Two-Port Laparoscopic Adrenalectomy in Dogs
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Selection and Data Collection
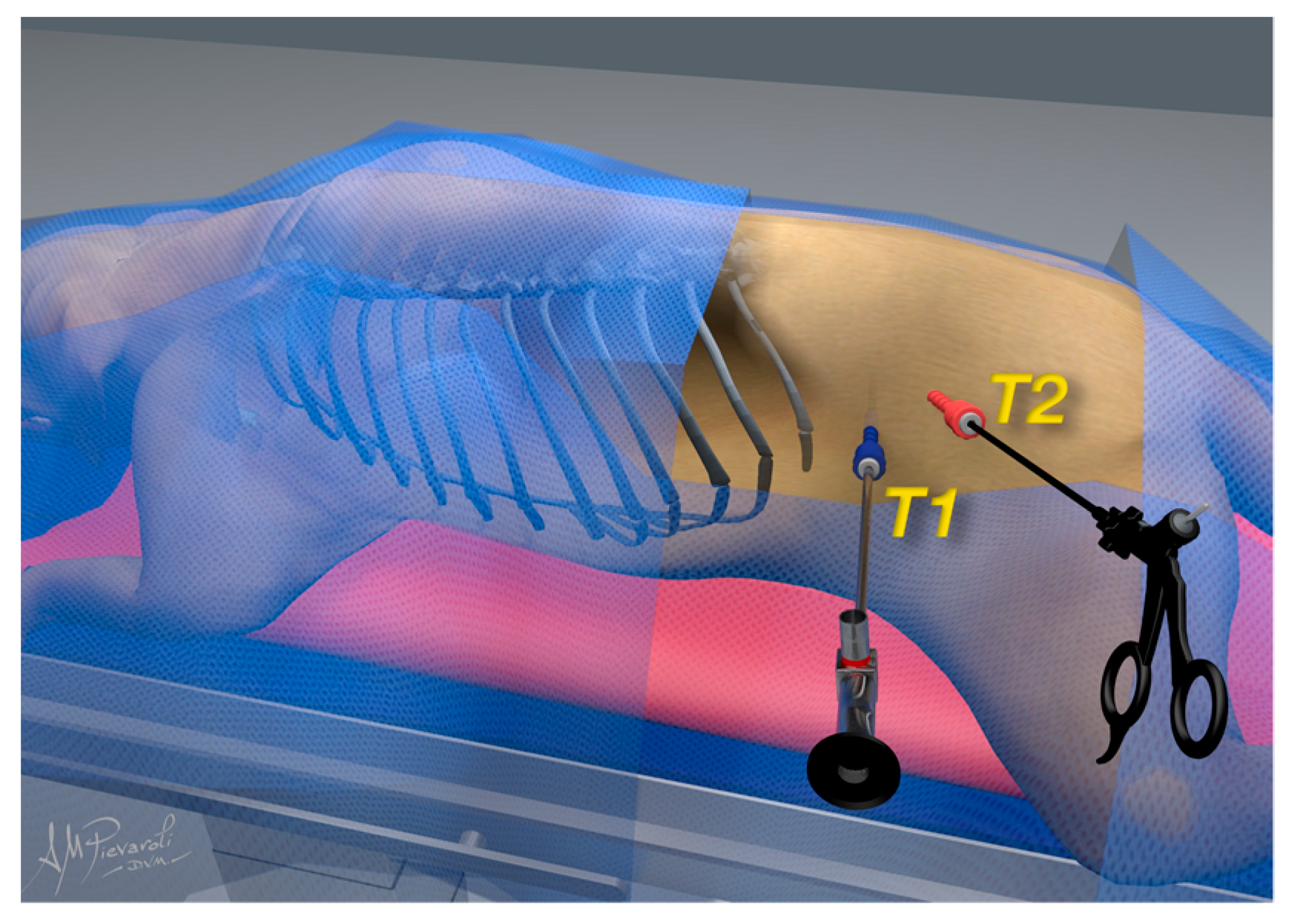
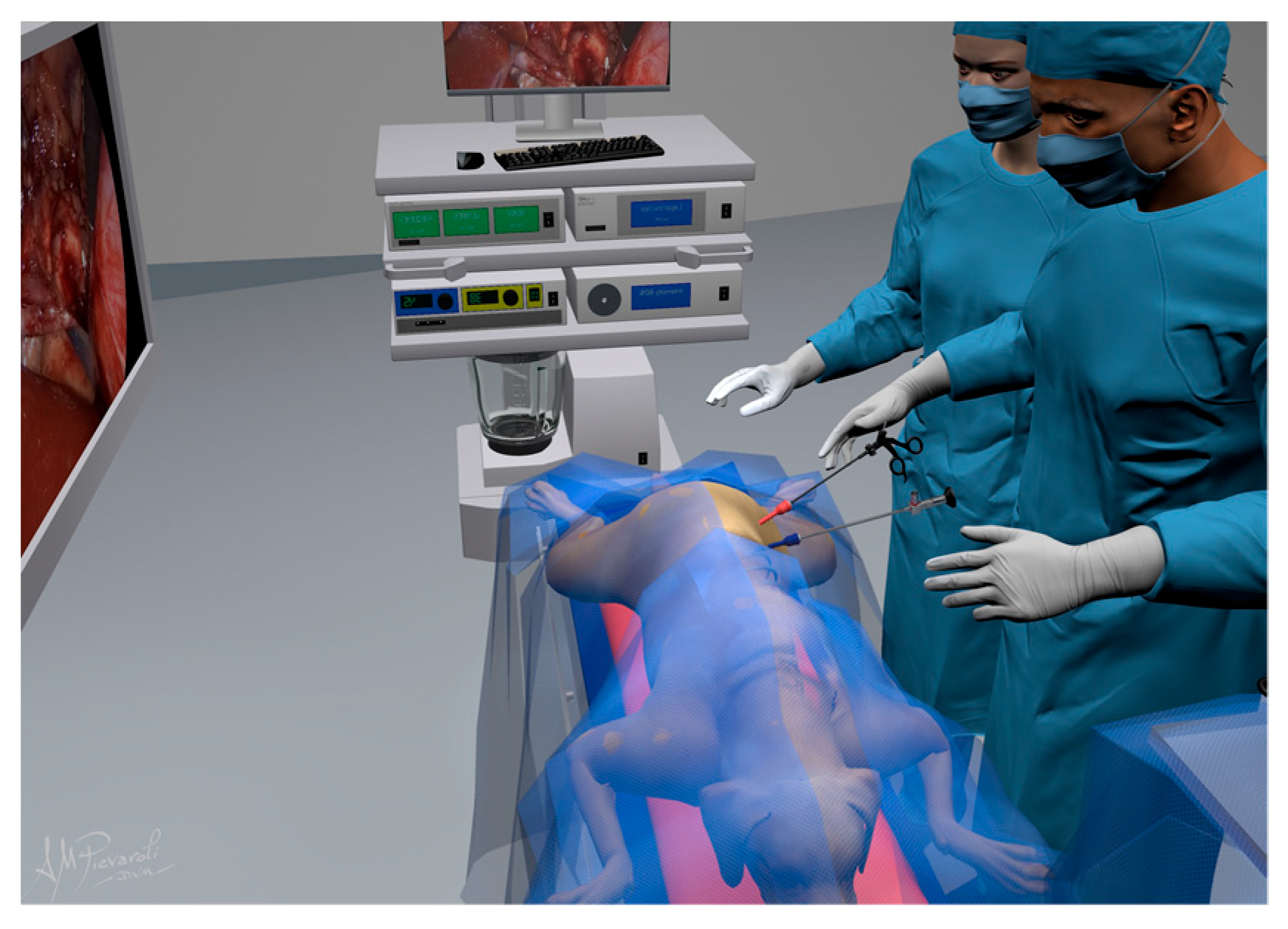
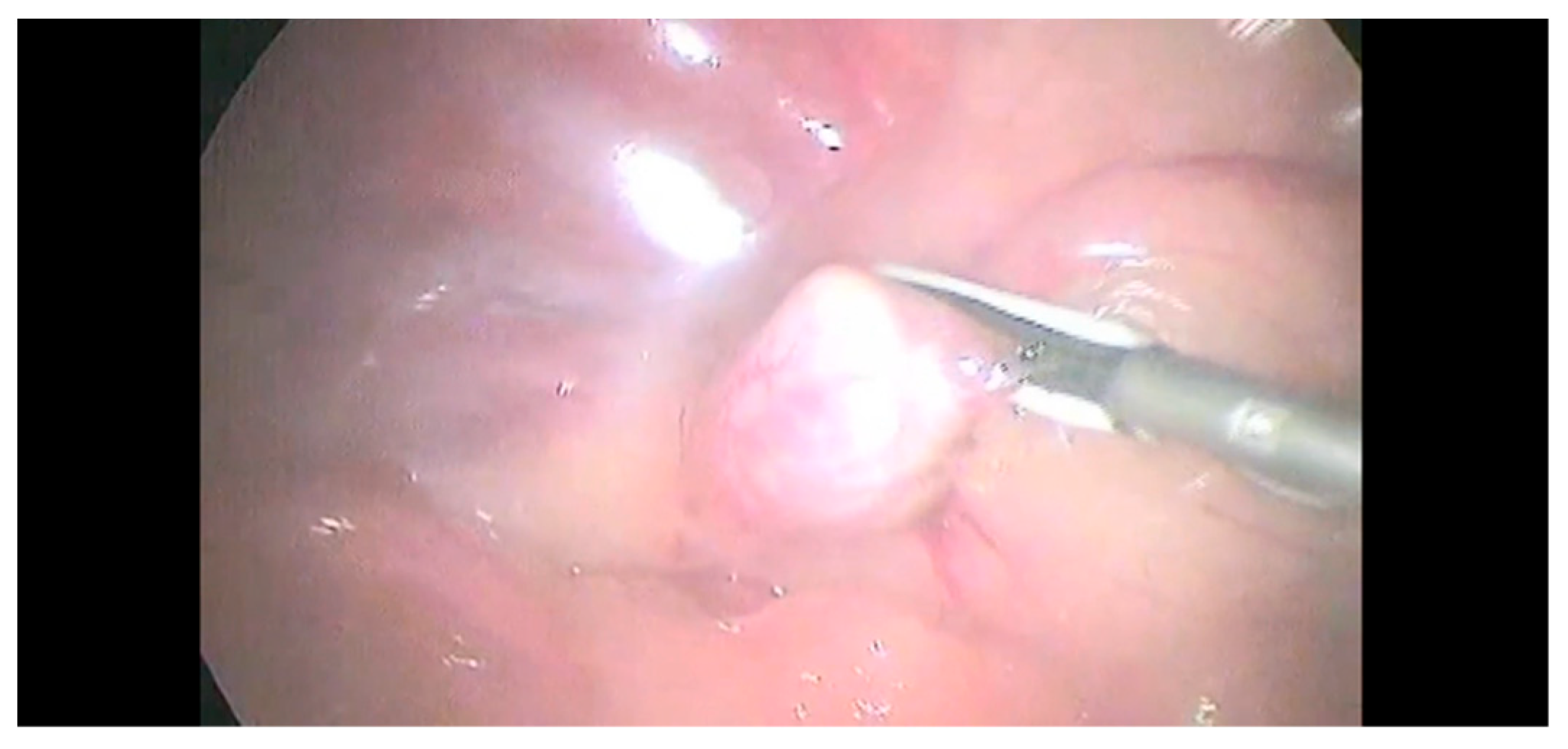
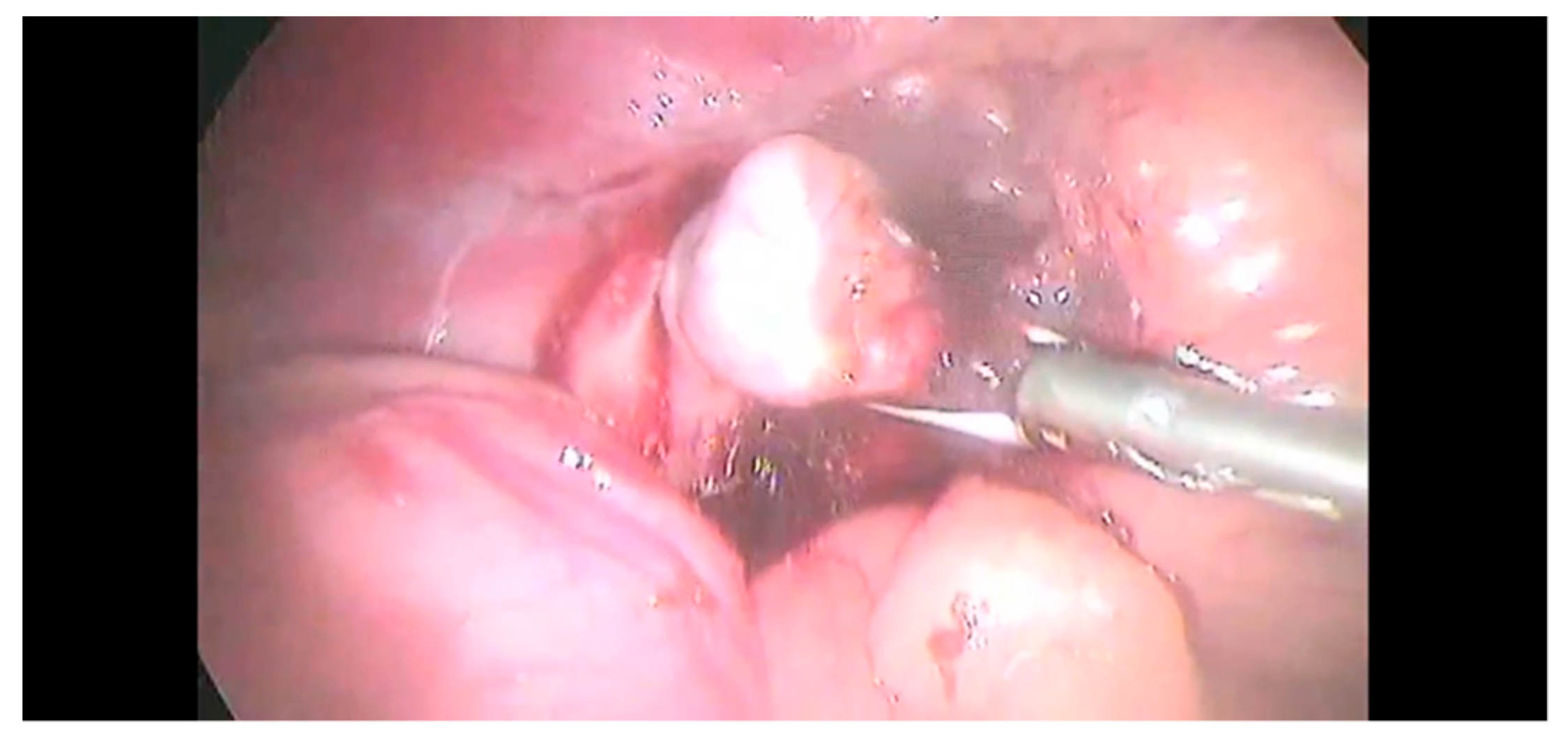
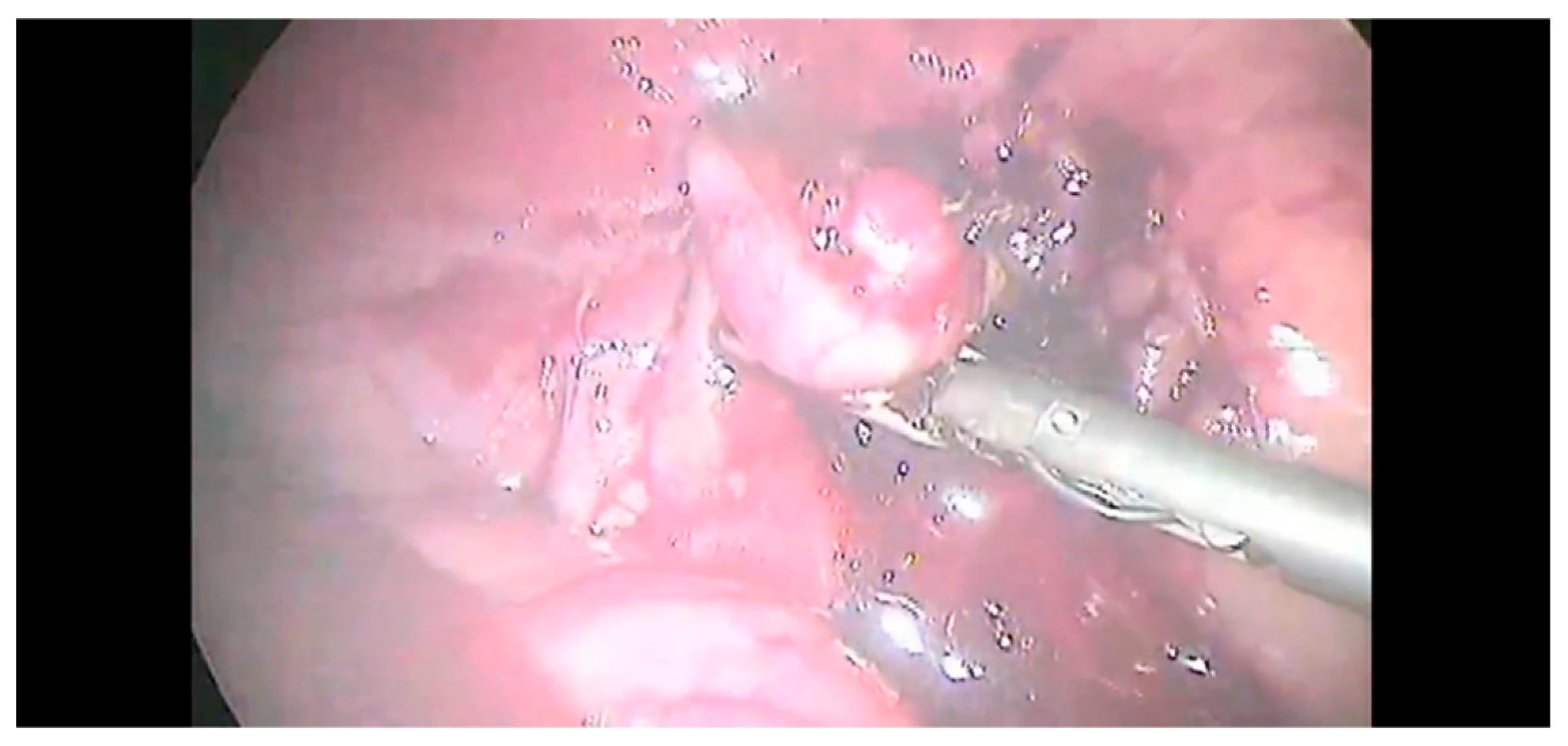
2.2. Surgical Procedure
2.3. Postoperative Care
2.4. Complications
2.5. Histopathology
2.6. Statistical Analysis
3. Results
3.1. Clinical Study
3.2. Complications
3.3. Outcomes
3.4. Histopathology
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lunn, K.F.; Boston, S.E. Tumors of the Endocrine System. In Withrow and MacEwen’s Small Animal Clinical Oncology; Elsevier: Amsterdam, The Netherlands, 2020; pp. 565–596. ISBN 978-0-323-59496-7. [Google Scholar]
- Mayhew, P.D. Advanced Laparoscopic Procedures (Hepatobiliary, Endocrine) in Dogs and Cats. Vet. Clin. N. Am. Small Anim. Pract. 2009, 39, 925–939. [Google Scholar] [CrossRef] [PubMed]
- Taylor, C.J.; Monnet, E. A Comparison of Outcomes between Laparoscopic and Open Adrenalectomies in Dogs. Vet. Surg. 2021, 50, O99–O107. [Google Scholar] [CrossRef] [PubMed]
- Cook, A.K.; Spaulding, K.A.; Edwards, J.F. Clinical Findings in Dogs with Incidental Adrenal Gland Lesions Determined by Ultrasonography: 151 Cases (2007–2010). J. Am. Vet. Med. Assoc. 2014, 244, 1181–1185. [Google Scholar] [CrossRef] [PubMed]
- Behrend, E.N.; Kooistra, H.S.; Nelson, R.; Reusch, C.E.; Scott-Moncrieff, J.C. Diagnosis of Spontaneous Canine Hyperadrenocorticism: 2012 ACVIM Consensus Statement (Small Animal). J. Vet. Intern. Med. 2013, 27, 1292–1304. [Google Scholar] [CrossRef] [PubMed]
- Balsa, I.; Culp, W. Use of Minimally Invasive Surgery in the Diagnosis and Treatment of Cancer in Dogs and Cats. Vet. Sci. 2019, 6, 33. [Google Scholar] [CrossRef]
