Diagnostic and Treatment of Spinal Fracture and Luxation in Italian Wolves (Canis lupus italicus)
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Statement
2.2. Clinical Examination
2.3. Diagnostic Procedures
2.4. Treatment and Follow-Up
3. Results
3.1. Case Analysis
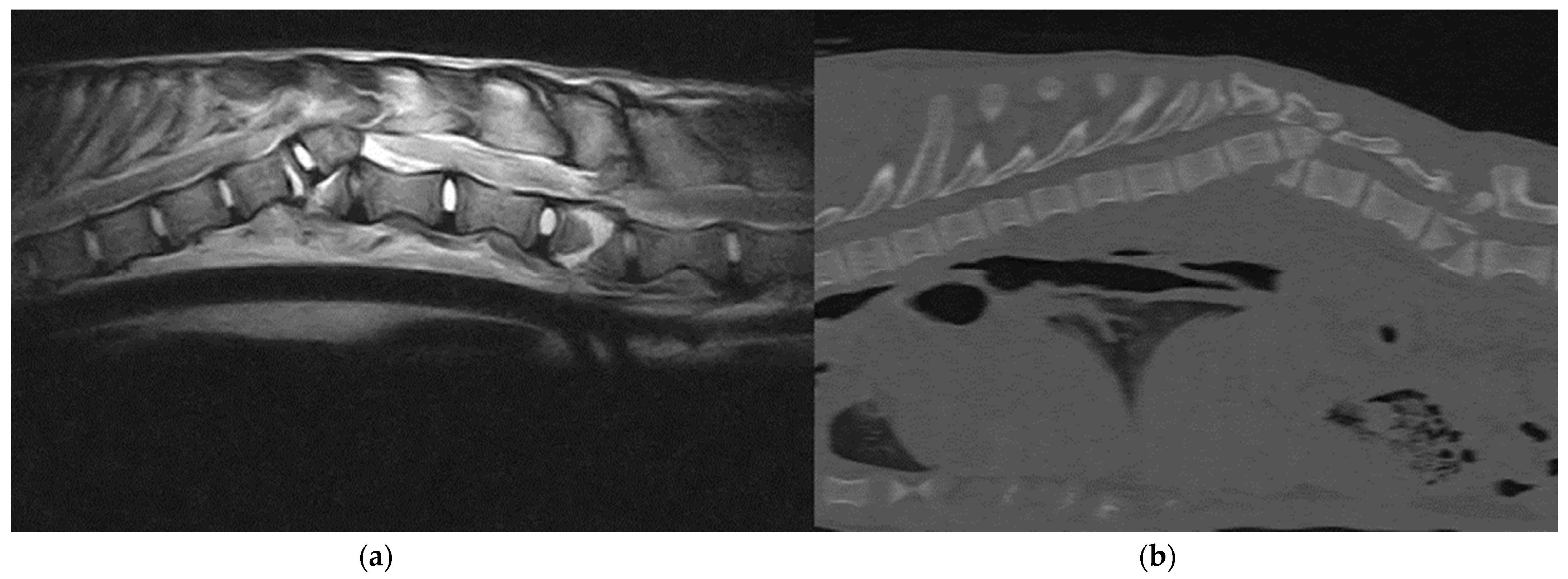
3.2. Imaging
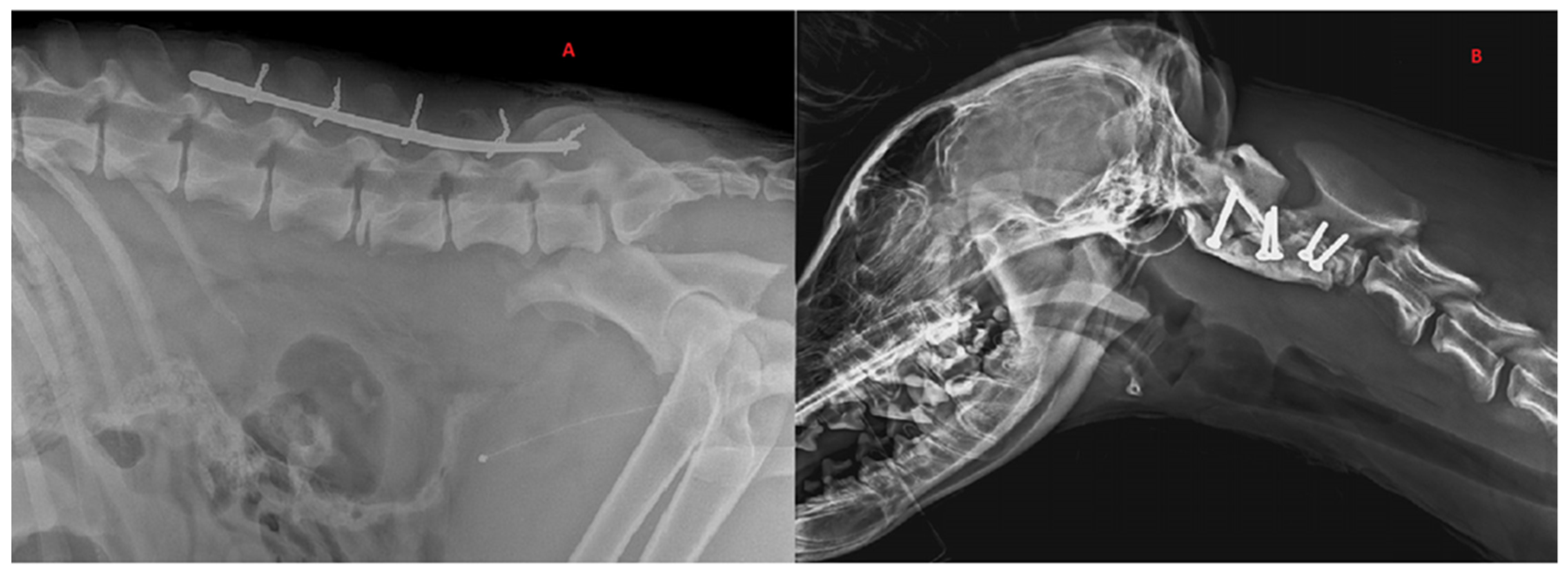
3.3. Treatments
3.4. Follow-Up
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fluehmann, G.; Doherr, M.; Jaggy, A. Canine neurological diseases in a referral hospital population between 1989 and 2000 in Switzerland. J. Small Anim. Pract. 2006, 47, 582–587. [Google Scholar] [CrossRef] [PubMed]
- Marioni-Henry, K.; Vite, C.H.; Newton, A.L.; Van Winkle, T.J. Prevalence of diseases of the spinal cord of cats. J. Veter-Intern. Med. 2005, 18, 851–858. [Google Scholar] [CrossRef]
- Matthiesen, D.T. Thoracolumbar spinal fractures/luxations: Surgical management. Compend. Contin. Educ. Pract. 1983, 5, 867–878. [Google Scholar]
- Turner, W.D. Fractures and fracture-luxations of the lumbar spine: A retrospective study in the dog. J. Am. Anim. Hosp. Assoc. 1987, 23, 459–464. [Google Scholar]
- McKee, W.M. Spinal trauma in dogs and cats: A review of 51 cases. Veter-Rec. 1990, 126, 285–289. [Google Scholar]
- Selcer, R.R.; Bubb, W.J.; Walker, T.L. Management of vertebral column fractures in dogs and cats: 211 cases (1977–1985). J. Am. Vet. Med. Assoc. 1991, 198, 1965–1968. [Google Scholar]
- Jeffery, N.D. Vertebral fracture and luxation in small animals. Vet. Clin. N. Am. Small Anim. Pract. 2010, 40, 809–828. [Google Scholar] [CrossRef]
- Bruce, C.W.; Brisson, B.A.; Gyselinck, K. Spinal fracture and luxation in dogs and cats. Vet. Comp. Orthop. Traumatol. 2008, 21, 280–284. [Google Scholar]
- Walker, T.M.; Pierce, W.A.; Welch, R.D. External fixation of the lumbar spine in a canine model. Veter-Surg. 2002, 31, 181–188. [Google Scholar] [CrossRef]
- Bruecker, K.A.; Seim, H.B.I. Principles of spinal fracture management. Semin. Vet. Med. Surg. (Small Anim.) 1992, 7, 71–84. [Google Scholar]
- Mace, G.M.; Collar, N.J.; Gaston, K.J.; Hilton-Taylor, C.; Akçakaya, H.R.; Leader-Williams, N.; Milner-Gulland, E.; Stuart, S.N. Quantification of Extinction Risk: IUCN’s System for Classifying Threatened Species. Conserv. Biol. 2008, 22, 1424–1442. [Google Scholar] [CrossRef] [PubMed]
