YPEL3 Negatively Regulates Endometrial Function via the Wnt/β-Catenin Pathways during Early Pregnancy in Goats
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Tissue Collection
2.2. Cell Culture and Drug Treatment
2.3. Immunohistochemistry
2.4. Real-time Quantitative PCR
2.5. Western Bolt Analysis
2.6. Immunofluorescent Staining
2.7. Cell Transfection with Interference Target Sequence
2.8. Enzyme Linked Immunosorbent Essay (ELISA)
2.9. Statistical Analysis
3. Results
3.1. YPEL3 Expression Was Downregulated in Early Pregnancy
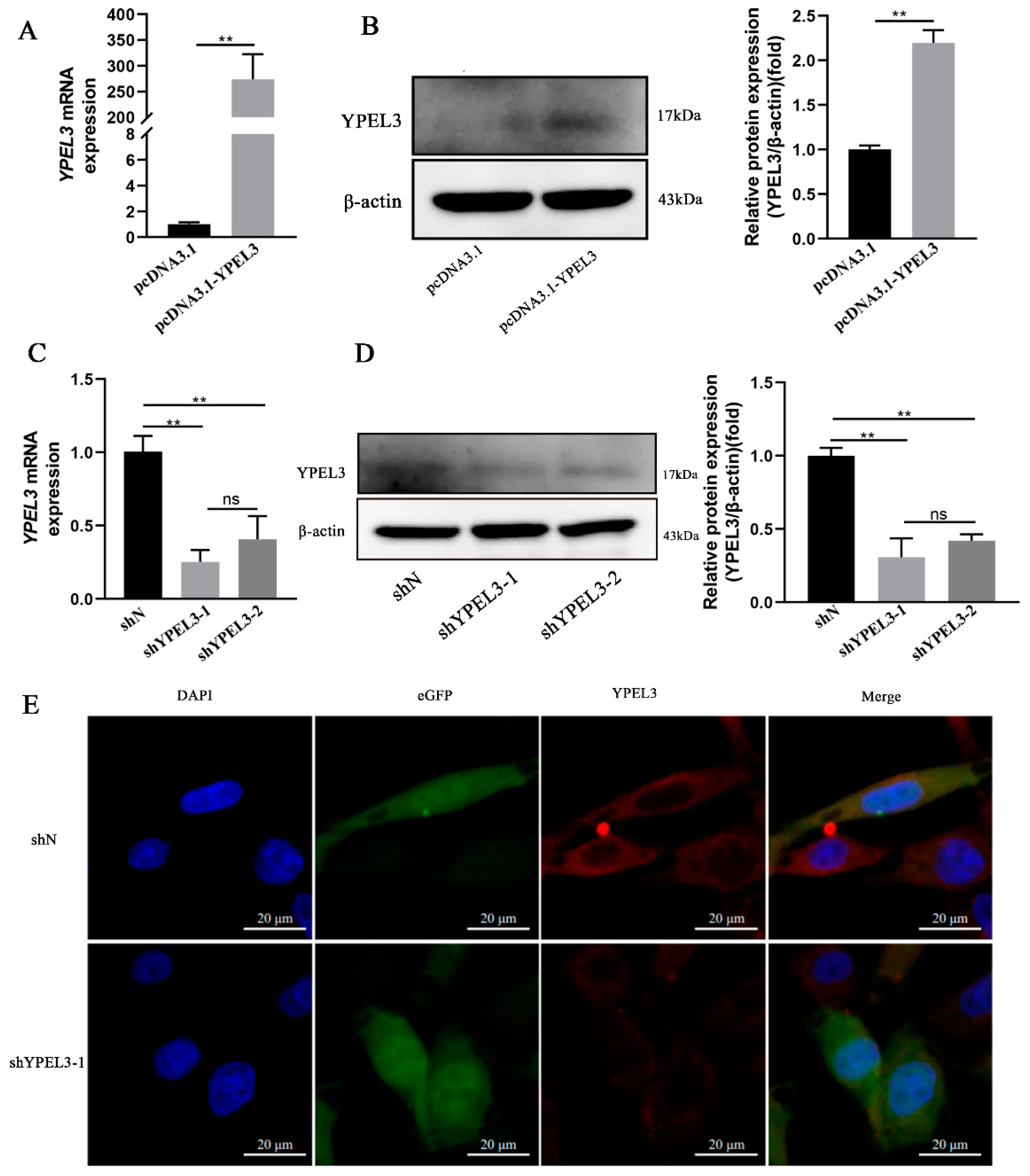
3.2. YPEL3 Expression in gEECs Was Significantly Affected by YPEL3 Silencing or Overexpression
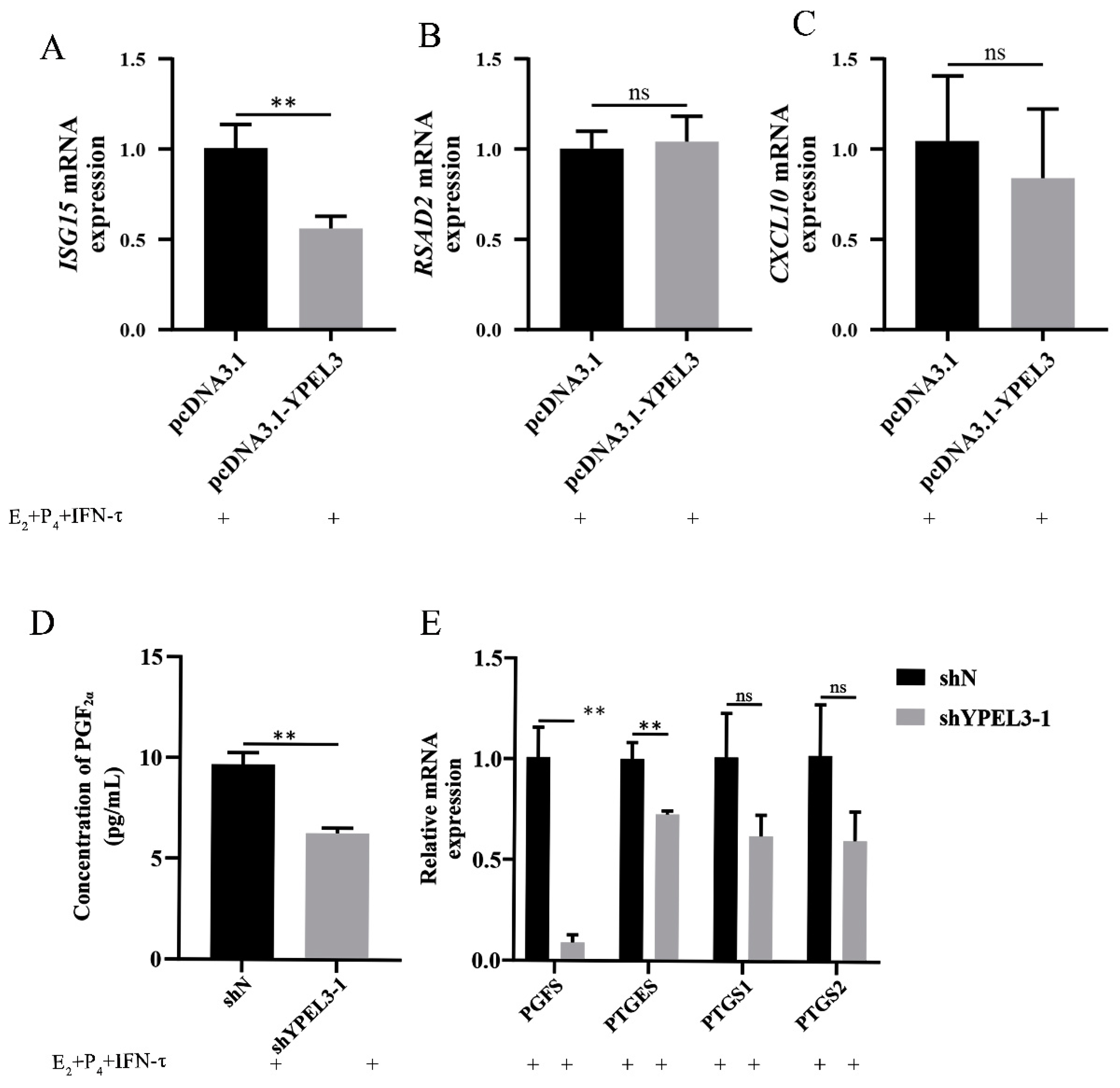
3.3. YPEL3 Affected Endometrial Receptivity and the Secretion of PGF2α
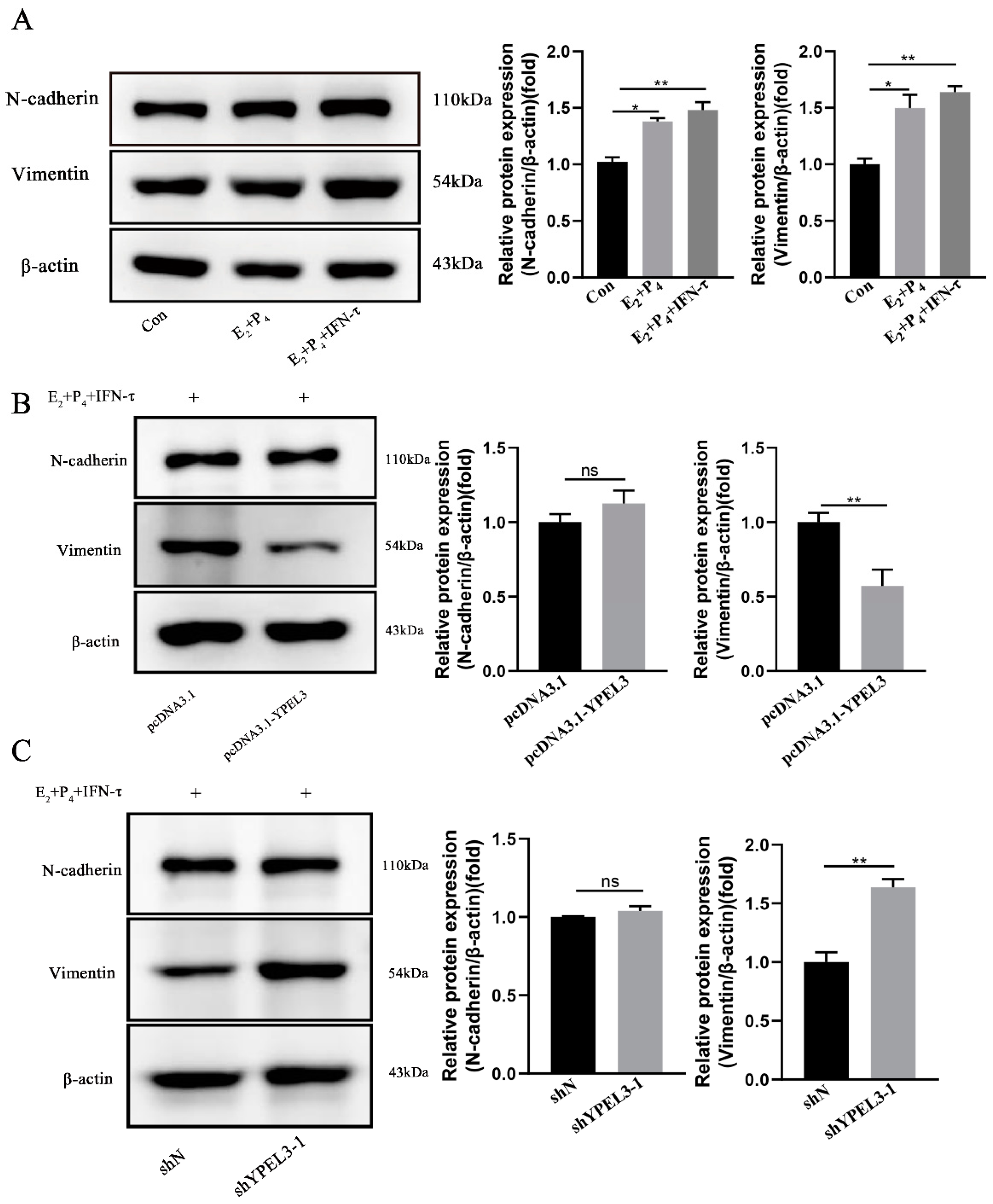
3.4. YPEL3 Significantly Affected the Expression of EMT Marker Proteins
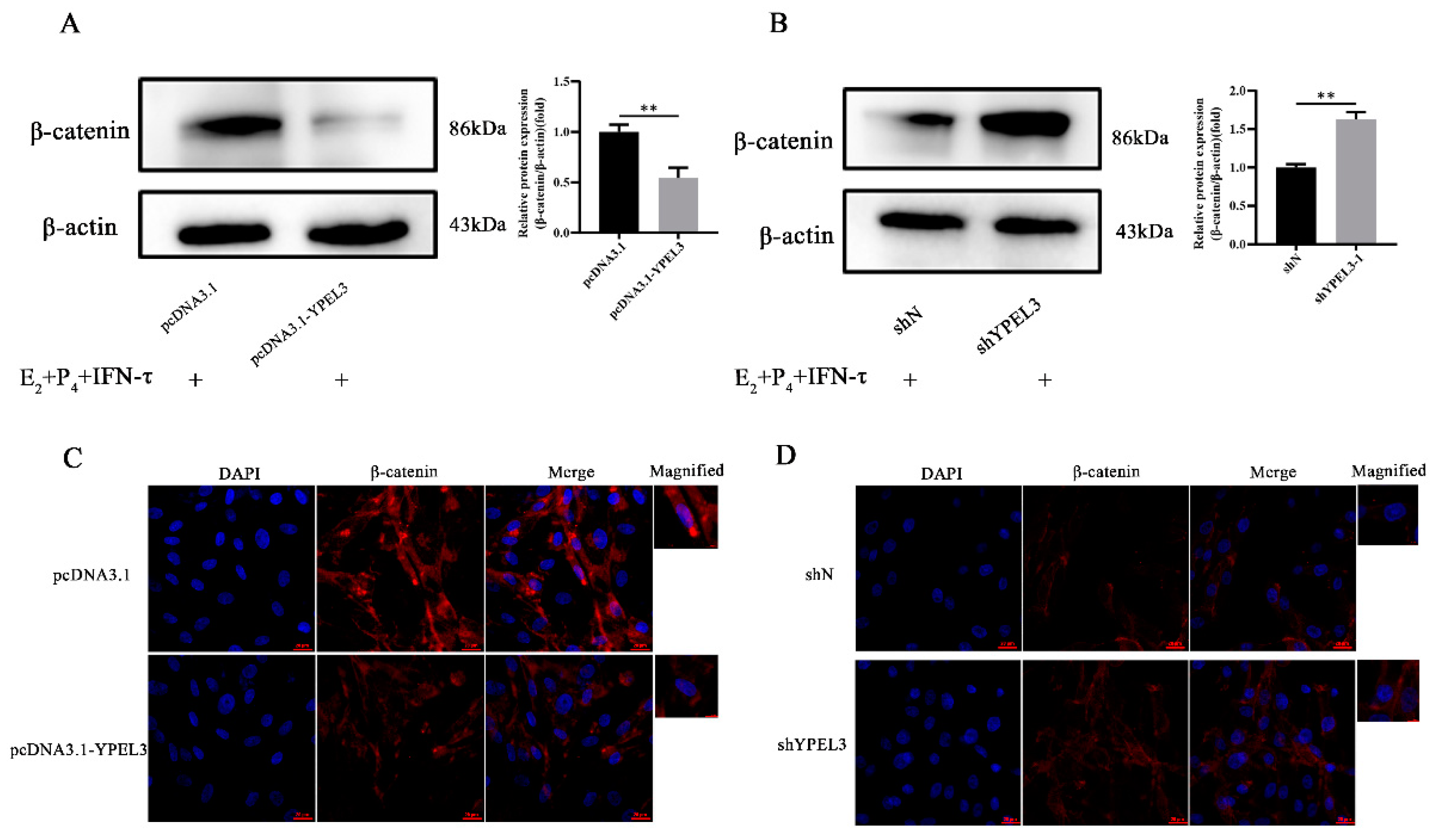
3.5. YPEL3 Was Involved in Regulation of the Wnt/β-Catenin Signaling Pathway
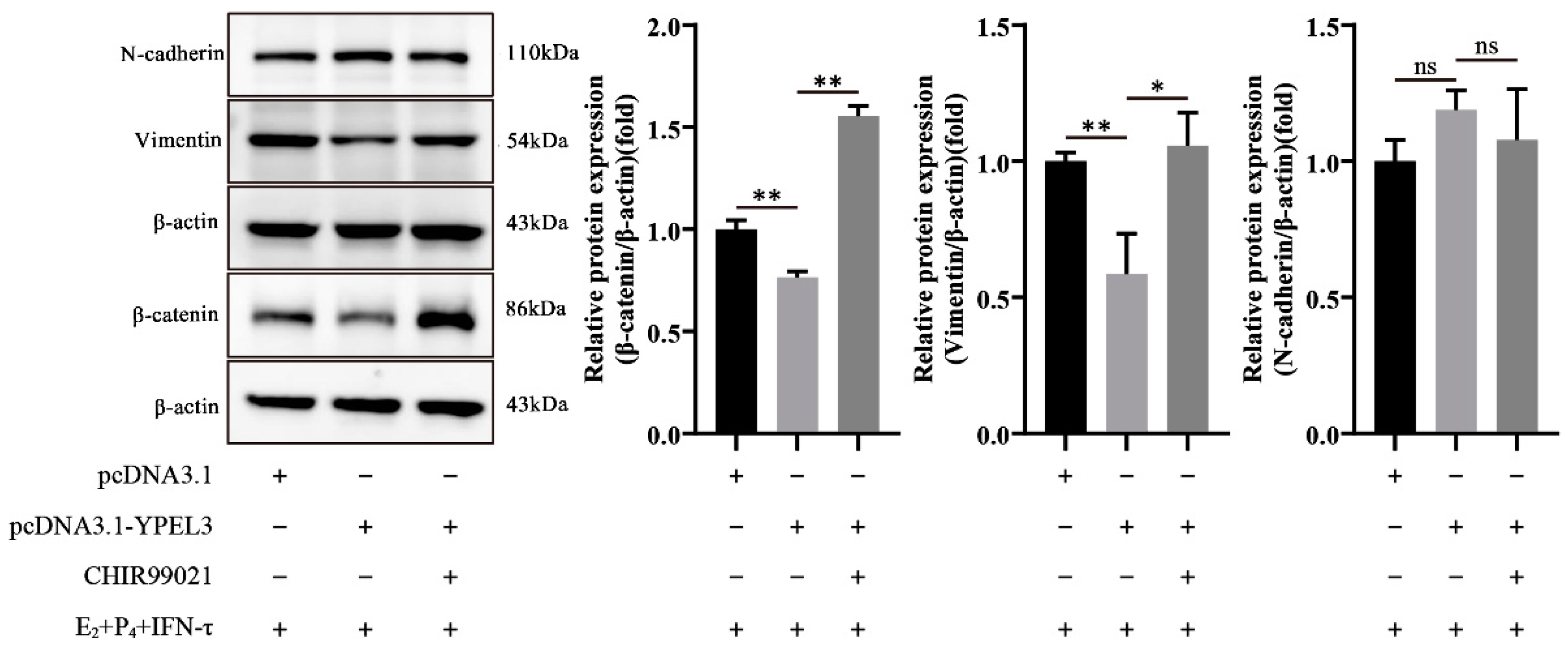
3.6. YPEL3 Regulated the Mesenchymal State of gEECs via the Wnt/β-Catenin Signaling Pathway
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Igwebuike, U.M. A review of uterine structural modifications that influence conceptus implantation and development in sheep and goats. Anim. Reprod. Sci. 2009, 112, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Miravet-Valenciano, J.A.; Rincon-Bertolin, A.; Vilella, F.; Simon, C. Understanding and improving endometrial receptivity. Curr. Opin. Obstet. Gynecol. 