- Mayhew, P.D.; Boston, S.E.; Zwingenberger, A.L.; Giuffrida, M.A.; Runge, J.J.; Holt, D.E.; Raleigh, J.S.; Singh, A.; Culp, W.T.N.; Case, J.B.; et al. Perioperative Morbidity and Mortality in Dogs with Invasive Adrenal Neoplasms Treated by Adrenalectomy and Cavotomy. Vet. Surg. 2019, 48, 742–750. [Google Scholar] [CrossRef]
- Kyles, A.E.; Feldman, E.C.; Cock, H.E.V.D.; Kass, P.H.; Mathews, K.G.; Hardie, E.M.; Nelson, R.W.; Ilkiw, J.E.; Gregory, C.R. Surgical Management of Adrenal Gland Tumors with and without Associated Tumor Thrombi in Dogs: 40 Cases (1994–2001). J. Am. Vet. Med. Assoc. 2003, 223, 654–662. [Google Scholar] [CrossRef]
- Mayhew, P.D.; Culp, W.T.N.; Hunt, G.B.; Steffey, M.A.; Mayhew, K.N.; Fuller, M.; Della-Maggiore, A.; Nelson, R.W. Comparison of Perioperative Morbidity and Mortality Rates in Dogs with Noninvasive Adrenocortical Masses Undergoing Laparoscopic versus Open Adrenalectomy. J. Am. Vet. Med. Assoc. 2014, 245, 1028–1035. [Google Scholar] [CrossRef]
- Jiménez Peláez, M.; Bouvy, B.M.; Dupré, G.P. Laparoscopic Adrenalectomy for Treatment of Unilateral Adrenocortical Carcinomas: Technique, Complications, and Results in Seven Dogs. Vet. Surg. 2008, 37, 444–453. [Google Scholar] [CrossRef]
- Naan, E.C.; Kirpensteijn, J.; Dupré, G.P.; Galac, S.; Radlinsky, M.G. Innovative Approach to Laparoscopic Adrenalectomy for Treatment of Unilateral Adrenal Gland Tumors in Dogs. Vet. Surg. 2013, 42, 710–715. [Google Scholar] [CrossRef]
- Ko, J.; Jeong, J.; Lee, S.; Son, H.; Kweon, O.-K.; Kim, W.H. Feasibility of Single-Port Retroperitoneoscopic Adrenalectomy in Dogs. Vet. Surg. 2018, 47, O75–O83. [Google Scholar] [CrossRef] [PubMed]
- Mayhew, P.D.; Araya, F.J.L.; Kirpensteijn, J. Laparoscopic Adrenalectomy. In Small Animal Laparoscopy and Thoracoscopy; Fransson, B.A., Mayhew, P.D., Eds.; Wiley: Hoboken, NJ, USA, 2022; pp. 199–213. ISBN 978-1-119-66685-1. [Google Scholar]
