Genetic Resistance of Bovines to Theileriosis
Abstract
Simple Summary
Abstract
1. Introduction
2. Bovine Theileriosis
2.1. Bovine Theileriosis—Definition
2.2. Prevalence
2.3. Economic Losses from Theileriosis
2.4. Pathogenesis
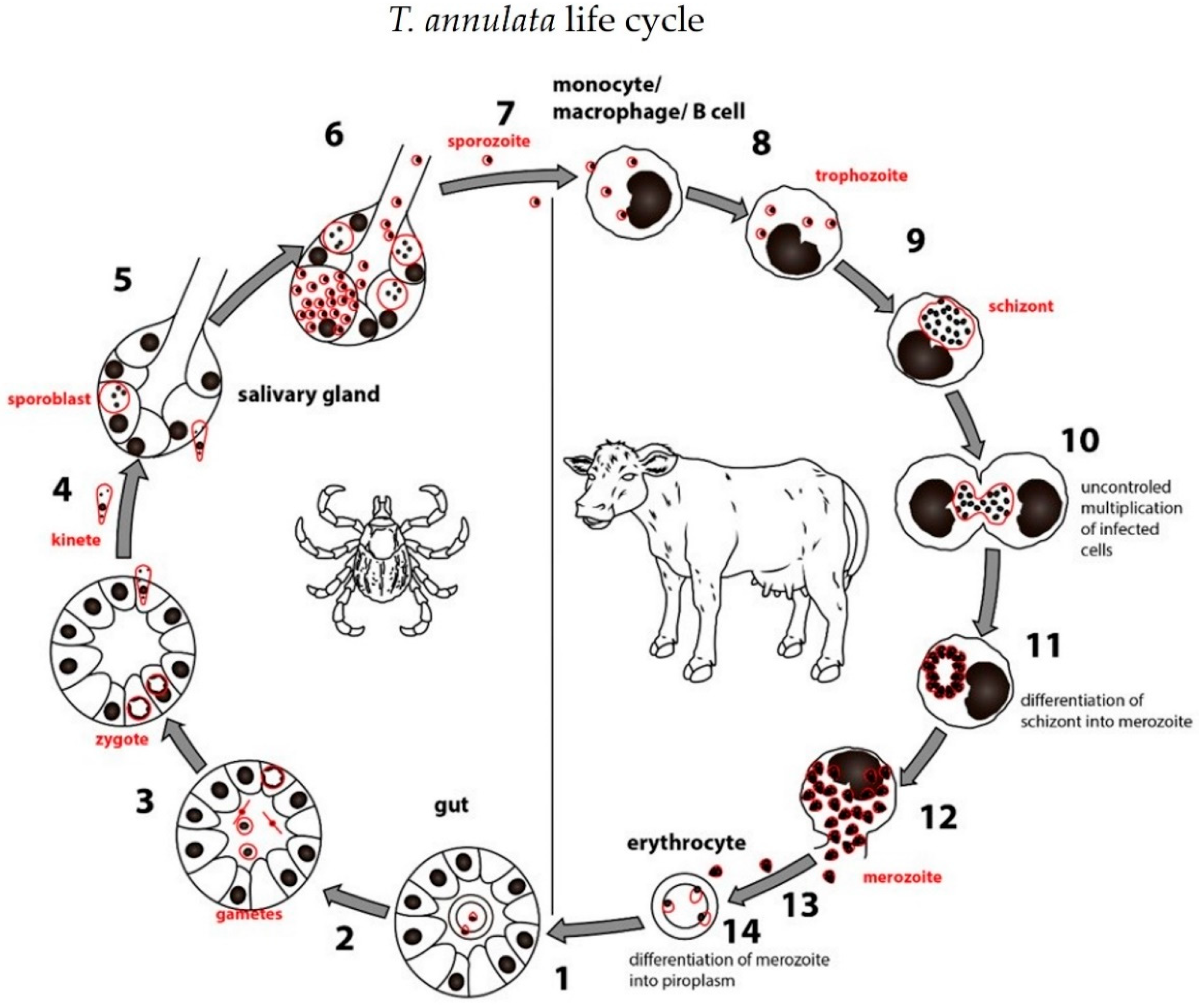
2.5. Life Cycle of Theileria spp.
2.6. Clinical Signs
2.7. Diagnosis
3. Theileriosis Control Strategies
4. Genetic Selection—Resistance and Tolerance
5. Immune Response Mechanisms
6. Theileriosis Resistance
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Knap, P.W.; Wilson, A.D. Why breed disease-resilient livestock, and how? Genet. Sel. Evol. 2020, 52, 60. [Google Scholar] [CrossRef] [PubMed]
- Wakchaure, R.; Ganguly, S. Disease Resistance in Livestock: A Review. J. Immunol. Immunopathol. 2017, 18, 94–99. [Google Scholar] [CrossRef]
- Bishop, S.C.; Woolliams, J.A. Genomics and disease resistance studies in livestock. Livest. Sci. 2014, 166, 190–198. [Google Scholar] [CrossRef] [PubMed]
- Morris, C.A. A review of genetic resistance to disease in Bos taurus cattle. Vet. J. 2007, 174, 481–491. [Google Scholar] [CrossRef]
- Ma, Z.; Lee, S.; Jeong, K.C. Mitigating Antibiotic Resistance at the Livestock-Environment Interface: A Review. J. Microbiol. Biotechnol. 2019, 29, 1683–1692. [Google Scholar] [CrossRef]
- Kaplan, R.M. Biology, Epidemiology, Diagnosis, and Management of Anthelmintic Resistance in Gastrointestinal Nematides of Livestock. Vet. Clin. N. Am. Food Anim. Pract. 2020, 36, 17–30. [Google Scholar] [CrossRef]
- Plastow, G.S. Invited Review Genomics to benefit livestock production: Improving animal health. Rev. Bras. Zootec. 2016, 45, 349–354. [Google Scholar] [CrossRef]
- Davies, G.; Genini, S.; Bishop, S.C.; Giuffra, E. An assessment of opportunities to dissect host genetic variation in resistance to infectious diseases in livestock. Anim. Consort. 2009, 3, 415–436. [Google Scholar] [CrossRef]
- Bishop, S.C.; Chesnais, J.; Stear, M.J. Breeding for disease resistance: Issues and opportunities. In Proceedings of the 7th Worlf Congress on Genetics Applied to Livestock Production, Montpellier, France, 19–23 August 2002; pp. 19–23. [Google Scholar]
- Dewangan, P.; Panigrahi, M.; Kumar, A.; Saravana, B.C.; Ghosh, S.; Asaf, V.N.M.; Parida, S.; Gaur, G.K.; Sharma, D.; Bhushan, B. The mRNA expression of immune-related genes in crossbred and Tharparkar cattle in response to in vitro infection with Theileria annulata. Mol. Biol. Rep. 2015, 42, 1247–1255. [Google Scholar] [CrossRef]
- Zeb, J.; Song, B.; Aziz, M.U.; Hussain, S.; Zarin, R.; Sparagano, O. Diversity and Distribution of Theileria Species and Their Vectors in Ruminants from India, Pakistan and Bangladesh. Diversity 2022, 14, 82. [Google Scholar] [CrossRef]