- Levine, G.J.; Levine, J.M.; Budke, C.M.; Kerwin, S.C.; Au, J.; Vinayak, A.; Hettlich, B.F.; Slater, M.R. Description and repeatability of a newly developed spinal cord injury scale for dogs. Prev. Veter-Med. 2009, 89, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Zeira, O.; Briola, C.; Konar, M.; Plonek, M.; Papa, V. Clinical and diagnostic imaging findings in an italian wolf (canis lupus italicus) with discospondylitis. J. Zoo Wildl. Med. 2013, 44, 1086–1089. [Google Scholar] [CrossRef]
- Denis, F. The Three Column Spine and Its Significance in the Classification of Acute Thoracolumbar Spinal Injuries. Spine 1983, 8, 817–831. [Google Scholar] [CrossRef] [PubMed]
- Johnson, P.; Beltran, E.; Dennis, R.; Taeymans, O. Magnetic resonance imaging characteristics of suspected vertebral instability associated with fracture or subluxation in eleven dogs. Vet. Radiol. Ultrasound 2012, 53, 552–559. [Google Scholar] [CrossRef]
- Orgonikova, I.; Brocal, J.; Cherubini, G.B.; Palus, V. Vertebral fractures and luxations in dogs and cats, part 1: Evaluation of diagnosis and prognosis. Companion Anim. 2021, 26, 1–10. [Google Scholar] [CrossRef]
- Kinns, J.; Mai, W.; Seiler, G.; Zwingenberger, A.; Johnson, V.; Cáceres, A.; Valdés-Martínez, A.; Schwarz, T. Radiographic sensitivity and negative predictive value for acute canine spinal trauma. Vet. Radiol. Ultrasound 2006, 47, 563–570. [Google Scholar] [CrossRef]
- Mann, F.; Cohen, W.A.; Linnau, K.F.; Hallam, D.K.; Blackmore, C. Evidence-based approach to using CT in spinal trauma. Eur. J. Radiol. 2003, 48, 39–48. [Google Scholar] [CrossRef]
- Sundgren, P.C.; Flanders, A.E. Acute spinal trauma. In Diseases of the Brain, Head & Neck, Spine 2012–2015; Springer: Milano, Italy, 2012; pp. 167–172. [Google Scholar] [CrossRef]
- Griffen, M.M.; Frykberg, E.R.; Kerwin, A.J.; Schinco, M.A.; Tepas, J.J.; Rowe, K.; Abboud, J. Radiographic Clearance of Blunt Cervical Spine Injury: Plain Radiograph or Computed Tomography Scan? J. Trauma: Inj. Infect. Crit. Care 2003, 55, 222–227. [Google Scholar] [CrossRef]
- Dai, L.-Y.; Jin, W.-J. Interobserver and intraobserver reliability in the load sharing classification of the assessment of thoracolumbar burst fractures. Spine 2005, 30, 354–358. [Google Scholar] [CrossRef]