- Sasaki, A.; Nitta, H.; Otsuka, K.; Nishizuka, S.; Baba, S.; Umemura, A.; Koeda, K.; Mizuno, M.; Wakabayashi, G. Laparoendoscopic Single Site Adrenalectomy: Initial Results of Cosmetic Satisfaction and the Potential for Postoperative Pain Reduction. BMC Urol. 2013, 13, 21. [Google Scholar] [CrossRef]
- Tamburro, R.; Brunetti, B.; Muscatello, L.V.; Mantovani, C.; De Lorenzi, D. Short-Term Surgical Outcomes and Histomorphological Evaluation of Thermal Injury Following Palatoplasty Performed with Diode Laser or Air Plasma Device in Dogs with Brachycephalic Airway Obstructive Syndrome. Vet. J. 2019, 253, 105391. [Google Scholar] [CrossRef]
- Labelle, P.; Kyles, A.E.; Farver, T.B.; de Cock, H.E.V. Indicators of Malignancy of Canine Adrenocortical Tumors: Histopathology and Proliferation Index. Vet. Pathol. 2004, 41, 490–497. [Google Scholar] [CrossRef] [PubMed]
- Case, J.B.; Marvel, S.J.; Boscan, P.; Monnet, E.L. Surgical Time and Severity of Postoperative Pain in Dogs Undergoing Laparoscopic Ovariectomy with One, Two, or Three Instrument Cannulas. J. Am. Vet. Med. Assoc. 2011, 239, 203–208. [Google Scholar] [CrossRef] [PubMed]
- Culp, W.T.N.; Mayhew, P.D.; Brown, D.C. The Effect of Laparoscopic versus Open Ovariectomy on Postsurgical Activity in Small Dogs. Vet. Surg. 2009, 38, 811–817. [Google Scholar] [CrossRef]
- Devitt, C.M.; Cox, R.E.; Hailey, J.J. Duration, Complications, Stress, and Pain of Open Ovariohysterectomy versus a Simple Method of Laparoscopic-Assisted Ovariohysterectomy in Dogs. J. Am. Vet. Med. Assoc. 2005, 227, 921–927. [Google Scholar] [CrossRef]
- Mayhew, P.D. Complications of Minimally Invasive Surgery in Companion Animals. Vet. Clin. N. Am. Small Anim. Pract. 2011, 41, 1007–1021. [Google Scholar] [CrossRef]