- Nene, V.; Morrison, W.I. Approaches to vaccination against Theileria parva and Theileria annulata. Parasite Immunol. 2016, 38, 724–734. [Google Scholar] [CrossRef] [PubMed]
- Agina, O.A.; Shaari, M.R.; Isa, N.M.M.; Ajat, M.; Zamri-Saad, M.; Hamzah, H. Clinical pathology, immunopathology and advanced vaccine technology in bovine theileriosis: A review. Pathogens 2020, 9, 697. [Google Scholar] [CrossRef]
- Bishop, R.; Musoke, A.; Morzaria, S.; Gardner, M.; Nene, V. Theileria: Intracellular protozoan parasites of wild and domestic ruminants transmitted by ixodid ticks. Parasitology 2004, 129, S271–S283. [Google Scholar] [CrossRef] [PubMed]
- Mans, B.J.; Pienaar, R.; Latif, A.A. A review of Theileria diagnostics and epidemiology. Int. J. Parasitol. Parasites Wildl. 2015, 4, 104–118. [Google Scholar] [CrossRef] [PubMed]
- Gharbi, M.; Darghouth, M.A.; Elati, K.; Al-Hosary, A.A.T.; Ayadi, O.; Salih, D.A.; El Hussein, A.M.; Mhadhbi, M.; Khbou, M.K.; Hassan, S.M.; et al. Current status of tropical theileriosis in Northern Africa: A review of recent epidemiological investigations and implications for control. Transbound. Emerg. Dis. 2020, 67, 8–25. [Google Scholar] [CrossRef]
- Gomes, J.; Soares, R.; Santos, M.; Santos-Gomes, G.; Botelho, A.; Amaro, A.; Inácio, J. Detection of Theileria and Babesia infections amongst asymptomatic cattle in Portugal. Ticks Tick Borne Dis. 2013, 4, 148–151. [Google Scholar] [CrossRef] [PubMed]
- Calleja-bueno, L.; Sainz, Á.; García-sancho, M.; Rodríguez-franco, F.; González-martín, J.V.; Villaescusa, A. Molecular, epidemiological, haematological and biochemical evaluation in asymptomatic Theileria annulata infected cattle from an endemic region in Spain. Ticks Tick Borne Dis. 2017, 8, 936–941. [Google Scholar] [CrossRef]
- Gargano, V.; Blanda, V.; Gambino, D.; La Russa, F.; Di Cataldo, S.; Gentile, A.; Schirò, G.; Torina, A.; Millán, A.; Vicari, D. Serological Survey and Molecular Characterization of Theileria annulata in Sicilian Cattle. Pathogens 2021, 10, 101. [Google Scholar] [CrossRef]
- Papadopoulos, B.; Brossard, M.; Made, N. Piroplasms of domestic animals in the Macedonia region of Greece-2 Piroplasms of cattle. Vet. Parasitol. 1996, 63, 57–66. [Google Scholar] [CrossRef]
- Zhou, M.; Cao, S.; Sevinc, F.; Sevinc, M.; Ceylan, O.; Moumouni, P.F.A.; Jirapattharasate, C.; Liu, M.; Wang, G.; Iguchi, A.; et al. Molecular detection and genetic identification of Babesia bigemina, Theileria annulata, Theileria orientalis and Anaplasma marginale in Turkey. Ticks Tick Borne Dis. 2016, 7, 126–134. [Google Scholar] [CrossRef]
