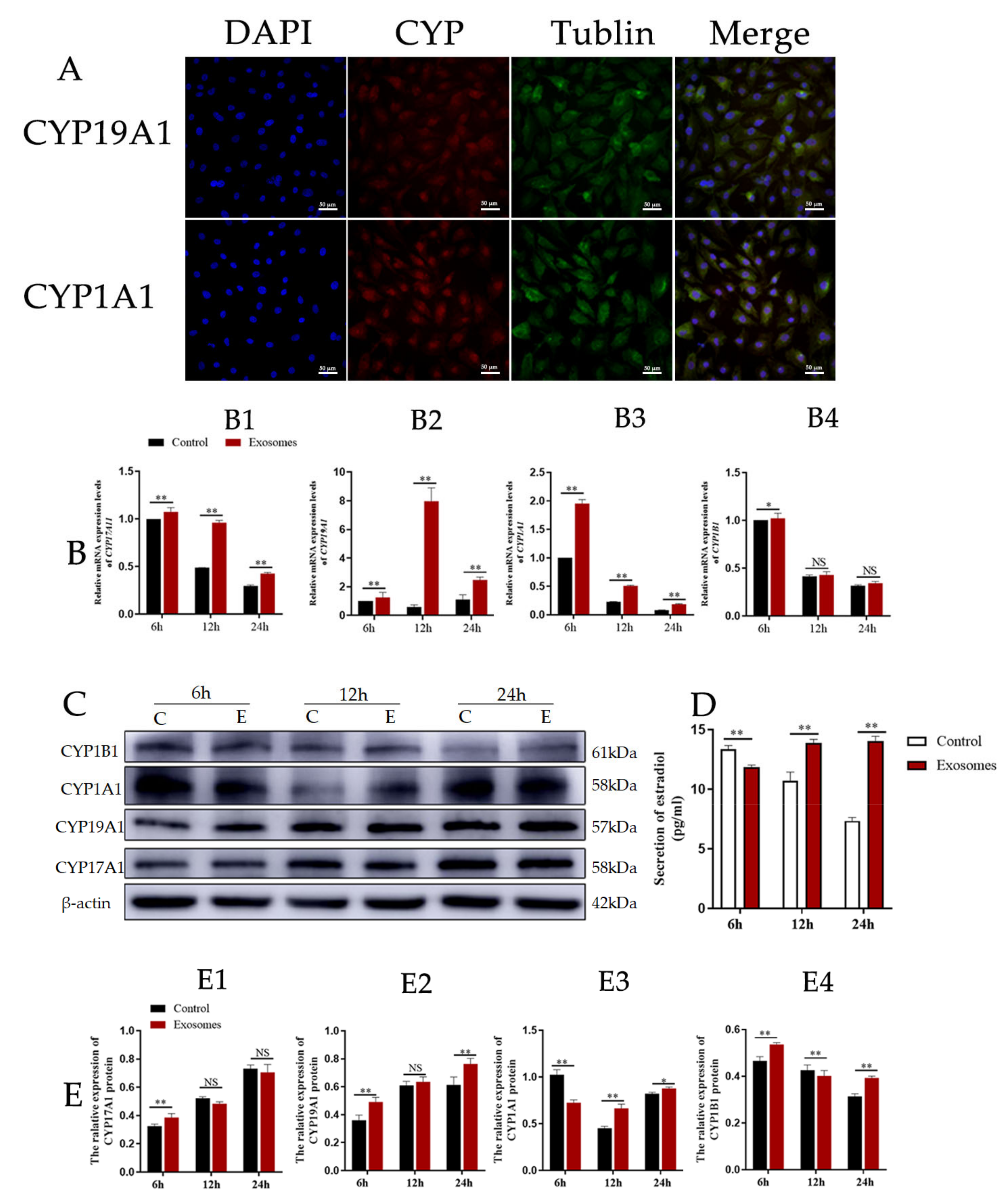
3.3. Exosomes Derived from Yak Follicular Fluid Could Increase 2-OHE2 Secretion in YCCs
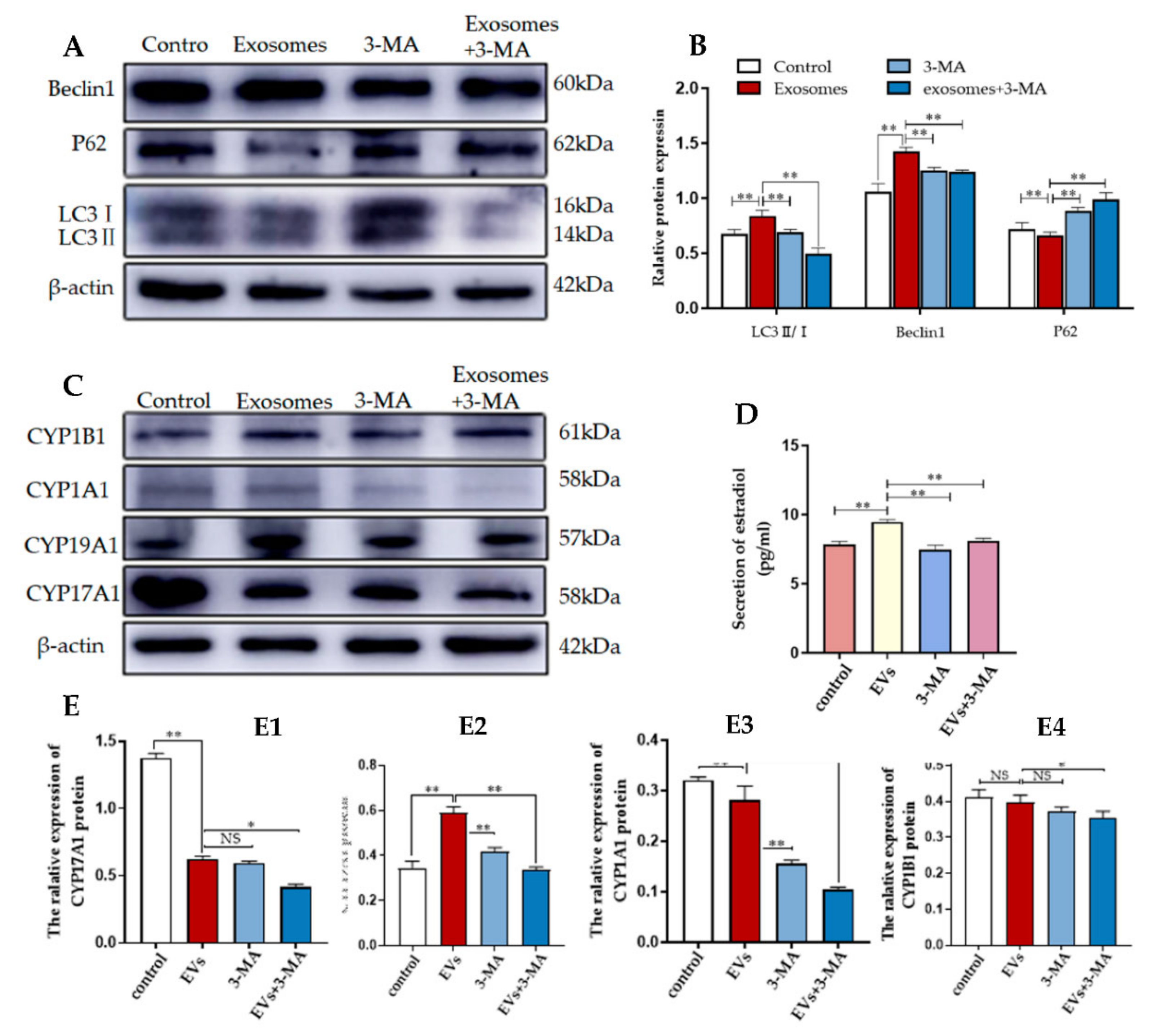
3.4. Autophagy Is Required for Yak Follicular Fluid Exosome-Mediated 2-OHE2 Secretion in YCCs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gu, X.; Li, Y.; Chen, K.; Wang, X.; Wang, Z.; Lian, H.; Lin, Y.; Rong, X.; Chu, M.; Lin, J.; et al. Exosomes derived from umbilical cord mesenchymal stem cells alleviate viral myocarditis through activating AMPK/mTOR-mediated autophagy flux pathway. J. Cell. Mol. Med. 2020, 24, 7515–7530. [Google Scholar] [CrossRef] [PubMed]
- Bister, N.; Pistono, C.; Huremagic, B.; Jolkkonen, J.; Giugno, R.; Malm, T. Hypoxia and extracellular vesicles: A review on methods, vesicular cargo and functions. J. Extracell. Vesicles 2020, 10, e12002. [Google Scholar] [CrossRef] [PubMed]
- Machtinger, R.; Laurent, L.C.; Baccarelli, A.A. Extracellular vesicles: Roles in gamete maturation, fertilization and embryo implantation. Hum. Reprod. Update 2016, 22, 182–193. [Google Scholar] [CrossRef]
- Klionsky, D.J.; Petroni, G.; Amaravadi, R.K.; Baehrecke, E.H.; Ballabio, A.; Boya, P.; Pedro, J.M.B.; Cadwell, K.; Cecconi, F.; Choi, A.M.K.; et al. Autophagy in major human diseases. EMBO J. 2021, 40, e108863. [Google Scholar] [CrossRef] [PubMed]
- Markaki, M.; Tavernarakis, N. Autophagy mechanisms and roles: Recent advances and implications. FEBS J. 2020, 287, 5024–5026. [Google Scholar] [CrossRef]
- Iqbal, I.K.; Bajeli, S.; Sahu, S.; Bhat, S.A.; Kumar, A. Hydrogen sulfide-induced GAPDH sulfhydration disrupts the CCAR2-SIRT1 interaction to initiate autophagy. Autophagy 2021, 17, 3511–3529. [Google Scholar] [CrossRef]
- Hariharan, N.; Zhai, P.; Sadoshima, J. Oxidative stress stimulates autophagic flux during ischemia/reperfusion. Antioxid. Redox Signal. 2011, 14, 2179–2190. [Google Scholar] [CrossRef]
- Li, H.P.; Liu, J.T.; Chen, Y.X.; Wang, W.B.; Han, Y.; Yao, Q.P.; Qi, Y.-X. Suppressed nuclear envelope proteins activate autophagy of vascular smooth muscle cells during cyclic stretch application. Biochimica et biophysica acta. Mol. Cell. Res. 2021, 1868, 118855. [Google Scholar]
- Wen, X.; Han, X.R.; Wang, Y.J.; Wang, S.; Shen, M.; Zhang, Z.F.; Fan, S.; Shan, Q.; Wang, L.; Li, M.; et al. MicroRNA-421 suppresses the apoptosis and autophagy of hippocampal neurons in epilepsy mice model by inhibition of the TLR/MYD88 pathway. J. Cell. Physiol. 2018, 233, 7022–7034. [Google Scholar] [CrossRef]
