1. Introduction
Energy-rich ingredients are expensive in broiler diets [
1,
2]. Maximizing the retention of energy for growth by applying a non-nutritive factor should therefore be considered. This can be achieved by enhancing the physical quality of pellets, or perhaps by increasing digestibility through feed processing, for instance by using the expander for compound feed [
3]. However, diets should achieve the broilers’ requirements for nutrients and energy during each growth phase, and it is necessary to consider that the expander conditioning achieved by applying steam, water, and pressure on the compound feed, affects the digestibility of nutrients and energy [
4,
5]. In this regard, the influence of feed processing should not be overlooked during feed formulation. However, estimating the contribution of feed processing for higher energy values of compound feed before diet formulation is still unclear. In addition to aspects of AME
N concentration, recent studies suggest that the energy content in compound feed for broilers may be reduced (lower than recommended by breeding lines) [
6,
7]. In fact, modern fast-growing broilers achieve the required slaughter weight over a shorter period. However, slaughtering the broilers at the correct time is important, and it is advantageous for it to be undertaken when the growth rate of the muscles is high in relation to the energy required for maintenance. After that, the requirements shift, with high energy required for a lower growth rate.
In the processing of compound feed for broilers, conditioning prior to pelleting is one of the most important steps in the processing line. The high-temperature short-time (HTST) technology with expanders, which apply heat, achieving temperatures above 110 °C for a short time, pressure, and shear forces to the compound feed, has the potential to modify the digestibility of fat [
8], amino acids, and starch [
9,
10]. Non-nutritive factors of compound feed offered to broilers, such as the pellet durability index (PDI) and low proportion of fines, have important contributions affecting feed intake, digestibility, and finally, the amount of energy retention by broilers [
3]. Higher energy retention is achieved by reducing the eating time. High pellet quality allows a high amount of feed apprehension per head movement. This contribution should already be considered during feed formulation, but, as mentioned above, the contribution that feed processing has on improvements in nutritive value and animal response on the increase in AME
N concentration has been poorly quantified until now. In fact, most broilers worldwide are fed with pelleted diets [
4], where modifications on the nutritive value of processed diets already occur. Therefore, the improvement of pellet quality using compound feed processing parameters should investigate the contribution of the different processing steps on the nutritive value of pelleted compound feed for broilers.
The objective of this study was to measure the effects of gradual AMEN reduction and expander conditioning prior to pelleting on nutrient digestibility, AMEN concentration in each feed phase, broiler performance, and blood serum markers. The main aim was to determine the optimal broiler-diet AMEN concentration promoted by expander conditioning prior to pelleting, while keeping the amino acid content constant (variable AMEN to amino acid ratio). We hypothesized that AMEN reduction decreases broiler performance and that using expander conditioning prior to pelleting may minimize this decrease, without negative influences on metabolic indices.
2. Materials and Methods
2.1. Diets
Corn-SBM-based broiler diets were formulated using previously analyzed amino acid content of corn (
Zea mays) and SBM (AMINONir
® Advanced, Evonik Operations GmbH, Hanau, Germany) for 6 treatments. The estimated digestibility coefficients of AA and AME
N concentration for broilers of corn and SBM was used for the calculation of the diet using digestible nutrients as a basis, following the AMINOChick
® recommendations [
11]. The ingredients composition of starter, grower, and finisher diets of calculated and analyzed nutrients are shown in
Table 1,
Table 2 and
Table 3, respectively. Nutritive value of these diets aimed to follow or exceed the lineage recommendations [
12]. The AME
N content in diets followed the requirements for the treatment Ross-0. For the other 5 diets, there was a 40 kcal/kg decrease performed step-by-step to reduce dietary AME
N for the starter, grower, and finisher feed phases (mainly by reducing the soybean oil). Each diet had the same and constant digestible AA content and the relationship between them as used in the Ross-0 treatment. This means that the formulation of feeds only decreased the AME
N concentration, changing the Lys:AME
N ratio among treatments.