- Barrera, J.S.; Bernard, F.; Ehrhart, E.J.; Withrow, S.J.; Monnet, E. Evaluation of Risk Factors for Outcome Associated with Adrenal Gland Tumors with or without Invasion of the Caudal Vena Cava and Treated via Adrenalectomy in Dogs: 86 Cases (1993–2009). J. Am. Vet. Med. Assoc. 2013, 242, 1715–1721. [Google Scholar] [CrossRef]
- Yoshida, O.; Kutara, K.; Seki, M.; Ishigaki, K.; Teshima, K.; Ishikawa, C.; Iida, G.; Edamura, K.; Kagawa, Y.; Asano, K. Preoperative Differential Diagnosis of Canine Adrenal Tumors Using Triple-Phase Helical Computed Tomography: Preoperative CT of Canine Adrenal Tumors. Vet. Surg. 2016, 45, 427–435. [Google Scholar] [CrossRef]
- Zini, E.; Nolli, S.; Ferri, F.; Massari, F.; Gerardi, G.; Nicoli, S.; Romanelli, G.; Montinaro, V.; Trez, D.; Cavicchioli, L.; et al. Pheochromocytoma in Dogs Undergoing Adrenalectomy. Vet. Pathol. 2019, 56, 358–368. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, A.; Collivignarelli, F.; Vignoli, M.; Scaletta, L.; Cuomo, A.; Falerno, I.; Paolini, A.; Tamburro, R. A Comparison of Times Taken for the Placement of the First Portal and Complication Rates between the Veress Needle Technique and the Modified Hasson Technique in Canine Ovariectomy Laparoscopic Surgery. Animals 2021, 11, 2936. [Google Scholar] [CrossRef] [PubMed]
- Anderson, C.; Birchard, S.; Powers, B.; Belandria, G.; Kuntz, C.; Withrow, S. Surgical Treatment of Adrenocortical Tumors: 21 Cases (1990–1996). J. Am. Anim. Hosp. Assoc. 2001, 37, 93–97. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, P.; Kovak, J.R.; Koprowski, A.; Ludwig, L.L.; Monette, S.; Bergman, P.J. Evaluation of Prognostic Factors in the Surgical Treatment of Adrenal Gland Tumors in Dogs: 41 Cases (1999–2005). J. Am. Vet. Med. Assoc. 2008, 232, 77–84. [Google Scholar] [CrossRef]