- Qu, Y.; Zhang, Q.; Cai, X.; Li, F.; Ma, Z.; Xu, M.; Lu, L. Exosomes derived from miR-181-5p-modified adipose-derived mesenchymal stem cells prevent liver fibrosis via autophagy activation. J. Cell. Mol. Med. 2017, 21, 2491–2502. [Google Scholar] [CrossRef]
- Baixauli, F.; López-Otín, C.; Mittelbrunn, M. Exosomes and autophagy: Coordinated mechanisms for the maintenance of cellular fitness. Front. Immunol. 2014, 5, 403. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Li, Y.; Zhang, W.; Qian, H.; Zhang, Z.; Xu, K. Alginic acid induces oxidative stress-mediated hormone secretion disorder, apoptosis and autophagy in mouse granulosa cells and ovaries. Toxicology 2022, 467, 153099. [Google Scholar] [CrossRef] [PubMed]
- Tofovic, S.P.; Dubey, R.; Salah, E.M.; Jackson, E.K. 2-Hydroxyestradiol attenuates renal disease in chronic puromycin aminonucleoside nephropathy. J. Am. Soc. Nephrol. 2002, 13, 2737–2747. [Google Scholar] [CrossRef] [PubMed]
- Merico, V.; Zanoni, M.; Parada-Bustamante, A.; Garagna, S.; Zuccotti, M. In vitro maturation of fully grown mouse antral follicles in the presence of 1 nM 2-hydroxyestradiol improves oocytes’ developmental competence. Reprod. Sci. 2021, 28, 121–133. [Google Scholar] [CrossRef]
- Chen, Y.; Zeng, R.; Kou, J.; Xiong, X.; Yao, Y.; Fu, W.; Yin, S.; Lan, D. GPR50 participates in and promotes yak oocyte maturation: A new potential oocyte regulatory molecule. Theriogenology 2022, 181, 34–41. [Google Scholar] [CrossRef]
- Liu, P.; Yu, S.; Cui, Y.; He, J.; Zhang, Q.; Sun, J.; Huang, Y.; Yang, X.; Cao, M.; Liao, B.; et al. Regulation by Hsp27/P53 in testis development and sperm apoptosis of male cattle (cattle-yak and yak). J. Cell Physiol. 2018, 234, 650–660. [Google Scholar] [CrossRef]
- Lan, D.; Xiong, X.; Huang, C.; Mipam, T.D.; Li, J. Toward understanding the genetic basis of yak ovary reproduction: A characterization and comparative analyses of estrus ovary transcriptiome in yak and cattle. PLoS ONE 2016, 11, e0152675. [Google Scholar] [CrossRef]
- Xiong, X.R.; Lan, D.L.; Li, J.; Wang, Y.; Zhong, J.C. Sodium butyrate improves the cloned yak embryo viability and corrects gene expression patterns. Zygote 2015, 23, 19–26. [Google Scholar] [CrossRef]