- Nourollahi-fard, S.R.; Khalili, M.; Ghalekhani, N. Detection of Theileria annulata in blood samples of native cattle by PCR and smear method in Southeast of Iran. J. Parasit. Dis. 2013, 39, 249–252. [Google Scholar] [CrossRef] [PubMed]
- Rashid, M.; Akbar, H.; Rashid, I.; Saeed, K.; Ahmad, L.; Ahmad, A.S.; Shehzad, W.; Islam, S.; Farooqi, S. Economic Significance of Tropical Theileriosis on a Holstein Friesian Dairy Farm in Pakistan. J. Parasitol. 2018, 104, 310–312. [Google Scholar] [CrossRef] [PubMed]
- George, N.; Bhandari, V.; Reddy, D.P.; Sharma, P. Molecular and Phylogenetic analysis revealed new genotypes of Theileria annulata parasites from India. Parasites Vectors 2015, 8, 468. [Google Scholar] [CrossRef]
- Ali, M.W.; Alauddin, M.; Azad, M.T.A.; Hasan, M.A.; Appiah-Kwarteng, C.; Takasu, M.; Baba, M.; Kitoh, K.; Rahman, M.; Takashima, Y. Theileria annulata seroprevalence among different cattle breeds in Rajshahi Division, Bangladesh. J. Vet. Med. Sci. 2016, 78, 1577–1582. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Yin, C.; Galon, E.M.; Du, J.; Gao, Y.; Moumouni, P.F.A.; Liu, M.; Efstratiou, A.; Lee, S.-H.; Li, J.; et al. Molecular survey and characterization of Theileria annulata and Ehrlichia ruminantium in cattle from Northwest China. Parasitol. Int. 2018, 67, 679–683. [Google Scholar] [CrossRef]
- Gebrekidan, H.; Hailu, A.; Kassahun, A.; Rohoušová, I.; Maia, C.; Talmi-Frank, D.; Warburg, A.; Baneth, G. Theileria infection in domestic ruminants in northern Ethiopia. Vet. Parasitol. 2014, 200, 31–38. [Google Scholar] [CrossRef]
- Salih, D.A.; Hussein AMEl Seitzer, U.; Ahmed, J.S. Epidemiological studies on tick-borne diseases of cattle in Central Equatoria State, Southern Sudan. Parasitol Res. 2007, 101, 1035–1044. [Google Scholar] [CrossRef]
- Mohamed, S.B.; Alagib, A.; AbdElkareim, T.B.; Hassan, M.M.; Johnson, W.C.; Hussein, H.E.; Taus, N.S.; Ueti, M.W. Molecular detection and characterization of Theileria spp. infecting cattle in Sennar State, Sudan. Parasitol. Res. 2018, 117, 1271–1276. [Google Scholar] [CrossRef] [PubMed]
- Al-hosary, A.; Ahmed, L.; Ahmed, J.; Nijhof, A.; Clausen, P. Epidemiological study on tropical theileriosis (Theileria annulata infection) in the Egyptian Oases with special reference to the molecular characterization of Theileria spp. Ticks Tick Borne Dis. 2018, 9, 1489–1493. [Google Scholar] [CrossRef]
- Gharbi, M.; Sassi, L.; Dorchies, P.; Darghouth, M.A. Infection of calves with Theileria annulata in Tunisia: Economic analysis and evaluation of the potential benefit of vaccination. Vet. Parasitol. 2006, 137, 231–241. [Google Scholar] [CrossRef]
- Ziam, H.; Kelanamer, R.; Aissi, M.; Abadou, A.; Berkvens, D.; Geysen, D. Prevalence of bovine theileriosis in North Central region of Algeria by real- time polymerase chain reaction with a note on its distribution. Trop. Anim. Health Prod. 2015, 47, 787–796. [Google Scholar] [CrossRef] [PubMed]