2015, 27, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Imakawa, K.; Bai, R.; Fujiwara, H.; Ideta, A.; Aoyagi, Y.; Kusama, K. Continuous model of conceptus implantation to the maternal endometrium. J. Endocrinol. 2017, 233, R53–R65. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Zhang, A.; Yu, F.; Gao, J.; Liu, Y.; Yu, C.; Zhou, H.; Xu, C. Label-free proteomics uncovers energy metabolism and focal adhesion regulations responsive for endometrium receptivity. J. Proteome Res. 2015, 14, 1831–1842. [Google Scholar] [CrossRef] [PubMed]
- Noel, S.; Herman, A.; Johnson, G.A.; Gray, C.A.; Stewart, M.D.; Bazer, F.W.; Gertler, A.; Spencer, T.E. Ovine placental lactogen specifically binds to endometrial glands of the ovine uterus. Biol. Reprod. 2003, 68, 772–780. [Google Scholar] [CrossRef][Green Version]
- Kelleher, A.M.; DeMayo, F.J.; Spencer, T.E. Uterine Glands: Developmental Biology and Functional Roles in Pregnancy. Endocr. Rev. 2019, 40, 1424–1445. [Google Scholar] [CrossRef]
- Bazer, F.W.; Burghardt, R.C.; Johnson, G.A.; Spencer, T.E.; Wu, G. Mechanisms for the establishment and maintenance of pregnancy: Synergies from scientific collaborations. Biol. Reprod. 2018, 99, 225–241. [Google Scholar] [CrossRef]
- Brooks, K.; Burns, G.; Spencer, T.E. Conceptus elongation in ruminants: Roles of progesterone, prostaglandin, interferon tau and cortisol. J. Anim. Sci. Biotechnol. 2014, 5, 53. [Google Scholar] [CrossRef]