- Van Sluijs, F.J.; Sjollema, B.E.; Voorhout, G.; van den Ingh, T.S.G.A.M.; Rijnberk, A. Results of Adrenalectomy in 36 Dogs with Hyperadrenocorticism Caused by Adrenocortical Tumour. Vet. Q. 1995, 17, 113–116. [Google Scholar] [CrossRef] [PubMed]
- Massari, F.; Nicoli, S.; Romanelli, G.; Buracco, P.; Zini, E. Adrenalectomy in Dogs with Adrenal Gland Tumors: 52 Cases (2002–2008). J. Am. Vet. Med. Assoc. 2011, 239, 216–221. [Google Scholar] [CrossRef]
- Cavalcanti, J.V.J.; Skinner, O.T.; Mayhew, P.D.; Colee, J.C.; Boston, S.E. Outcome in Dogs Undergoing Adrenalectomy for Small Adrenal Gland Tumours without Vascular Invasion. Vet. Comp. Oncol. 2020, 18, 599–606. [Google Scholar] [CrossRef]
- Lacitignola, L.; Trisciuzzi, R.; Imperante, A.; Fracassi, L.; Crovace, A.M.; Staffieri, F. Comparison of Laparoscopic Steerable Instruments Performed by Expert Surgeons and Novices. Vet. Sci. 2020, 7, 135. [Google Scholar] [CrossRef]
- Gonzalez-Gasch, E.; Monnet, E. Comparison of Single Port Access Versus Multiple Port Access Systems in Elective Laparoscopy: 98 Dogs (2005–2014). Vet. Surg. 2015, 44, 895–899. [Google Scholar] [CrossRef]
- Wright, T.; Singh, A.; Mayhew, P.D.; Runge, J.J.; Brisson, B.A.; Oblak, M.L.; Case, J.B. Laparoscopic-Assisted Splenectomy in Dogs: 18 Cases (2012–2014). J. Am. Vet. Med. Assoc. 2016, 248, 916–922. [Google Scholar] [CrossRef]
- Runge, J.J.; Mayhew, P.D.; Case, J.B.; Singh, A.; Mayhew, K.N.; Culp, W.T.N. Single-Port Laparoscopic Cryptorchidectomy in Dogs and Cats: 25 Cases (2009–2014). J. Am. Vet. Med. Assoc. 2014, 245, 1258–1265. [Google Scholar] [CrossRef] [PubMed]
- Lacitignola, L.; Guadalupi, M.; Massari, F. Single Incision Laparoscopic Surgery (SILS) in Small Animals: A Systematic Review and Meta-Analysis of Current Veterinary Literature. Vet. Sci. 2021, 8, 144. [Google Scholar] [CrossRef] [PubMed]