- Elhachimi, L.; Rogiers, C.; Casaert, S.; Fellahi, S.; Van Leeuwen, T.; Dermauw, W.; Valcárcel, F.; Olmeda, S.; Daminet, S.; Khatat, S.E.H.; et al. Ticks and Tick-Borne Pathogens Abound in the Cattle Population of the Rabat-Sale Kenitra Region, Morocco. Pathogens 2021, 10, 1594. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.; Hussain, A.; Ho, J.; Li, J.; George, D.; Rehman, A.; Zeb, J.; Sparagano, O. An Epidemiological Survey Regarding Ticks and Tick-Borne Diseases among Livestock Owners in Punjab, Pakistan: A One Health Context. Pathogens 2021, 10, 361. [Google Scholar] [CrossRef]
- Kho, K.L.; Amarajothi, A.D.G.; Koh, F.X.; Panchadcharam, C.; Hassan Nizam, Q.N.; Tay, S.T. The first molecular survey of theileriosis in Malaysian cattle, sheep and goats. Vet. Parasitol. Reg. Stud. Rep. 2017, 10, 149–153. [Google Scholar] [CrossRef] [PubMed]
- Moumouni, P.F.A.; Aboge, G.O.; Terkawi, M.A.; Masatani, T.; Cao, S.; Kamyingkird, K.; Jirapattharasate, C.; Zhou, M.; Wang, G.; Liu, M.; et al. Molecular detection and characterization of Babesia bovis, Babesia bigemina, Theileria species and Anaplasma marginale isolated from cattle in Kenya. Parasites Vectors 2015, 8, 496. [Google Scholar] [CrossRef]
- Perera, P.K.; Gasser, R.B.; Firestone, S.M.; A Anderson, G.; Malmo, J.; Davis, G.; Beggs, D.S.; Jabbar, A. Oriental theileriosis in dairy cows causes a significant milk production loss. Parasit Vectors 2014, 7, 73. [Google Scholar] [CrossRef]
- Inci, A.; Ica, A.; Yildirim, A.; Vatansever, Z.; Cakmak, A.; Albasan, H.; Cam, Y.; Atasever, A.; Sariozkan, S.; Duzlu, O. Economical impact of tropical theileriosis in the Cappadocia region of Turkey. Parasitol Res. 2007, 101, 171–174. [Google Scholar] [CrossRef]
- Spooner, R.L.; Innes, E.A.; Glass, E.J.; Arfc, C.G.D.B.; Physiology, A. Theileria annulata and T. parva infect and transform different bovine mononuclear cells. Immunology 1989, 66, 284–288. [Google Scholar]
- Tajeri, S.; Langsley, G. Theileria secretes proteins to subvert its host leukocyte. Biol Cell. 2020, 113, 220–233. [Google Scholar]
- Jalovecka, M.; Hajdusek, O.; Sojka, D.; Kopacek, P. The Complexity of Piroplasms Life Cycles. Front. Cell. Infect. Microbiol. 2018, 8, 248. [Google Scholar] [CrossRef]
- Shaw, M.K. Cell invasion by Theileria sporozoites. Trends Parasitol. 2003, 19, 2–6. [Google Scholar] [CrossRef]
- Von Schubert, C.; Xue, G.; Schmuckli-maurer, J.; Woods, K.L.; Erich, A.; Dobbelaere, D.A.E. The Transforming Parasite Theileria Co-opts Host Cell Mitotic and Central Spindles to Persist in Continuously Dividing Cells. PLoS Biol. 2010, 8, e1000499. [Google Scholar] [CrossRef] [PubMed]