2.2. Feed Processing Design
The above diets were submitted to two kinds of compound feed processing, resulting in a 6 × 2 factorial design with six AMEN levels and two expander conditionings prior to pelleting (OE 15 expander, Amandus Kahl, Reinbek, Germany). The corn was ground with a roller mill (LWM 400-1, Amandus Kahl, Reinbeck, Germany) to obtain 1.6 mm average particle size for all diets, which were mixed for 5 min in a ploughshare mixer (Lödige FKM 1200 D, Amandus Kahl, Reinbek, Germany). Pellets were produced using a 3 mm diameter hole die, installed in a pellet press (Type 33-390, Amandus Kahl, Reinbek, Germany), with 1:4 press ratio (3 mm diameter × 12 mm hole length). Starter, grower, and finisher diets were used as replicates for the feed processing step of the trial, considering each phase (diet) a block in the statistical model (Equation (4)). The starter diet was fed as crumbled, as was the grower diet from 15 to 21 days of age. From day 22 to the end of the trial the broilers received pellet diets with 3 mm of diameter and ~0.8 cm of length.
2.3. Pellet Quality Measurements
Pellet durability index (PDI) was measured on cooled pellets by the P-fost method [
13] and pellet hardness with the automatic pellet hardness tester (Amandus Kahl, Reinbek, Germany). Briefly, 10 randomly selected pellets from each replication were submitted to a force (kg/cm
2) until the first fracture. This force was recorded, and the mean value was used for statistical analysis.
2.4. Broiler Experimental Design and Housing
A total of 1008 one-day-old male Ross 308 broiler chickens vaccinated against Marek were placed in 36 pens (5.5 birds/m2) littered with wood shavings and equipped with one bell drinker and one tubular feeder. Birds had ad libitum access to water and feed. The environmental temperature was controlled automatically using infrared lamps (one per box) as a heat source and exhaust fans to supply ventilation and cooling.
The temperature of 32 °C used in the feeding trial follows the strain recommendations, reducing by one degree every two days until achieving 24 °C. The light regime was 23 h dark and 1 h light until 7 days of age and then 7 h dark and 17 h light afterward.
2.5. Performance Data
Body weight (BW) and feed intake (FI) were recorded at the end of each feed phase, by weighing the birds without fasting and the non-consumed feed. BWG and FCR were calculated for each phase and overall period. FCR was adjusted by including the weight of dead birds.
2.6. Determination of Apparent Total Tract Digestibility and Metabolized Energy
Titanium dioxide (TiO2) was used as an indigestible marker. Partial excreta samples were taken, placing all the birds of each pen in metal cages with steel plates for two h at the end of each feed phase. Excreta were packed into plastic bags prior to being stored in freezer at −20 °C for further analyses.
2.7. Determination of the Coefficients of Apparent Ileal Digestibility, pH of Gizzard Content, and Blood Sampling
On day 36 of age, 4 birds with average weight ± 2.5% from each pen were taken, stunned by percussive blow to the head, then bled through the jugular vein cut for 3 min. Blood was sampled at the bled moment in tubes with coagulation activator, then centrifuged and stored in cooled boxes and sent to an external laboratory. The gastrointestinal tract was removed, and the gizzard opened for pH measurement of the gizzard content, then weighed empty. The weight of the pancreas and liver were also determined and expressed as relative values to the body weight. Digesta samples were taken from the ileum between Merkel diverticulum and 2 cm cranial to the ileo-cecal junction by flushing with distilled water, packed in plastic bags, and stored in a freezer at −20 °C for further analyses. Prior to analyses, samples were freeze-dried.
2.8. Slaughter and Commercial Cuts
On day 37, all remaining broilers were fasted for 8 h, individually weighed, stunned by percussive blow to the head, then bled through a jugular vein cut for 3 min, scalded at 60 °C for 45 s, and defeathered. Evisceration was performed manually, and carcasses were statically chilled in the cooling room at 4 °C for 24 h. Commercial cuts were performed by a crew of industry-trained personnel into bone-in legs, wings, and also as deboned breast fillets with tenders. Abdominal fat was weighed separately. Carcass yield was expressed relative to the live weight, while commercial cuts and abdominal fat were expressed relative to eviscerated carcass weight.