- Walz, M.K.; Groeben, H.; Alesina, P.F. Single-Access Retroperitoneoscopic Adrenalectomy (SARA) Versus Conventional Retroperitoneoscopic Adrenalectomy (CORA): A Case–Control Study. World J. Surg. 2010, 34, 1386–1390. [Google Scholar] [CrossRef] [PubMed]
- Beiša, V.; Kildušis, E.; Strupas, K. Single Access Retroperitoneoscopic Adrenalectomy: Initial Experience. Wideochir. Inne Tech. Maloinwazyjne 2012, 7, 45–49. [Google Scholar] [CrossRef]
- Jeong, J.; Ko, J.; Lim, H.; Kweon, O.; Kim, W.H. Retroperitoneal Laparoscopy in Dogs: Access Technique, Working Space, and Surgical Anatomy. Vet. Surg. 2016, 45, O102–O110. [Google Scholar] [CrossRef]
| Patient | Signalment | Clinical Signs | Laboratory Findings | Ultrasound Findings | LDDS Test Results |
|---|---|---|---|---|---|
| 1 | 11y, NF, 37 Kg, German shepherd | PU/PD, alopecia, polyphagia | - | 30 × 40 mm LS, mild hepatomegaly | T0: 12.4 mg/dL T4: 16.3 mg/dL T8: 11.5 mg/dL |
| 2 | 8y, NM, 40 Kg, German shepherd | Cutaneous calcinosis, alopecia | ALP 4512 U/L | 34 × 40 mm RS, mild hepatomegaly | T0: 3.2 mg/dL T4: 3.1 mg/dL T8: 4.5 mg/dL |
| 3 | 9y, NF, 38 Kg, Golden retriever | PU/PD, polyphagia, alopecia, thin skin | WBC 22 × 1010/L ALP 328 U/L TCHOL 3g/L | 25 × 40 mm LS | T0: 4.6 mg/dL T4: 5.7 mg/dL T8: 5.4 mg/dL |
| 4 | 8y, NM, 37 Kg, Golden retriever | Incidental finding | - | 33 × 33 mm LS, prostatic cysts, mild hepatomegaly | T0: 2.7 mg/dL T4: <1.0 mg/dL T8: <1.0 mg/dL |
| 5 | 11y, NM, 27 Kg, Mixed breed | PU/PD; hematuria, stranguria, astenia | WBC 18 × 1010/L ALP 2907 U/L | 25 × 35 mm RS | T0: 3.4 mg/dL T4: 3.4 mg/dL T8: 3.8 mg/dL |
| 6 | 9y, NF, 14 Kg, Mixed breed | PU/PD | - | 34 × 40 mm LS | T0: 5.5 mg/dL T4: 5.4 mg/dL T8: 6.5 mg/dL |
| 7 | 12y, N, 9 Kg, Mixed breed | Incidental finding | ALP 4712 U/L | 35 × 40 mm LS, hepatomegaly, reduction cortical medullary ratio | T0: 8.9 mg/dL T4: 7.4 mg/dL T8: 6.4 mg/dL |
| 8 | 9y, M, 10 Kg, Yorkshire terrier | PU/PD | - | 24 × 30 RS | T0: 9.3 mg/dL T4: 9.0 mg/dL T8: 8.7 mg/dL |