- Gallastegui, A.; Davies, E.; Zwingenberger, A.L.; Nykamp, S.; Rishniw, M.; Johnson, P.J. MRI has limited agreement with CT in the evaluation of vertebral fractures of the canine trauma patient. Veter-Radiol. Ultrasound 2019, 60, 533–542. [Google Scholar] [CrossRef] [PubMed]
- Wang-Leandro, A.; Siedenburg, J.; Hobert, M.; Dziallas, P.; Rohn, K.; Stein, V.; Tipold, A. Comparison of preoperative quantitative magnetic resonance imaging and clinical assessment of deep pain perception as prognostic tools for early recovery of motor function in paraplegic dogs with intervertebral disk herniations. J. Veter-Intern. Med. 2017, 31, 842–848. [Google Scholar] [CrossRef] [PubMed]
- Aikawa, T.; Fujita, H.; Kanazono, S.; Shibata, M.; Yoshigae, Y. Long-term neurologic outcome of hemilaminectomy and disk fenestration for treatment of dogs with thoracolumbar intervertebral disk herniation: 831 cases (2000–2007). J. Am. Veter-Med. Assoc. 2012, 241, 1617–1626. [Google Scholar] [CrossRef] [PubMed]
- Castel, A.; Olby, N.J.; Ru, H.; Mariani, C.L.; Muñana, K.R.; Early, P.J. Risk factors associated with progressive myelomalacia in dogs with complete sensorimotor loss following intervertebral disc extrusion: A retrospective case-control study. BMC Veter-Res. 2019, 15, 1–9. [Google Scholar] [CrossRef]
- Roaf, R. A study of the mechanics of spinal injuries. J. Bone Jt. Surgery. Br. Vol. 1960, 42, 810–823. [Google Scholar] [CrossRef]
- Waldron, D.R.; Shires, P.K.; McCain, W.; Hedlund, C.; Blass, C.E. The rotational stabilizing effect of spinal fixation techniques in an unstable vertebral model. Prog. Vet. Neurol. (USA) 1991, 2, 105–110. [Google Scholar]
- Voss, K.; Montavon, P.M. Tension band stabilization of fractures and luxations of the thoracolumbar vertebrae in dogs and cats: 38 cases (1993–2002). J. Am. Veter-Med. Assoc. 2004, 225, 78–83. [Google Scholar] [CrossRef]
- Bojrab, J. Mechanisms of Disease in Small Animal Surgery, 3rd ed.; Apple Books; Lea & Febiger: Philadelphia, PA, USA, 2012. [Google Scholar]
- Hambly, M.; Lee, C.K.; Gutteling, E.; Zimmerman, M.C.; Langrana, N.; Pyun, Y. Tension Band Wiring-Bone Grafting for Spondylolysis and Spondylolisthesis. Spine 1989, 14, 455–460. [Google Scholar] [CrossRef]