2.9. Chemical and Blood Analyses
All diets were analyzed for dry matter (DM) (method 3.1.4), ash (method no. 8.1.1), ether extract after acid hydrolysis (EEh) (method 5.1.2), starch (method 7.2.1) according to standard procedures of VDLUFA [
14]. Total N was determined by Dumas’ method (method 968.06) [
15] (Büchi, DuMaster D-480, Flawil, Switzerland) and the result multiplied by 6.25 to obtain crude protein. Gross energy concentration was determined by a calorimeter calibrated with benzoic acid as a standard (IKA C 200, Parr instruments, Staufen, Germany). Amino acids content was determined by ion-exchange chromatography with post-column derivatization with ninhydrin, as described by Figueiredo-Silva et al. [
16]. Wet-ashing in a microwave oven (CEM Mars 6, CEM Corp. Matthews, NC, USA) was applied to analyze Ca by flame atomic absorption spectrophotometry (AAnalyst 200, Perkin Elmer Inc., Waltham, MA, USA), and P photometrically (Tecan Group Ltd., Männedorf, Switzerland) using the vanado-molybdate method at 436 nm. TiO
2 content in feed, digesta, and excreta was analyzed following Jagger et al. [
17]. In ileal content, DM, AA, starch, and TiO
2 were analyzed. However, in excreta, only DM, EEh, gross energy, N, ash, and TiO
2 were analyzed. From blood serum, total cholesterol (method: CHOD-PAP), triglycerides (method: GPO-PAP), lipase (method: enzymatic and colorimetric), gamma-glutamyl transferase (GGT) (method: IFCC), alpha-amylase (method: modified IFCC), and total protein (method: Biuret) were analyzed.
2.10. Calculations
Total tract digestibility and AME
N were calculated using the equations suggested by Kong and Adeola [
18]:
where M
d represents the concentration of TiO
2 in the diet in g/kg; M
e represents the concentration of TiO
2 in the excreta and ileal digesta in g/kg; E
d represents the content of total fat, starch, amino acids in g/kg, and gross energy (kcal/kg) in the diet. E
e represents the amount of total fat (g/kg) in excreta; and starch and amino acids (g/kg) in ileal digesta. GE
d represents the gross energy of diet (kcal/kg), and GE
e represents the gross energy of excreta (kcal/kg). N
d represents nitrogen (g/kg) in diet and N
e represents nitrogen (g/kg) in excreta. All the nutrients and energy were used in DM basis for the calculations.
2.11. Statistical Analyses
The experiment design was a completely randomized factorial arrangement of 6 AME
N levels and 2 expander conditionings prior to pelleting (with and without expander). All data were subjected to a normality test using Shapiro–Wilk test [
19] prior to the 2-way ANOVA using the GLM procedures from SAS Institute [
20]. When significant, means were separated by Tukey–Kramer [
21] and accepted as different when
p < 0.05.
The model used was:
where Y
ijk = observation, µ = population mean, γ
i = broiler performance period effect (i = 1, 2), α
j = AME
N concentration effect (j = Ross-0, Ross-40, Ross-80, Ross-120, Ross-160, Ross-200), β
k = expander effect (k = with, without) and (αβ)
jk = interaction between AME
N concentration and expander effect, and ε
ijk = residual error.
Statistical analysis for feed processing parameters followed the following model:
where Y
ijk = observation, µ = population mean, γ
i = block effect (i = starter, grower, finisher), α
j = AME
N concentration effect (i = Ross-0, Ross-40, Ross-80, Ross-120, Ross-160, Ross-200), β
j = expander effect (j = with, without), and (αβ)
ij = interaction between AME
N concentration and expander effect, and ε
ijk = residual error. Compound feed for starter, grower, and finisher was used as blocks (r = 3).