| 9 | 9y, NF, 13 Kg, Yorkshire terrier | Incidental finding | - | 24 × 40 mm LS, mild hepatomegaly | T0: 4.3 mg/dL T4: 6.5 mg/dL T8: 6.2 mg/dL |
| 10 | 12y, NF, 9 Kg, Poodle | PU/PD, polyphagia, alopecia | ALP 3223 U/L ALT 340 U/L | 23 × 33 mm RS, mild hepatomegaly | T0: 4.8 mg/dL T4: 3.6 mg/dL T8: 4.2 mg/dL |
| 11 | 10y, M, 10 Kg, Cocker spaniel | PU/PD, calcinosis cutis, alopecia | WBC 20 × 1010/L ALP 2858 U/L ALT 437 U/L | 23 × 30 mm LS | T0: 15.5 mg/dL T4: 13.1 mg/dL T8: 10.9 mg/dL |
| 12 | 9y, NF, 12 Kg, Cocker spaniel | PU/PD, calcinosis cutis, alopecia | ALP 2850 U/L | 32 × 35 mm LS | T0: 9.7 mg/dL T4: 8.4 mg/dL T8: 8.9 mg/dL |
| 13 | 9y, 27 Kg, Epagneul Breton | Incidental finding | - | 28 × 38 mm LS | T0: 5.5 mg/dL T4: <1.0 mg/dL T8: <1.0 mg/dL |
| 14 | 6y, 18 Kg, English bulldog | PU/PD; hematuria, calcinosis cutis | ALP 4340 U/L | 36 × 30 mm LS | T0: 7.7 mg/dL T4: 6.6 mg/dL T8: 6.5 mg/dL |
| 15 | 7y, 33 Kg, Weimaraner | Incidental finding | - | 34 × 40 mm LS | T0: 2.6 mg/dL T4: <1.0 mg/dL T8: <1.0 mg/dL |
| 16 | 12y, NF, 9 Kg, Dachshund | PU/PD | WBC 18 × 1010/L ALP 3858 U/L ALT 473 U/L | 24 × 40 mm LS | T0: 3.1 mg/dL T4: 2.9 mg/dL T8: 3.7 mg/dL |
| Dog | Breed | Intraoperative Complications | Postoperative Complications | Surgical Time (Min) | Outcome | Histological Diagnosis |
|---|---|---|---|---|---|---|
| 1 | German shepherd | - | - | 100 | Alive | Carcinoma |
| 2 | German shepherd | - | - | 120 | Alive | Carcinoma |
| 3 | Golden retriever | Capsule rupture | - | 85 | Lost follow-up | Carcinoma with vascular emboli |
| 4 | Golden retriever | - | - | 100 | Alive | Adenoma |
| 5 | Mixed breed | - | - | 85 | 18 months, euthanasia for oral melanoma | Carcinoma |
| 6 | Mixed breed | - | - | 80 | 12 months | Carcinoma with vascular emboli |
| 7 | Mixed breed | - | Periportal cellulitis | 74 | Alive | Carcinoma |
