Effects of Feeding 5-Aminolevulinic Acid on Iron Status in Weaned Rats from the Female Rats during Gestation and Lactation
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal, Dietary Treatment and Experimental Design
2.2. Determination of Blood Physiological Parameter
2.3. Determination of Serum Iron Index and Liver Iron Content
2.4. Determination of Hepcidin Content in Serum and Liver
2.5. Immunohistochemistry
2.6. Total RNA Extraction, cDNA Preparation, and Quantitative Real-Time Reverse Transcription–Polymerase Chain Reaction (qRT-PCR)
2.7. Data Calculations and Statistical Analysis
3. Results
3.1. Blood Physiological Parameters of Weaned Rats
3.2. Liver and Serum Iron-Related Indicators of Weaned Rats
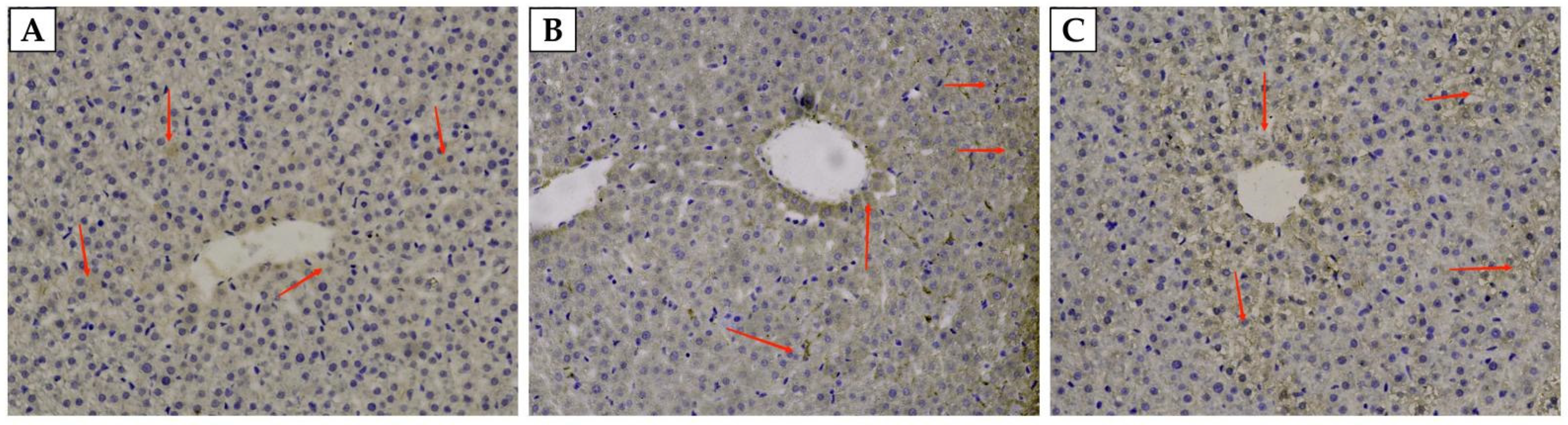
3.3. The Content of Hepcidin in the Liver and Serum of Weaned Rats
3.4. The Expression of Iron-Related Genes in the Liver of Weaned Rats
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Starzyński, R.R.; Laarakkers, C.M.; Tjalsma, H.; Swinkels, D.W.; Pieszka, M.; Styś, A.; Mickiewicz, M.; Lipiński, P. Iron supplementation in suckling piglets: How to correct iron deficiency anemia without affecting plasma hepcidin levels. PLoS ONE 2013, 8, e64022. [Google Scholar] [CrossRef] [PubMed]
- KiSzudzik, M.; Starzyński, R.R.; Jończy, A.; Mazgaj, R.; Lenartowicz, M.; Lipiński, P. Iron supplementation in suckling piglets: An ostensibly easy therapy of neonatal iron deficiency anemia. Pharmaceuticals 2018, 11, 128. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yang, W.; Dong, D.; Jiang, S.; Yang, Z.; Wang, Y. Effect of different sources and levels of iron in the diet of sows on iron status in neonatal pigs. Anim. Nutr. 2018, 4, 197–202. [Google Scholar] [CrossRef]
- Leyshon, B.J.; Ji, P.; Caputo, M.P.; Matt, S.M.; Johnson, R.W. Dietary iron deficiency impaired peripheral immunity but did not alter brain microglia in PRRSV-infected neonatal piglets. Front. Immunol. 2019, 9, 3150. [Google Scholar] [CrossRef]
- Bruininx, E.M.A.M.; Swinkels, J.W.G.M.; Parmentier, H.K.; Jetten, C.W.J.; Gentry, J.L.; Schrama, J.W. Effects of an additional iron injection on growth and humoral immunity of weanling pigs. Livest. Prod. Sci. 2000, 67, 31–39. [Google Scholar] [CrossRef]