3. Results
The main feed processing parameters are shown in
Table 4. No effect of the interaction AME
N concentration vs. expander conditioning prior to pelleting was observed, except for PDI. The statistical analyses for main effects showed a clear influence of reduction in AME
N concentration on the pressure (
p < 0.001) needed in the pellet press to achieve a similar output of 2 t/h. The specific mechanical energy (SME) used by the pellet press increased (
p < 0.001) when AME
N concentration was reduced in the diet. Reducing AME
N concentration in diets increased pellet hardness (
p < 0.001) without significantly changing the proportion of fines (
Table 4).
In contrast, expander conditioning prior to pelleting affected diet processing. Using expander conditioning prior to pelleting showed clear differences: more steam was added to the compound feed prior to expanding. In addition, lower SME input (kWh/t) was necessary in the pellet press when expander conditioning was used prior to pelleting. The proportion of fines is positively affected (reduced) by expanding prior to pelleting (p < 0.001).
Table 5 shows the interaction effects for the PDI values. The PDI values of the compound feed were, in general, lower when expander conditioning prior to pelleting was not used. For treatments Ross-0 and Ross-40, expander conditioning prior to pelleting could significantly increase the PDI value.
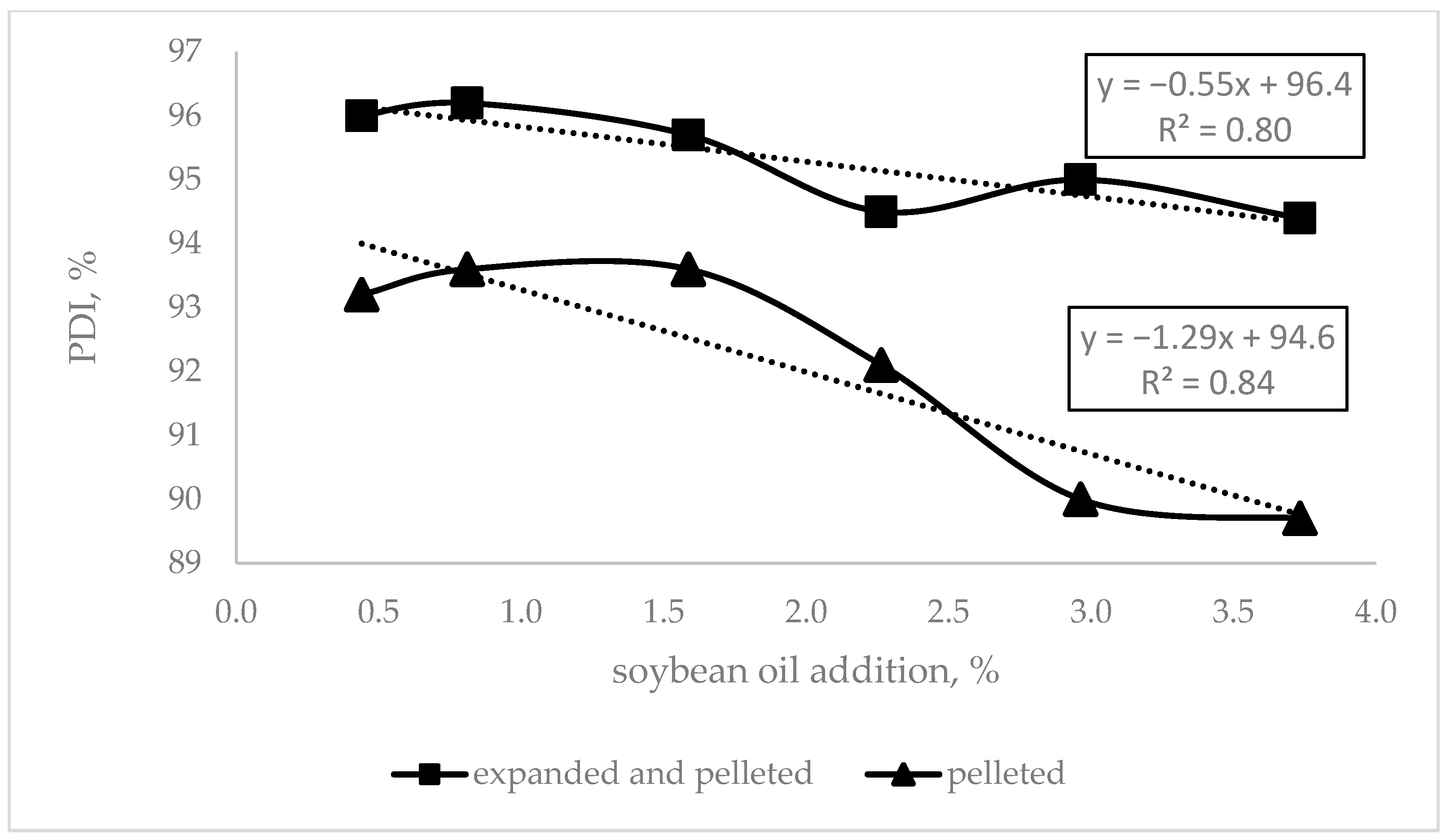
Evaluating the effect of the diets’ oil inclusion,
Figure 1 shows that the higher the oil content, the poorer the pellets.
The apparent total tract digestibility (ATTD) and AME
N concentration measured at the end of each feed phase are shown in
Table 6. The effects detected were mostly the main effects and there were only a few interactions. The ATTD of starter diets was affected by AME
N reduction, showing 3.7%-point higher organic matter digestibility in treatment Ross-0 compared to Ross-200. Organic matter ATTD and AME
N concentration were both unaffected by the expander prior to pelleting in the starter phase. However, EEh digestibility was improved when expander conditioning prior to pelleting was used (
p = 0.046). In the grower phase, only the EEh was positively affected by expander conditioning prior to pelleting (
p = 0.027). In the finisher phase, the AME
N concentration reduction decreased the organic matter.