- Imakawa, K.; Bai, R.; Kusama, K. Integration of molecules to construct the processes of conceptus implantation to the maternal endometrium. J. Anim. Sci. 2018, 96, 3009–3021. [Google Scholar] [CrossRef]
- Song, G.; Bazer, F.W.; Spencer, T.E. Pregnancy and interferon tau regulate RSAD2 and IFIH1 expression in the ovine uterus. Reproduction 2007, 133, 285–295. [Google Scholar] [CrossRef]
- Ulbrich, S.E.; Schulke, K.; Groebner, A.E.; Reichenbach, H.D.; Angioni, C.; Geisslinger, G.; Meyer, H.J.R. Quantitative characterization of prostaglandins in the uterus of early pregnant cattle. Reproduction 2009, 138, 371–382. [Google Scholar] [CrossRef] [PubMed]
- Wathes, D.C.; Abayasekara, D.R.E.; Aitken, R.J. Polyunsaturated Fatty Acids in Male and Female Reproduction1. Biol. Reprod. 2007, 77, 190–201. [Google Scholar] [CrossRef] [PubMed]
- Dorniak, P.; Bazer, F.W.; Spencer, T.E. Prostaglandins regulate conceptus elongation and mediate effects of interferon tau on the ovine uterine endometrium. Biol. Reprod. 2011, 84, 1119–1127. [Google Scholar] [CrossRef] [PubMed]
- Owusu-Akyaw, A.; Krishnamoorthy, K.; Goldsmith, L.T.; Morelli, S.S. The role of mesenchymal-epithelial transition in endometrial function. Hum. Reprod. Update. 2019, 25, 114–133. [Google Scholar] [CrossRef] [PubMed]