- Andrade, P.S. Cattle Theileriosis: Insight on the Occurrence of Theileria orientalis and the Putative Resistance of Autochthonous Cattle Breeds to Theileria Species; Universidade de Lisboa: Lisboa, Portugal, 2019. [Google Scholar]
- Naik, B.S.; Maiti, S.K.; Raghuvanshi, P.D.S. Prevalence of Tropical Theileriosis in Cattle in Chhattisgarh State. J. Anim. Res. 2016, 6, 1043–1045. [Google Scholar] [CrossRef]
- Khawale, T.S.; Siddiqui MF, M.F.; Borikar, S.T.; Sakhare, M.P.; Rajurkar, S.R.; Chigure, G.M.; Shafi, T.A. Study of occurrence of theileriosis in cattle from Parbhani district, Maharashtra, India. Pharma Innov. 2019, 8, 171–173. [Google Scholar]
- Gebrekidan, H.; Perera, P.K.; Ghafar, A.; Abbas, T.; Gasser, R.B.; Jabbar, A. An appraisal of oriental theileriosis and the Theileria orientalis complex, with an emphasis on diagnosis and genetic characterization. Parasitology 2020, 119, 11–22. [Google Scholar] [CrossRef]
- El Damaty, H.M.; Yousef, S.G.; Mahmmod, Y.S.; El-Balkemy, F.A.; Mweu, M.M. Sensitivity and specificity of piroplasm indirect fluorescent antibody test and PCR for Theileria annulata infection in clinically asymptomatic large ruminants using Bayesian latent class analysis. Vet. Parasitol. Reg. Stud. Rep. 2021, 24, 100563. [Google Scholar] [CrossRef] [PubMed]
- Salih, D.A.; Hassan, S.M.; El Hussein, A.M. Comparisons among two serological tests and microscopic examination for the detection of Theileria annulata in cattle in northern Sudan. Prev. Vet. Med. 2007, 81, 323–326. [Google Scholar] [CrossRef]
- Perera, P.K.; Gasser, R.B.; Pulford, D.J.; A Stevenson, M.; Firestone, S.M.; McFadden, A.M.J.; Jabbar, A. Comparison of the performance of three PCR assays for the detection and differentiation of Theileria orientalis genotypes. Parasit Vectors 2015, 8, 192. [Google Scholar] [CrossRef]
- Jay, K.P.S.; Gupta, P. Breeding strategies for tick resistance in tropical cattle: A sustainable approach for tick control. J. Parasit. Dis. 2015, 39, 1–6. [Google Scholar]
- Jenkins, C. Bovine theileriosis in Australia: A decade of disease. Microbiol. Aust. 2018, 39, 215–219. [Google Scholar] [CrossRef]
- Watts, J.G.; Playford, M.C.; Hickey, K.L. Theileria orientalis: A review. N Z Vet. J. 2015, 64, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Dzemo, W.D.; Thekisoe, O.; Vudriko, P. Development of acaricide resistance in tick populations of cattle: A systematic review and meta-analysis. Heliyon 2022, 8, e08718. [Google Scholar] [CrossRef] [PubMed]
- Morrison, W.I. The aetiology, pathogenesis and control of theileriosis in domestic animals Diseases caused by Theileria species. Rev. Sci. Técnica. Int. Off Epizoot. 2015, 34, 599–611. [Google Scholar] [CrossRef]