- Zi, X.D.; Lu, H.; Yin, R.H.; Chen, S.W. Development of embryos after in vitro fertilization of bovine oocytes with sperm from either yaks (Bos grunniens) or cattle (Bos taurus). Anim. Reprod. Sci. 2008, 108, 208–215. [Google Scholar] [CrossRef]
- Qiao, G.Y.; Dong, B.W.; Zhu CJYan, C.Y.; Chen, B.L. Deregulation of WNT2/FZD3/β-catenin pathway compromises the estrogen synthesis in cumulus cells from patients with polycystic ovary syndrome. Biochem. Biophys. Res. Commun. 2017, 493, 847–854. [Google Scholar] [CrossRef]
- Turathum, B.; Gao, E.M.; Chian, R.C. The function of cumulus cells in oocyte growth and maturation and in subsequent ovulation and fertilization. Cells 2021, 10, 2292. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.X.; Tian, Y.Q.; Hao, H.S.; Zou, H.Y.; Pang, Y.W.; Zhao, S.J.; Zhao, X.-M.; Zhu, H.-B.; Du, W.-H. Knockdown of ASH1L methyltransferase induced apoptosis inhibiting proliferation and H3K36 methylation in bovine cumulus cells. Theriogenology 2021, 161, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Pan, Y.; Wang, M.; Wang, J.; Wang, Y.; Han, X.; Wang, J.; Zhang, T.; Zhao, T.; He, H.; et al. Integrated analysis of the expression profiles of the lncRNA-miRNA-mRNA ceRNA network in granulosa and cumulus cells from yak ovaries. BMC Genom. 2022, 23, 633. [Google Scholar] [CrossRef]
- Liu, Y.; Denisov, I.G.; Sligar, S.G.; Kincaid, J.R. Substrate-specific allosteric effects on the enhancement of CYP17A1 lyase efficiency by cytochrome. J. Am. Chem. Soc. 2021, 143, 3729–3733. [Google Scholar] [CrossRef]
- Ping, X.; Liang, J.; Shi, K.; Bao, J.; Wu, J.; Yu, X.; Tang, X.; Zou, J.; Shentu, X. Rapamycin relieves the cataract caused by ablation of Gja8b through stimulating autophagy in zebrafish. Autophagy 2021, 17, 3323–3337. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Lin, F.; Dong, J.; Liu, J.; Ding, Z.; Xu, J. Peripheral macrophage-derived exosomes promote repair after spinal cord injury by inducing local anti-inflammatory type microglial polarization via increasing autophagy. Int. J. Biol. Sci. 2021, 17, 1339–1352. [Google Scholar] [CrossRef]
- Xu, R.; Rai, A.; Chen MSuwakulsiri, W.; Greening, D.W.; Simpson, R.J. Extracellular vesicles in cancer—Implications for future improvements in cancer care. Nat. Rev. Clin. Oncol. 2018, 15, 617–638. [Google Scholar] [CrossRef] [PubMed]