- Thiery, J.P.; Sleeman, J.P. Complex networks orchestrate epithelial-mesenchymal transitions. Rev. Mol. Cell Biol. 2006, 7, 131–142. [Google Scholar] [CrossRef]
- Kokudo, T.; Suzuki, Y.; Yoshimatsu, Y.; Yamazaki, T.; Watabe, T.; Miyazono, K. Snail is required for TGFbeta-induced endothelial-mesenchymal transition of embryonic stem cell-derived endothelial cells. J. Cell Sci. 2008, 121, 3317–3324. [Google Scholar] [CrossRef]
- Kokkinos, M.I.; Murthi, P.; Wafai, R.; Thompson, E.W.; Newgreen, D.F. Cadherins in the human placenta--epithelial-mesenchymal transition (EMT) and placental development. Placenta 2010, 31, 747–755. [Google Scholar] [CrossRef]
- Roxström-Lindquist, K.; Faye, I. The Drosophila gene Yippee reveals a novel family of putative zinc binding proteins highly conserved among eukaryotes. Insect. Mol. Biol. 2001, 10, 77–86. [Google Scholar] [CrossRef]
- Hosono, K.; Sasaki, T.; Minoshima, S.; Shimizu, N. Identification and characterization of a novel gene family YPEL in a wide spectrum of eukaryotic species. Gene 2004, 340, 31–43. [Google Scholar] [CrossRef]
- Zhang, W.; Xu, L.; Park, H.B.; Hwang, J.; Kwak, M.; Lee, P.C.W.; Liang, G.; Zhang, X.; Xu, J.; Jin, J.O. Escherichia coli adhesion portion FimH functions as an adjuvant for cancer immunotherapy. Nat. Commun. 2020, 11, 1187. [Google Scholar] [CrossRef]
- Kong, X.; Li, Y.; Zhang, X. Increased Expression of the YPEL3 Gene in Human Colonic Adenocarcinoma Tissue and the Effects on Proliferation, Migration, and Invasion of Colonic Adenocarcinoma Cells In Vitro via the Wnt/b-Catenin Signaling Pathway. Med. Sci. Monit. 2018, 24, 4767–4775. [Google Scholar] [CrossRef] [PubMed]