- Azhahianambi, P.; Madhanmohan, M.; Madan, N.; Kumaran, D.; Mala Priyadharshini, M.L.; Bharathi, R.; Senthilkumar, T.; Manoharan, S. Successful treatment of severe form of bovine tropical theileriosis in dairy cattle and genotyping of Theileria annulata isolates of Tamil Nadu, India. Vet. Parasitol. Reg. Stud. Rep. 2021, 26, 100628. [Google Scholar] [CrossRef] [PubMed]
- Nampoothiri, V.M. Theileriosis in cattle: Treatment and management. Int. J. Vet. Sci. Anim. Husb. 2021, 6, 1–3. [Google Scholar] [CrossRef]
- Verma, A.K.; Singh, S.K. Control and therapeutic management of bovine tropical theileriosis in crossbred cattle. J Parasit Dis. 2016, 40, 208–210. [Google Scholar] [CrossRef][Green Version]
- EMA. Use of glycylcyclines in animals in the European Union: Development of Resistance and Possible Impact on Human and Animal Health; EMA: Zuidas, Amsterdam, 2016; Volume 44. [Google Scholar]
- Long, T.E.; Short, T.H.; Bates, R.O. Estimating Genetic Merit. Swine Genet. 1988, 8. [Google Scholar]
- Berry, D.P.; Bermingham, M.L.; Good, M.; More, S.J. Genetics of animal health and disease in cattle. Ir. Vet. J. 2011, 64, 5. [Google Scholar] [CrossRef]
- Berry, D.P.; Meade, K.G.; Mullen, M.P.; Butler, S.; Diskin, M.G.; Morris, D.; Creevey, C.J. The integration of ‘omic’ disciplines and systems biology in cattle breeding. Anim. Int. J. Anim. Biosci. 2011, 5, 493–505. [Google Scholar] [CrossRef]
- Wakchaure, R.; Ganguly, S.; Praveen, P.K.; Kumar, A.; Sharma, S.; Mahajan, T. Marker Assisted Selection (MAS) in Animal Breeding: A Review. J. Drug. Metab. Toxicol. 2015, 6, e127. [Google Scholar] [CrossRef]
- Ibtisham, F.; Zhang, L.; Xiao, M.; An, L.; Bilal, M.; Nawab, A.; Zhao, Y.; Li, G.; Xu, Y. Genomic selection and its application in animal breeding. Thai J. Vet. Med. 2017, 47, 301–310. [Google Scholar]
- Glass, E.J.; Crutchley, S.; Jensen, K. Living with the enemy or uninvited guests: Functional genomics approaches to investigating host resistance or tolerance traits to a protozoan parasite, Theileria annulata, in cattle. Vet. Immunol. Immunopathol. 2012, 148, 178–189. [Google Scholar] [CrossRef] [PubMed]
- Wakchaure, R.; Ganguly, S.; Preveen PKGenotype, X. Environment Interaction in Animal Breeding: A Review. In Biodiversity Conservation in Changing Climate; Khan, M.M.A., Abid, M., Omar, A.M., Zaidi, N.H., Maheshwari, R.K., Eds.; Lenin Media Private Limited: Delhi, India, 2016; pp. 60–73. [Google Scholar]
- McCarville, J.L.; Ayres, J.S. Disease tolerance: Concept and mechanisms. Curr. Opin. Immunol. 2018, 50, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Louie, A.; Song, K.H.; Hotson, A.; Thomas Tate, A.; Schneider, D.S. How Many Parameters Does It Take to Describe Disease Tolerance? PLoS Biol. 2016, 14, e1002435. [Google Scholar] [CrossRef]