- Martinez, R.M.; Hauser, R.; Liang, L.; Adir, A.M.; Dioni, L.; Racowsky, C.; Bollati, V.; Baccarelli, A.A.; Machtinger, R. Urinary concentrations of phenols and phthalate metabolites reflect extracellular vesicle microRNA expression in follicular fluid. Environ. Int. 2019, 123, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Hua, T.; Yang, M.; Song, H.; Kong, E.; Deng, M.; Li, Y.; Li, J.; Liu, Z.; Fu, H.; Wang, Y.; et al. Huc-MSCs-derived exosomes attenuate inflammatory pain by regulating microglia pyroptosis and autophagy via the miR-146a-5p/TRAF6 axis. J. Nanobiotech. 2022, 20, 324. [Google Scholar] [CrossRef]
- Wang, B.; Mao, J.H.; Wang, B.Y.; Wang, L.X.; Wen, H.Y.; Xu, L.G.; Fu, J.; Yang, H. Exosomal miR-1910-3p promotes proliferation, metastasis, and autophagy of breast cancer cells by targeting MTMR3 and activating the NF-κB signaling pathway. Cancer Lett. 2020, 489, 87–99. [Google Scholar] [CrossRef]
- Zhang, X.; Shi, H.; Yuan, X.; Jiang, P.; Qian, H.; Xu, W. Tumor-derived exosomes induce N2 polarization of neutrophils to promote gastric cancer cell migration. Mol. Cancer 2018, 17, 146. [Google Scholar] [CrossRef] [PubMed]
- Simon, C.; Greening, D.W.; Bolumar, D.; Balaguer, N.; Salamonsen, L.A.; Vilella, F. Extracellular vesicles in human reproduction in health and disease. Endocr. Rev. 2018, 39, 292–332. [Google Scholar] [CrossRef] [PubMed]
- Gabryś, S.; Kij-Mitka, B.; Sawicki, S.; Kochan, J.; Nowak, A.; Lojko, J.; Karnas, E.; Bugno-Poniewierska, M. Extracellular vesicles from follicular fluid may improve the nuclear maturation rate of in vitro matured mare oocytes. Theriogenology 2022, 188, 116–124. [Google Scholar] [CrossRef]
- Hung, W.T.; Hong, X.; Christenson, L.K.; McGinnis, L.K. extracellular vesicles from bovine follicular fluid support cumulus expansion. Biol. Reprod. 2015, 93, 117. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Zhang, D.; Tong, X.; Wang, Y.; Qi, X.; Ning, W.; Xu, T.; Gao, D.; Zhang, L.; Ma, Y.; et al. Cumulus cell-derived and maternal SIRT6 differentially regulates porcine oocyte meiotic maturation. Theriogenology 2020, 142, 158–168. [Google Scholar] [CrossRef] [PubMed]
- Kabeya, Y.; Mizushima, N.; Ueno, T.; Yamamoto, A.; Kirisako, T.; Noda, T.; Kominami, E.; Ohsumi, Y.; Yoshimori, T. LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. EMBO J. 2000, 19, 5720–5728. [Google Scholar] [CrossRef]