- Clevers, H.; Nusse, R. Wnt/β-catenin signaling and disease. Cell 2012, 149, 1192–1205. [Google Scholar] [CrossRef] [PubMed]
- Raza, S.H.A.; Khan, R.; Cheng, G.; Long, F.; Bing, S.; Easa, A.A.; Schreurs, N.M.; Pant, S.D.; Zhang, W.; Li, A.; et al. RNA-Seq reveals the potential molecular mechanisms of bovine KLF6 gene in the regulation of adipogenesis. Int. J. Biol. Macromol. 2022, 195, 198–206. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Jiang, T.; Liu, J.; Hong, J.; Lin, P.; Chen, H.; Zhou, D.; Tang, K.; Wang, A.; Jin, Y. Hormone regulates endometrial function via cooperation of endoplasmic reticulum stress and mTOR-autophagy. J. Cell Physiol. 2018, 233, 6644–6659. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Lu, M.; Zhang, R.; Guo, W.; Lin, P.; Yang, D.; Chen, H.; Tang, K.; Zhou, D.; Wang, A.; et al. Inhibition of Luman/CREB3 expression leads to the upregulation of testosterone synthesis in mouse Leydig cells. J. Cell Physiol. 2019. online ahead of print. [Google Scholar] [CrossRef]
- Yang, D.; Zhang, B.; Wang, Z.; Zhang, L.; Chen, H.; Zhou, D.; Tang, K.; Wang, A.; Lin, P.; Jin, Y. COPS5 negatively regulates goat endometrial function via the ERN1 and mTOR-autophagy pathways during early pregnancy. J. Cell Physiol. 2019, 234, 18666–18678. [Google Scholar] [CrossRef]