- Bishop, S.C. A consideration of resistance and tolerance for ruminant nematode infections. Front. Genet. 2012, 3, 168. [Google Scholar] [CrossRef]
- Ahmed, J.S.; Glass, E.J.; Salih, D.A.; Seitzer, U. Innate immunity to tropical theileriosis. Innate Immun. 2008, 14, 5–11. [Google Scholar] [CrossRef]
- Ahmed, J.S.; Mehlhorn, H. Review: The cellular basis of the immunity to and immunopathogenesis of tropical theileriosis. Parasitol. Res. 1999, 85, 539–549. [Google Scholar] [CrossRef]
- Bilgic, H.B.; Karagenc, T.; Bak, S.; Shiels, B. Identification and Analysis of Immunodominant Antigens for ELISA-Based Detection of Theileria annulata. PLoS ONE 2016, 1, e0156645. [Google Scholar] [CrossRef]
- Glass, E.J.; Jensen, K. Resistance and susceptibility to a protozoan parasite of cattle—Gene expression differences in macrophages from different breeds of cattle. Vet. Immunol. Immunopathol. 2007, 120, 20–30. [Google Scholar] [CrossRef]
- Jang, J.; Terefe, E.; Kim, K.; Lee, Y.H.; Belay, G.; Tijjani, A.; Han, J.-L.; Hanotte, O.; Kim, H. Population differentiated copy number variation of Bos taurus, Bos indicus and their African hybrids. BCM Genom. 2022, 22, 531. [Google Scholar] [CrossRef]
- Glass, E.J.; Preston, P.M.; Springbett, A.; Craigmile, S.; Kirvar, E.; Wilkie, G.; Brown, C.D. Bos taurus and Bos indicus (Sahiwal) calves respond differently to infection with Theileria annulata and produce markedly different levels of acute phase proteins. Int. J. Parasitol. 2005, 35, 337–347. [Google Scholar] [CrossRef] [PubMed]
- Rehman, Z.; Khan, M.S.; Mirza, M.A. Factors affecting performance of Sahiwal cattle—A review. J. Anim. Plant Sci. 2014, 24, 1–12. [Google Scholar]
- Eshonqulovich, A.M.; Askarovich, Y.A. Milk Productivity of Holstein Breed of Different Selections in the Conditions of Uzbekistan. J. Dairy Vet. Sci. 2019, 10, 10–12. [Google Scholar] [CrossRef]
- Jensen, K.; Paxton, E.; Waddington, D.; Talbot, R.; Darghouth, M.A.; Glass, E.J. Differences in the transcriptional responses induced by Theileria annulata infection in bovine monocytes derived from resistant and susceptible cattle breeds. Int. J. Parasitol. 2008, 38, 313–325. [Google Scholar] [CrossRef]
- Vlasova, A.N.; Saif, L.J. Bovine Immunology: Implications for Dairy Cattle. Front. Immunol. 2021, 12, 643206. [Google Scholar] [CrossRef]
- Kumar, A.; Gaur, G.K.; Gandham, R.K.; Panigrahi, M.; Ghosh, S.; Saravanan, B.; Bhushan, B.; Tiwari, A.K.; Sulabh, S.; Priya, B.; et al. Global gene expression profile of peripheral blood mononuclear cells challenged with Theileria annulata in crossbred and indigenous cattle. Infect. Genet. Evol. 2017, 47, 9–18. [Google Scholar] [CrossRef]