The interactions of AME
N reduction vs. expander are shown in
Table 7 for the AME
N concentration in the starter phase and EEh digestibility in the finisher phase. The measured AME
N concentration was numerically higher when the expander conditioning prior to pelleting was used. Considering the pelleted-only diet (without expander conditioning), the EEh digestibility was reduced, being lowest at the lowest AME
N concentration (Ross-200).
Results of starch and amino acids apparent ileal digestibility (AID) of the compound diets, as measured at the end of the performance trial, are summarized in
Table 8. No interaction between the factors AME
N reduction and expander conditioning prior to pelleting was observed. In contrast, the main effect of AME
N concentration reduction was significant for a range of amino acids, whereas expander conditioning prior to pelleting poorly affected the AID of amino acids. Being lower as the AME
N concentration was reduced.
Detailed examination of AID values showed that treatment Ross-200 resulted in the highest starch digestibility, whereas TSAA, Thr, Arg, Leu, Phe, Gly, Ser, Pro, and Asp all had lower AID values when treatment Ross-160 was fed. Only the digestibility of TSAA (
Table 8) and Pro were negatively affected by expander conditioning prior to pelleting.
Diet composition hardly changed the broiler performance (
Table 9). Body weight gain was not affected by the main effects of AME
N concentration reduction or expander conditioning prior to pelleting. However, the factors interacted for BWG for the overall period (1–35 d). The treatment Ross-40 without expander conditioning prior to pelleting promoted the highest BWG (
Table 10). In contrast, BWG was similar among the different AME
N concentration reductions (Ross-0 to Ross-200) when an expander prior to pelleting was used. The feed intake (FI) was not affected by treatments in grower, finisher, and overall feed phases. However, at the starter phase, the AME
N reduction interacted with expander conditioning prior to pelleting (
Table 10). The highest FI was observed in treatment Ross-160 after expander conditioning prior to pelleting. Without expander conditioning prior to pelleting, the FI increased also with decreasing AME
N concentration in the diet. There were no differences in the comparison between expander conditioning within each AME
N concentration reduction.