- Shores, A.; Brisson, B.A. (Eds.) Current Techniques in Canine and Feline Neurosurgery; John Wiley & Sons: Hoboken, NJ, USA, 2017; pp. 216–217. [Google Scholar]
- Bagley, R.S. Spinal Fracture or Luxation. Veter-Clin. N. Am. Small Anim. Pract. 2000, 30, 133–153. [Google Scholar] [CrossRef]
- Garcia, J.N.P.; Milthorpe, B.K.; Russell, D.; Johnson, K.A. Biomechanical study of canine spinal fracture fixation using pins or bone screws with polymethylmethacrylate. Veter-Surg. 1994, 23, 322–329. [Google Scholar] [CrossRef]
- Beaver, D.P.; MacPhersont, G.C.; Muir, P.; Johnson, K.A. Methyl-methacrylate and bone screw repair of seventh lumbar vertebral fracture-luxations in dogs. J. Small Anim. Pract. 1996, 37, 381–386. [Google Scholar] [CrossRef] [PubMed]
- Caterino, C.; Aragosa, F.; Della Valle, G.; Fatone, G. Canine Seventh Lumbar Vertebra Fracture: A Systematic Review. Animals 2022, 12, 193. [Google Scholar] [CrossRef] [PubMed]
- Hawthorne, J.C.; Blevins, W.E.; Wallace, L.J.; Glickman, N.; Waters, D.J. Cervical vertebral fractures in 56 dogs: A retrospective study. J. Am. Anim. Hosp. Assoc. 1999, 35, 135–146. [Google Scholar] [CrossRef] [PubMed]
- Kepler, C.K.; Vaccaro, A.R.; Fleischman, A.N.; Traynelis, V.C.; Patel, A.A.; Dekutoski, M.B.; Harrop, J.; Wood, K.B.; Schroeder, G.D.; Bransford, R.; et al. Treatment of Axis Body Fractures. Clin. Spine Surgery: A Spine Publ. 2017, 30, 442–456. [Google Scholar] [CrossRef] [PubMed]
- Stone, E.A.; Betts, C.W.; Chambers, J.N. Cervical fractures in the dog: A literature and case review. J. Am. Anim. Hosp. Assoc. 1979, 15, 463–471. [Google Scholar]
- Schmidli, F.E.; Stein, V.M.; Aikawa, T.; Boudrieau, R.J.; Jeandel, A.; Jeffery, N.; Jurina, K.; Moissonnier, P.; Rupp, S.; Vidondo, B.; et al. Fractures of the Second Cervical Vertebra in 66 Dogs and 3 Cats: A Retrospective Study. Veter-Comp. Orthop. Traumatol. 2019, 32, 200–206. [Google Scholar] [CrossRef]
- Krauss, M.W.; Theyse, L.F.H.; Tryfonidou, M.A.; Hazewinkel, H.A.W.; Meij, B.P. Treatment of spinal fractures using Lubra plates. Veter-Comp. Orthop. Traumatol. 2012, 25, 326–331. [Google Scholar] [CrossRef]
- Välkki, K.J.; Thomson, K.H.; Grönthal, T.S.C.; Junnila, J.J.T.; Rantala, M.H.J.; Laitinen-Vapaavuori, O.M.; Mölsä, S.H. Antimicrobial prophylaxis is considered sufficient to preserve an acceptable surgical site infection rate in clean orthopaedic and neurosurgeries in dogs. Acta Veter-Scand. 2020, 62, 1–10. [Google Scholar] [CrossRef]