- Tesfaigzi, Y.; Daheshia, M. CD14. In Encyclopedia of Respiratory Medicine; Elsevier: Amsterdam, The Netherlands, 2006; pp. 343–347. [Google Scholar]
- Nazifi, S.; Razavi, S.M.; Reiszadeh, M.; Esmailnezhad, Z.; Ansari-Lari, M.; Hasanshahi, F. Evaluation of the resistance of indigenous Iranian cattle to Theileria annulata compared with Holstein cattle by measurement of acute phase proteins. Comp. Clin. Pathol. 2010, 19, 155–161. [Google Scholar] [CrossRef]
- Chaussepied, M.; Janski, N.; Baumgartner, M.; Lizundia, R.; Jensen, K.; Weir, W.; Shiels, B.; Weitzman, J.; Glass, E.; Werling, D.; et al. TGF-b2 Induction Regulates Invasiveness of Theileria-Transformed Leukocytes and Disease Susceptibility. PLoS Pathog. 2010, 6, e1001197. [Google Scholar] [CrossRef]
| Prevalence of Theileria annulata | ||||
|---|---|---|---|---|
| Country | Region | Technique | Prevalence | Reference |
| Southern Europe | ||||
| Portugal | All country | RLB | 17.8% | [17] |
| Spain | Madrid | PCR | 22.4% | [18] |
| Italy | Sicilia | IFA | 26.0% | [19] |
| Greece | Macedonia | IFA | 2.0% | [20] |
| Asia | ||||
| Turkey | All country | PCR | 6.6% | [21] |
| Iran | Kerman | PCR | 45.3% | [22] |
| Pakistan | Punjab | PCR | 8.0% | [23] |
| India | Andhra Pradesh | PCR | 32.4% | [24] |
| Bangladesh | Natore District | ELISA | 80.0% | [25] |
| Rajshahi District | ELISA | 20.4% | [25] | |
| China | Xinjiang Uygur Autonomous region | PCR | 18.2% | [26] |
| Northern Africa | ||||
| Ethiopia | Humera | PCR | 2.0% | [27] |
| South Sudan | Juba | RLB | 0.2% | [28] |
| Sudan | Sennar State | PCR | 50.0% | [29] |
| Egypt | Egyptian Oases | PCR | 63.6% | [30] |
| Tunisia | Ariana | IFA | 92.9% | [31] |
| Algeria | Central Algeria | FRET-PCR | 30.0% | [32] |
| Morocco | Northwest of Morocco | PCR and DNA Sequencing | 15.9% | [33] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valente, D.; Gomes, J.; Coelho, A.C.; Carolino, I. Genetic Resistance of Bovines to Theileriosis. Animals 2022, 12, 2903. https://doi.org/10.3390/ani12212903
Valente D, Gomes J, Coelho AC, Carolino I. Genetic Resistance of Bovines to Theileriosis. Animals. 2022; 12(21):2903. https://doi.org/10.3390/ani12212903
Chicago/Turabian StyleValente, Diana, Jacinto Gomes, Ana Cláudia Coelho, and Inês Carolino. 2022. "Genetic Resistance of Bovines to Theileriosis" Animals 12, no. 21: 2903. https://doi.org/10.3390/ani12212903
APA StyleValente, D., Gomes, J., Coelho, A. C., & Carolino, I. (2022). Genetic Resistance of Bovines to Theileriosis. Animals, 12(21), 2903. https://doi.org/10.3390/ani12212903