The feed conversion ratio (FCR) was mostly influenced by treatment factors among performance parameters. Regarding main effects, the reduction in AMEN concentration affected FCR in the starter and finisher phases, and in the overall experimental period, getting worse as a lower AMEN concentration was calculated in the diet.
The FCR was reduced by 0.056 points for the overall period (
Table 9). The expander conditioning prior to pelleting improved FCR in the overall period (1–35 d) regardless of the reduction in AME
N concentration. In the grower phase, the factors interacted. FCR was lowest when the combination of treatments Ross-120 and expander conditioning prior to pelleting were applied (
Table 10). No differences in FCR were observed in the comparison between expander conditioning within each AME
N concentration reduction.
Internal organs and health indicators from blood serum were measured on day 36 of life. As shown in
Table 11, just the liver weight, presented as a percentage of the non-fasted live body weight, was changed by the AME
N concentration reduction. Treatment Ross-200 increased the liver weight, relative to that of treatment Ross-40. For blood serum indicators, only the α-amylase activity showed higher values in broilers of treatment Ross-160 compared to Ross-0. Expander conditioning prior to pelleting did not influence these animal response parameters. No interaction was observed.
Commercial cuts were hardly changed by treatments (
Table 12); although, total carcass yield was slightly higher when diets were just pelleted, and the AME
N concentration reduction resulted in lower abdominal fat deposition. There were no interactions observed for carcass yield, abdominal fat, and commercial cuts.
4. Discussion
One of the goals of the present study was to find out if energy coming from nutrients could be replaced by energy made available from non-nutritional sources, i.e., by the processing of compound feed with modifications at the processing line that could help to improve the pellet quality and animal response. As observed for the feed processing indicators (
Table 4), the AME
N reduction is implied by the higher pressure applied by the pellet press to the compound feed. This resulted in higher consumption of electric power when diets had less AME
N. The lower amount of soybean oil added to the diet may explain this. Oil greases the die holes, reducing friction, so the mechanical energy to push the compound feed through the die holes is lower, reflected in lower kWh/t, PDI, hardness, and higher content of fines (
Figure 1). As explained by Abdollahi et al. [
4] with increasing fat added to the compound feed, the starch particles become covered, restricting the steam penetration to the granules, and reducing starch gelatinization during the conditioning and later in the pellet die. In consequence, the friction is reduced, and agglomeration capacity is lowered, which weakens the resulting pellets, as found in this trial (
Figure 1). On the one hand, although the AME
N concentration of the compound feed increased when fat was added, the higher fat content decreased pellet quality, as observed by decreasing PDI values (
Figure 1). This result is supported by McKinney and Teeter [
3]. However, fat is included to cover the animal requirements of fatty acids and energy, but sometimes these inclusions are high, challenging the pellet quality. On the other hand, expander conditioning prior to pelleting allows higher fat inclusion in the compound feed without substantial changes in the pellet quality [
22], as can be observed in
Table 5 and
Figure 1. Our results showed that an average 4.7%-point lower PDI was achieved when 3.73% of soybean oil was included and diets were processed on the line without expander conditioning. However, with expander conditioning prior to pelleting, the decrease in PDI was only about 1.5% points. The results suggest that expander conditioning prior to pelleting enables the higher inclusion of fat in compound feed for broilers without losses in pellet quality, a goal for the feed mill.