- Qu, X.L.; Ming, Z.; Yuan, F.; Wang, H.; Zhang, Y.Z. Effect of 2,3′,4,4′,5-Pentachlorobiphenyl Exposure on Endometrial Receptivity and the Methylation of HOXA10. Reprod. Sci. 2018, 25, 256–268. [Google Scholar] [CrossRef]
- Craciunas, L.; Gallos, I.; Chu, J.; Bourne, T.; Quenby, S.; Brosens, J.J.; Coomarasamy, A. Conventional and modern markers of endometrial receptivity: A systematic review and meta-analysis. Hum. Reprod. Update 2019, 25, 202–223. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, X.; Liu, J.; Ma, L.; Zhou, Z.; Song, Y.; Cao, B. The developmental transcriptome landscape of receptive endometrium during embryo implantation in dairy goats. Gene 2017, 633, 82–95. [Google Scholar] [CrossRef]
- Zhang, J.; Wen, X.; Ren, X.Y.; Li, Y.Q.; Tang, X.R.; Wang, Y.Q.; He, Q.M.; Yang, X.J.; Sun, Y.; Liu, N.; et al. Correction to: YPEL3 suppresses epithelial-mesenchymal transition and metastasis of nasopharyngeal carcinoma cells through the Wnt/β-catenin signaling pathway. J. Exp. Clin. Cancer Res. 2021, 40, 400. [Google Scholar] [CrossRef]
- Liu, H.; Wang, C.; Li, Z.; Shang, C.; Zhang, X.; Zhang, R.; Wang, A.; Jin, Y.; Lin, P. Transcriptomic Analysis of STAT1/3 in the Goat Endometrium During Embryo Implantation. Front. Vet. Sci. 2021, 8, 757–759. [Google Scholar] [CrossRef] [PubMed]
- Dorniak, P.; Bazer, F.W.; Spencer, T.E. Physiology and Endocrinology Symposium: Biological role of interferon tau in endometrial function and conceptus elongation. J. Anim. Sci. 2013, 91, 1627–1638. [Google Scholar] [CrossRef]