- Jiang, T.; Chen, X.; Ren, X.; Yang, J.M. Emerging role of autophagy in anti-tumor immunity: Implications for the modulation of immunotherapy resistance. Drug Res. Updates 2021, 56, 100752. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.; Livingston, M.J.; Liu, Z.; Dong, Z. Autophagy in kidney homeostasis and disease. Nature reviews. Nephrology 2020, 16, 489–508. [Google Scholar]
- Komatsu, M.; Waguri, S.; Koike, M.; Sou, Y.S.; Ueno, T.; Hara, T.; Mizushima, N.; Iwata, J.; Ezaki, J.; Murata, S.; et al. Homeostatic levels of p62 control cytoplasmic inclusion body formation in autophagy-deficient mice. Cell 2007, 131, 1149–1163. [Google Scholar] [CrossRef]
- Juris, L.; Montino, M.; Rube, P.; Schlotterhose, P.; Thum, M.; Krick, R. PI3P binding by Atg21 organises Atg8 lipidation. EMBO J. 2015, 34, 955–973. [Google Scholar] [CrossRef]
- Huang, Q.; Liu, Y.; Zhang, S.; Yap, Y.T.; Li, W.; Zhang, D.; Gardner, A.; Zhang, L.; Song, S.; Hess, R.A.; et al. Autophagy core protein ATG5 is required for elongating spermatid development, sperm individualization and normal fertility in male mice. Autophagy 2021, 17, 1753–1767. [Google Scholar] [CrossRef] [PubMed]
- Shimada, M. Regulation of oocyte meiotic maturation by somatic cells. Reprod. Med. Biol. 2012, 11, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Li, G.; Liu, C.; Gao, H.; Wang, H.; Liu, W.; Chen, M.; Shang, Y.; Wang, L.; Shi, J.; et al. Autophagy regulates testosterone synthesis by facilitating cholesterol uptake in Leydig cells. J. Cell Biol. 2018, 217, 2103–2119. [Google Scholar] [CrossRef] [PubMed]
- Yi, J.; Tang, X.M. Functional implication of autophagy in steroid-secreting cells of the rat. Anat. Rec. 1995, 242, 137–146. [Google Scholar] [CrossRef]
- Texada, M.J.; Malita, A.; Rewitz, K. Autophagy regulates steroid production by mediating cholesterol trafficking in endocrine cells. Autophagy 2019, 15, 1478–1480. [Google Scholar] [CrossRef]
- Texada, M.J.; Malita, A.; Christensen, C.F.; Dall, K.B.; Faergeman, N.J.; Nagy, S.; Halberg, K.A.; Rewitz, K. Autophagy-mediated cholesterol trafficking controls steroid production. Dev. Cell 2019, 48, 659–671.e4. [Google Scholar] [CrossRef]
- Hamilton, K.J.; Hewitt, S.C.; Arao, Y.; Korach, K.S. Estrogen hormone biology. Curr. Top. Dev. Biol. 2017, 125, 109–146. [Google Scholar]
- Xiang, J.; Liu, X.; Ren, J.; Chen, K.; Wang, H.I.; Miao, Y.Y.; Qi, M.M. How does estrogen work on autophagy? Autophagy 2019, 15, 197–211. [Google Scholar] [CrossRef]