| 8 | Yorkshire terrier | - | - | 40 | 6 months | Carcinoma with vascular emboli |
| 9 | Yorkshire terrier | - | - | 50 | Lost follow-up | Adenoma |
| 10 | Poodle | - | - | 74 | 4 months | Carcinoma with vascular and lymphatic emboli |
| 11 | Cocker spaniel | Capsule rupture | Periportal cellulitis | 80 | 8 months | Carcinoma with vascular and lymphatic emboli |
| 12 | Cocker spaniel | Capsule rupture | Periportal cellulitis | 55 | 14 months | Carcinoma |
| 13 | Epagneul breton | - | - | 35 | Alive | Carcinoma |
| 14 | English bulldog | Capsule rupture | Mild peritonitis, periportal cellulitis | 60 | 3 years, metastasis | Carcinoma |
| 15 | Weimaraner | - | - | 40 | Alive | Adenoma |
| 16 | Dachshund | Capsule rupture | - | 50 | 5 years, metastasis | Carcinoma |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Collivignarelli, F.; Bianchi, A.; Paolini, A.; Vignoli, M.; Crisi, P.E.; Falerno, I.; Bonis, A.D.; Rosto, M.; Tamburro, R. Two-Port Laparoscopic Adrenalectomy in Dogs. Animals 2022, 12, 2917. https://doi.org/10.3390/ani12212917
Collivignarelli F, Bianchi A, Paolini A, Vignoli M, Crisi PE, Falerno I, Bonis AD, Rosto M, Tamburro R. Two-Port Laparoscopic Adrenalectomy in Dogs. Animals. 2022; 12(21):2917. https://doi.org/10.3390/ani12212917
Chicago/Turabian StyleCollivignarelli, Francesco, Amanda Bianchi, Andrea Paolini, Massimo Vignoli, Paolo Emidio Crisi, Ilaria Falerno, Andrea De Bonis, Martina Rosto, and Roberto Tamburro. 2022. "Two-Port Laparoscopic Adrenalectomy in Dogs" Animals 12, no. 21: 2917. https://doi.org/10.3390/ani12212917
APA StyleCollivignarelli, F., Bianchi, A., Paolini, A., Vignoli, M., Crisi, P. E., Falerno, I., Bonis, A. D., Rosto, M., & Tamburro, R. (2022). Two-Port Laparoscopic Adrenalectomy in Dogs. Animals, 12(21), 2917. https://doi.org/10.3390/ani12212917