Furthermore, it should be noted that the diet with the lowest PDI in this trial was the one without energy reduction (Ross-0) and pelleted without expander conditioning, as observed in
Table 4. However, compound feed with a PDI of 89.7% and 6.3% of fines still represents a diet with well-performed processing, especially when animal responses are highlighted. McKinney and Teeter [
3] showed that diets containing 60% of pellets and 40% fines had similar broiler performance to broilers fed with 100% pellets, supporting the absence of well-defined differences in feed intake and body weight gain, observed in the current trial. During feed processing in this trial, the aim was to produce pellets with a PDI as high as possible to demonstrate that higher digestibility and broiler performance with expander conditioning prior to pelleting is possible. However, PDI did not substantially affect animal performance in the present study, as the correlations with animal responses showed (
Table 13,
Table 14,
Table 15,
Table 16 and
Table 17). This finding is supported by Svihus [
23], who explained the high ability of broilers to digest corn starch, such as was observed in this trial. In fact, the diets had on average 449 g/kg of starch, being the highest source of energy, but the changes promoted by the expander conditioning prior to pelleting were not enough to improve starch digestibility. However, starch digestibility did not decrease with expander conditioning, showing that expander conditioning prior to pelleting contributes to producing high-quality pellets.
The effect of the expander conditioning prior to pelleting can be seen in the disruption of grain particles, resulting in the higher availability of nutrients for digestion. In fact, the EEh digestibility was higher in the starter and grower phases when expander conditioning prior to pelleting was applied, but the effect disappeared in the finisher phase. Younger birds have a reduced digestion capacity of the gastrointestinal tract, as described by Furlan and Macari [
24]. On the one hand, the expander conditioning prior to pelleting may have increased the access of digestion enzymes to the lipid fraction of ingredients of the compound feed, improving fat digestibility. On the other hand, the experimental factors hardly changed the measured AME
N, which could be explained by the similar results observed for the rearing of broilers in pens littered with wood shavings, which could have been eaten by the birds, affecting the excreta used to measure energy. These findings support the notion that expander conditioning prior to pelleting plays an important role in providing energy to the broilers by raising fat digestibility and likely enhancing starch digestibility.
The absence of substantial effects of AME
N reduction suggests that the energy concentration of diets may be reduced at feed formulation. Both processing lines, with or without an expander prior to pelleting, promoted high-quality pellets and showed few changes in pellet physical aspects as well as in digestibility. However, the energy requirements of modern broiler strains may support the reduction in AME
N concentration without substantial negative effects. Aftab [
6] supports these findings by reviewing the energy and amino acid requirements of broilers. In that publication, the author suggests that the current strains require diets with lower AME
N and higher amino acid concentrations than recommended, especially due to the shorter time of approximately 35 days required to achieve slaughter weight. Therefore, the AME
N of diets for broilers can be reduced preserving the amino acid inclusion levels.
The apparent ileal amino acids digestibility of compound diets was also affected by treatments (
Table 8). The diet without AME
N reduction was for most amino acids, the one with the higher digestibility compared to treatment Ross-160. The reduction in AME
N was responsible for lower amino acid digestibility. In contrast, the expander conditioning prior to pelleting did not affect AA digestibility (exceptions for TSAA and Pro) at similar amino acid compositions of diets. However, only TSAA and proline were negatively affected by expander conditioning prior to pelleting. This means that shear forces, heat, residence time, and moisture content, all features resulting from expander conditioning [
25,
26], poorly affected the digestion of amino acids in the present study. The AID of amino acids also supports the reduction in AME
N and the use of expander conditioning.
Considering the difficulties in using digestibility values of processed compound feeds for feed formulation, the gains by high-quality pellets produced by expander conditioning prior to pelleting in broiler performance are associated with the shorter time [
27] and energy expenditure during ingestion of feed [
28,
29]. Broiler performance was thereby affected only slightly by experimental factors (
Table 9). In fact, the feed intake was higher in the starter phase when diets had lower energy. Birds are likely to try to compensate for the lower energy with higher feed intake, as observed by Leeson et al. [
30,
31]. These authors demonstrated that broilers eat to compensate for the limiting nutrients in their diet, which is reflected in poor FCR in these feeding phases, as observed in our study.