- Brooks, K.; Spencer, T.E. Biological roles of interferon tau (IFNT) and type I IFN receptors in elongation of the ovine conceptus. Biol. Reprod. 2015, 92, 47. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Wu, Y.; Gao, H.; Evans, A.; Zeng, S.M. Roles of interferon-stimulated gene 15 protein in bovine embryo development. Reprod. Fertil. Dev. 2017, 29, 1209–1216. [Google Scholar] [CrossRef] [PubMed]
- Oghbaei, F.; Zarezadeh, R.; Jafari-Gharabaghlou, D.; Ranjbar, M.; Nouri, M.; Fattahi, A.; Imakawa, K. Epithelial-mesenchymal transition process during embryo implantation. Cell Tissue Res. 2022, 388, 1–17. [Google Scholar] [CrossRef]
- Habas, K.; Shang, L. Alterations in intercellular adhesion molecule 1 (ICAM-1) and vascular cell adhesion molecule 1 (VCAM-1) in human endothelial cells. Tissue Cell 2018, 54, 139–143. [Google Scholar] [CrossRef]
- Jeong, J.W.; Lee, H.S.; Franco, H.L.; Broaddus, R.R.; Taketo, M.M.; Tsai, S.Y.; Lydon, J.P.; DeMayo, F.J. beta-catenin mediates glandular formation and dysregulation of beta-catenin induces hyperplasia formation in the murine uterus. Oncogene 2009, 28, 31–40. [Google Scholar] [CrossRef]
- Chronopoulou, E.; Koika, V.; Tsiveriotis, K.; Stefanidis, K.; Kalogeropoulos, S.; Georgopoulos, N.; Adonakis, G.; Kaponis, A. Wnt4, Wnt6 and β-catenin expression in human placental tissue—is there a link with first trimester miscarriage? Results from a pilot study. Reprod. Biol. Endocrinol. 2022, 20, 51. [Google Scholar] [CrossRef]