- Boag, A.K.; Otto, C.M.; Drobatz, K.J. Complications of Methylprednisolone Sodium Succinate Therapy in Dachshunds with Surgically Treated Intervertebral Disc Disease. J. Veter-Emerg. Crit. Care 2001, 11, 105–110. [Google Scholar] [CrossRef]
- Levine, J.M.; Levine, G.J.; Boozer, L.; Schatzberg, S.J.; Platt, S.R.; Kent, M.; Kerwin, S.C.; Fosgate, G.T. Adverse effects and outcome associated with dexamethasone administration in dogs with acute thoracolumbar intervertebral disk herniation: 161 cases (2000–2006). J. Am. Veter-Med. Assoc. 2008, 232, 411–417. [Google Scholar] [CrossRef]
- Elsamadicy, A.A.; Wang, T.Y.; Back, A.G.; Sergesketter, A.; Warwick, H.; Karikari, I.O.; Gottfried, O.N. Impact of Intraoperative Steroids on Postoperative Infection Rates and Length of Hospital Stay: A Study of 1200 Spine Surgery Patients. World Neurosurg. 2016, 96, 429–433. [Google Scholar] [CrossRef] [PubMed]
- Tihista, M.; Gu, A.; Wei, C.; Weinreb, J.H.; Rao, R.D. The impact of long-term corticosteroid use on acute postoperative complications following lumbar decompression surgery. J. Clin. Orthop. Trauma 2020, 11, 921–927. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, N.D.; Ruska, T.; Austin, T.M.; Guisse, N.F.; Murphy, J.S.; Bruce, R.W. Postoperative dexamethasone following posterior spinal fusion for adolescent idiopathic scoliosis. J. Bone Jt. Surg. 2020, 102, 1807–1813. [Google Scholar] [CrossRef] [PubMed]
- Gardiner, D.; McShane, B.J.; Kerr, M.; Agarwal, P.; Saylany, A.; Sharma, N.; Joshi, D.; Pierce, J.; Pasao-Pham, N.; Dante, S.J.; et al. Low-Dose Steroids to Decrease Postoperative Pain and Opioid Use. J. Nurse Pract. 2020, 16, 523–527. [Google Scholar] [CrossRef]
| Case No | Gender | Age (months) | Weight (Kg) | Cause | Vertebral Site | Other Injuries | Neurological Grade | Treatment | Final Outcome |
|---|---|---|---|---|---|---|---|---|---|
| 1 | M | 23 | 34 | HBC | L6 | Hip Fracture | Grade 0 | None | Euthanasia |
| 2 | M | 28 | 31 | HBC | T12, T13 | None | Grade 0 | None | Euthanasia |
| 3 | F | 21 | 28 | Discospondylitis | T12-T13 | None | Grade 4 | Body-splint and antibiotics treatment | 10 weeks: Normal, Release into the wild (grade 6). |