- Jobe, S.O.; Tyler, C.T.; Magness, R.R. Aberrant synthesis, metabolism, and plasma accumulation of circulating estrogens and estrogen metabolites in preeclampsia implications for vascular dysfunction. Hypertension 2013, 61, 480–487. [Google Scholar] [CrossRef]
- Zacharia, L.C.; Jackson, E.K.; Gillespie, D.G.; Dubey, R.K. Catecholamines abrogate antimitogenic effects of 2-hydroxyestradiol on human aortic vascular smooth muscle cells. Arterioscler. Thromb. Vasc. Biol. 2001, 21, 1745–1750. [Google Scholar] [CrossRef]
- Takanashi, K.; Honma, T.; Kashiwagi, T.; Honjo, H.; Yoshizawa, I. Detection and measurement of urinary 2-hydroxyestradiol 17-sulfate, a potential placental antioxidant during pregnancy. Clin. Chem. 2000, 46, 373–378. [Google Scholar] [CrossRef] [PubMed]
- Payne, A.H.; Hales, D.B. Overview of steroidogenic enzymes in the pathway from cholesterol to active steroid hormones. Endocr. Rev. 2004, 25, 947–970. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, H.; Hirai, S.; Tateno, H.; Fukui, Y. Variation of cholesterol contents in porcine cumulus-oocyte complexes is a key factor in regulation of fertilizing capacity. Theriogenology 2013, 79, 680–686. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fu, X.H.; Chen, C.Z.; Wang, Y.; Peng, Y.X.; Wang, W.H.; Yuan, B.; Gao, Y.; Jiang, H.; Zhang, J.-B. COL1A1 affects apoptosis by regulating oxidative stress and autophagy in bovine cumulus cells. Theriogenology 2019, 139, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Fester, L.; Zhou, L.; Bütow, A.; Huber, C.; von Lossow, R.; Prange-Kiel, J.; Jarry, H.; Rune, G.M. Cholesterol-promoted synaptogenesis requires the conversion of cholesterol to estradiol in the hippocampus. Hippocampus 2009, 19, 692–705. [Google Scholar] [CrossRef]
- Senthilkumaran, B.; Joy, K.P. Periovulatory changes in catfish ovarian oestradiol-17beta, oestrogen-2-hydroxylase and catechol-O-methyltransferase during GnRH analogue-induced ovulation and in vitro induction of oocyte maturation by catecholoestrogens. J. Endocrinol. 2001, 168, 239–247. [Google Scholar] [CrossRef]
- Mishra, A.; Joy, K.P. Effects of gonadotrophin in vivo and 2-hydroxyoestradiol-17beta in vitro on follicular steroid hormone profile associated with oocyte maturation in the catfish Heteropneustes fossilis. J. Endocrinol. 2006, 189, 341–353. [Google Scholar] [CrossRef][Green Version]
- Zhang, T.; BaSang, W.-D.; Chang, W.; Huo, S.; Ma, X.; Ju, X.; Yu, S.; Cui, S. Dynamics of apoptosis-related gene expression during follicular development in yak. J. Anim. Physiol. Anim. Nutr. 2021, 105, 1002–1013. [Google Scholar] [CrossRef]