- Chen, J.J.; Xiao, Z.J.; Meng, X.; Wang, Y.; Yu, M.K.; Huang, W.Q.; Sun, X.; Chen, H.; Duan, Y.G.; Jiang, X.; et al. MRP4 sustains Wnt/β-catenin signaling for pregnancy, endometriosis and endometrial cancer. Theranostics 2019, 9, 5049–5064. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Qiu, R.; Liu, R.; Song, P.; Lin, P.; Chen, H.; Zhou, D.; Wang, A.; Jin, Y. YPEL3 Negatively Regulates Endometrial Function via the Wnt/β-Catenin Pathways during Early Pregnancy in Goats. Animals 2022, 12, 2973. https://doi.org/10.3390/ani12212973
Liu J, Qiu R, Liu R, Song P, Lin P, Chen H, Zhou D, Wang A, Jin Y. YPEL3 Negatively Regulates Endometrial Function via the Wnt/β-Catenin Pathways during Early Pregnancy in Goats. Animals. 2022; 12(21):2973. https://doi.org/10.3390/ani12212973
Chicago/Turabian StyleLiu, Jianguo, Rendong Qiu, Ran Liu, Pengjie Song, Pengfei Lin, Huatao Chen, Dong Zhou, Aihua Wang, and Yaping Jin. 2022. "YPEL3 Negatively Regulates Endometrial Function via the Wnt/β-Catenin Pathways during Early Pregnancy in Goats" Animals 12, no. 21: 2973. https://doi.org/10.3390/ani12212973
APA StyleLiu, J., Qiu, R., Liu, R., Song, P., Lin, P., Chen, H., Zhou, D., Wang, A., & Jin, Y. (2022). YPEL3 Negatively Regulates Endometrial Function via the Wnt/β-Catenin Pathways during Early Pregnancy in Goats. Animals, 12(21), 2973. https://doi.org/10.3390/ani12212973