| 4 | F | 6 | 13 | HBC | T12, L2 | None | Grade 0 | None | Euthanasia |
| 5 | M | 15 | 27 | HBC | L2 | None | Grade 0 | None | Euthanasia |
| 6 | M | 10 | 20 | HBC | T13 | None | Grade 0 | None | Euthanasia |
| 7 | M | 12 | 28 | HBC | L3 | Hip fracture | Grade 3 | Screws-PMMA implant | 12 weeks: Normal, Release into the wild (grade 6). |
| 8 | F | 7 | 11 | HBC | C2 | Humerus fracture Radius/ulna fracture | Grade 3 | Screws-PMMA implant | 15 weeks: Normal, Release into the wild (grade 6). |
| 9 | M | 8 | 13 | HBC | T11 | None | Grade 4 | Dorsal spinal staple | 7 weeks: Normal, Release in the wild (grade 6). |
| 10 | M | 7 | 16 | Unknown | T5, T6, T7 | None | Grade 3 | Body-splint | 3 weeks: Euthanized for poor quality of life |
| 11 | M | 50 | 30 | HBC | L5 | Hip fracture | Grade 3 | Dorsal spinal staple | 16 weeks: Normal, Release into the wild (grade 6). |
| 12 | M | 34 | 29 | HBC | L2 | None | Grade 3 | Dorsal spinal staple | At present ambulatory with spinal ataxia. Stay in protection center (grade 4). |
| 13 | F | 4 | 8 | HBC | L2, L3 | Ribs | Grade 0 | None | Euthanasia |
| 14 | F | 26 | 26 | HBC | L2 | Radius/ulna fracture | Grade 4 | Dorsal spinal staple | 6 weeks: Normal, Release into the wild (grade 6). |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fugazzotto, D.; Costa Devoti, C.; Dumas, M.P.; Teani, C.; Berti, E.; Zeira, O. Diagnostic and Treatment of Spinal Fracture and Luxation in Italian Wolves (Canis lupus italicus). Animals 2022, 12, 3044. https://doi.org/10.3390/ani12213044
Fugazzotto D, Costa Devoti C, Dumas MP, Teani C, Berti E, Zeira O. Diagnostic and Treatment of Spinal Fracture and Luxation in Italian Wolves (Canis lupus italicus). Animals. 2022; 12(21):3044. https://doi.org/10.3390/ani12213044
Chicago/Turabian StyleFugazzotto, Domenico, Chiara Costa Devoti, Maria Pia Dumas, Chiara Teani, Elisa Berti, and Offer Zeira. 2022. "Diagnostic and Treatment of Spinal Fracture and Luxation in Italian Wolves (Canis lupus italicus)" Animals 12, no. 21: 3044. https://doi.org/10.3390/ani12213044
APA StyleFugazzotto, D., Costa Devoti, C., Dumas, M. P., Teani, C., Berti, E., & Zeira, O. (2022). Diagnostic and Treatment of Spinal Fracture and Luxation in Italian Wolves (Canis lupus italicus). Animals, 12(21), 3044. https://doi.org/10.3390/ani12213044