The compensation of low energy in diets with higher feed intake was not clearly observed in the grower or finisher phases in the present study (
p < 0.10). Aftab [
6] suggests that modern broiler genetic lines may no longer regulate their feed intake by diet nutrient density, but instead by just physical distension of the gastrointestinal tract. This is reflected in our study by the lack of a statistical effect on feed intake and in body weight gain, although AME
N concentration was reduced. In addition, the abdominal fat accumulated proportionally to the lower AME
N concentration in the diet. The expander conditioning prior to pelleting did not affect these results (
Table 12). Reducing the energy concentration in diets resulted in the AME
N to amino acid ratio being affected. Our results support the reduction in AME
N concentration by 80, 120, and 160 kcal/kg in starter, grower, and finisher feed phases, respectively, without negative effects on animal performance and the proportion of commercial cuts.
Furthermore, the use of expander conditioning prior to pelleting is favorable to optimize FCR, being 1.376 without and 1.352 g/g in the overall period when expander conditioning prior to pelleting was used. There was a tendency shown (
p < 0.10) for improved FCR when an expander was not used in the starter phase; however, this changes as broilers get older, resulting in the benefit observed for the overall period. The higher FCR observed using an expander in the starter phase could be explained by the changes in the gastrointestinal tract that happened due to aging and due to adaptation to the diet, such as lower exogen pancreatic enzymes and bile production for this at up to 14-day-old birds. The older birds were later well-adapted [
24].
Looking closer at the relationship between the physical pellet quality and the nutrient digestibility in the starter phase by correlation analysis (
Table 13), the PDI had a positive correlation with FCR in the starter phase, suggesting that higher physical quality is important for birds up to 14 days of age, even if these pellets were crumbled to achieve an edible size that was ingestible by chickens of just a few days old. Later, in the grower and finisher feed phases these correlations disappear (
Table 14 and
Table 15). Although some correlations were observed, the PDI in this trial was higher than 89%, and fines were lower than 7%, showing therefore the high feed quality of all diets. Low fines amounts were also used by McKinney and Teeter [
3], who did not find differences in FCR when birds were consuming diets with 0 to 80% of fines. Stocking density was not an experimental factor, but was low, promoting animal welfare. However, industrial production systems normally use higher housing densities, raising the importance of good pellets allowing fast feed intake, highlighting again the importance of producing pellets of high physical quality.
Animal physiological answers to the treatments were also measured, aiming to evaluate variables related to animal health (
Table 11). The blood serum variables measured show that fat digestibility changed the lipase secretion positively, which is physiologically expected, and it is important to see that the levels of fat in diets may be measured in blood (
Table 16). The serum concentration of α-amylase increased as AME
N concentration was reduced (
Table 11). This can be associated with the starch content in the diet, which increased slightly as AME
N was reduced. However, the starch digestibility was only slightly affected by the treatments. On the one hand, soybean oil content reduction was the biggest driver for AME
N variation among treatments, increasing liver size as the AME
N concentration decreased (
Table 11). On the other hand, cholesterol and triglyceride concentrations were not affected by treatments, showing that the health status was not changed by AME
N reduction and compound feed processing. Ivanovich et al. [
32] conducted a study of changing AME
N and amino acid concentrations, and no changes in amylase and lipase were found. However, their study was conducted at up to 10 days of age, which represents a period with strong intestinal tract development and enzymatic adaptations. The longer time of eating diets with less energy may help to explain the differences observed in serum amylase. Thus, treatments hardly change serum markers, showing the metabolic adaptation to maintain body homeostasis.
Besides the correlation between physical properties and animal response, the correlation analyses between pellet quality variables and size of gastrointestinal organs and abdominal fat (
Table 17) showed that abdominal fat is the variable that is most sensitive in relation to variations in pellet quality, AME
N concentration, and nutrient digestibility. Among them, the most powerful factor influencing abdominal fat deposition is the AME
N concentration (
Table 12), especially when the AME
N to amino acid ratio is increasing. However, higher fat content (soybean oil added) reduces pellet quality. Together with high abdominal fat deposition, the poultry industry is avoiding both. Furthermore, the abdominal fat accumulation clearly shows that gradual AME
N reduction was achieved and how sensitive the body composition is to the energy content in the diet.