| Primer | Forward or Reverse | Sequence (5’–3’) | GenBank No. |
|---|---|---|---|
| LC3B | Forward | AACCAAGCCTTCTTCCTCCT | NM_001001169 |
| Reverse | ATTGCTGTCCCGAATGTCTC | ||
| BECLIN1 | Forward | AGTACCAGCGGGAGTATAGTGA | NM_001033627 |
| Reverse | CAAGCGACCCAGTCTGAAAT | ||
| ATG5 | Forward | ATCAATCGGAAACTCATG | NM_001034579 |
| Reverse | AGATGTTCACTCAGCCAC | ||
| ATG12 | Forward | CCCCTTCTTCTGCTGC | NM_001076982 |
| Reverse | GGGTCCCAACTTCCTG | ||
| P62 | Forward | AGGAGCTTTGGTTCGTGGAA | NM_001205519 |
| Revers | CCCCTTGACTCTGGCTGTAATA | ||
| CYP17A1 | Forward | GCCCAAGACCAAGCACTC | NM_174304 |
| Reverse | CCCAAACGAAAGGAATAGATG | ||
| CYP19A1 | Forward | TGCTGGACACCTCTAACATGC | NM_174305 |
| Reverse | AAAATCAACTCAGTGGCGAAAT | ||
| CYP1A1 | Forward | GTCCCCTTCACCATCCCA | AB060696 |
| Reverse | CCAAGCCGAAAATAATCACC | ||
| CYP1B1 | Forward | GCTTCCGTCTTGGGCTAC | NM_001192294 |
| Reverse | GGTCAAAGTCCTCTGGGTTC | ||
| β-actin | Forward | ATCGTGCGTGACATCAAAGA | AY141970 |
| Reverse | CAAGAAGGAAGGCTGGAAAA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, R.; Wang, J.; Wang, M.; Gao, L.; Zhang, R.; Zhao, L.; Liu, B.; Han, X.; Baloch, A.R.; Cui, Y.; et al. Exosomes Derived from Yak Follicular Fluid Increase 2-Hydroxyestradiol Secretion by Activating Autophagy in Cumulus Cells. Animals 2022, 12, 3174. https://doi.org/10.3390/ani12223174
Xu R, Wang J, Wang M, Gao L, Zhang R, Zhao L, Liu B, Han X, Baloch AR, Cui Y, et al. Exosomes Derived from Yak Follicular Fluid Increase 2-Hydroxyestradiol Secretion by Activating Autophagy in Cumulus Cells. Animals. 2022; 12(22):3174. https://doi.org/10.3390/ani12223174
Chicago/Turabian StyleXu, Ruihua, Jinglei Wang, Meng Wang, Liqing Gao, Rui Zhang, Ling Zhao, Bin Liu, Xiaohong Han, Abdul Rasheed Baloch, Yan Cui, and et al. 2022. "Exosomes Derived from Yak Follicular Fluid Increase 2-Hydroxyestradiol Secretion by Activating Autophagy in Cumulus Cells" Animals 12, no. 22: 3174. https://doi.org/10.3390/ani12223174
APA StyleXu, R., Wang, J., Wang, M., Gao, L., Zhang, R., Zhao, L., Liu, B., Han, X., Baloch, A. R., Cui, Y., Yu, S., & Pan, Y. (2022). Exosomes Derived from Yak Follicular Fluid Increase 2-Hydroxyestradiol Secretion by Activating Autophagy in Cumulus Cells. Animals, 12(22), 3174. https://doi.org/10.3390/ani12223174