- Maes, D.; Steyaert, M.; Vanderhaeghe, C.; López Rodríguez, A.; de Jong, E.; del Pozo Sacristán, R.; Vangroenweghe, F.; Dewulf, J. Comparison of oral versus parenteral iron supplementation on the health and productivity of piglets. Vet. Rec. 2011, 168, 188. [Google Scholar] [CrossRef]
- Brown, K.R.; Brown, B.M.; Hoagland, E.; Mayne, C.L.; Hegg, E.L. Heme A synthase does not incorporate molecular oxygen into the formyl group of heme A. Biochemistry 2004, 43, 8616–8624. [Google Scholar] [CrossRef]
- Miura, M.; Ito, K.; Hayashi, M.; Nakajima, M.; Tanaka, T.; Ogura, S.I. The effect of 5-aminolevulinic acid on cytochrome P450-mediated prodrug activation. PLoS ONE 2015, 10, e0131793. [Google Scholar] [CrossRef]
- Hendawy, A.O.; Khattab, M.S.; Sugimura, S.; Sato, K. Effects of 5-Aminolevulinic Acid as a Supplement on Animal Performance, Iron Status, and Immune Response in Farm Animals: A Review. Animals 2020, 10, 1352. [Google Scholar] [CrossRef]
- Chang, M.; Li, M.; Li, M.; Xie, Y.; Li, Y.; Yang, W.; Gao, Z. Changes of gut microbiota in pregnant sows induced by 5-Aminolevulinic acid. Res. Vet. Sci. 2021, 136, 57–65. [Google Scholar] [CrossRef]
- Min, B.J.; Hong, J.W.; Kwon, O.S.; Kang, D.K.; Kim, I.H. Influence of dietary δ-aminolevulinic acid supplement on growth performance and hematological changes in weaned pigs. J. Korean Soc. Food Sci. Nutr. 2004, 33, 1606–1610. [Google Scholar]
- Wang, J.P.; Kim, I.H. Effects of iron injection at birth on neonatal iron status in young pigs from first-parity sows fed delta-aminolevulinic acid. Anim. Feed. Sci. Tech. 2012, 178, 151–157. [Google Scholar] [CrossRef]
- Chen, Y.J.; Cho, J.H.; Yoo, J.S.; Wang, Y.; Huang, Y.; Kim, I.H. Evaluation of δ-aminolevulinic acid on serum iron status, blood characteristics, egg performance and quality in laying hens. Asian Austral. J. Anim. 2008, 21, 1355–1360. [Google Scholar] [CrossRef]
- Liu, L.; Chu, L.C.; Ceng, X.F.; Qiao, S.Y. Effects of Decreasing Dietary Protein Level on Growth Performance, Nitrogen Balance and Skeletal Muscle Protein Turnover of Adult Rats. Chin. J. Anim. Nutr. 2019, 31, 2222–2231. (In Chinese) [Google Scholar]
- Association of Official Analytical Chemists (AOAC). Official Methods of Analysis, 18th ed.; AOAC: Washington, DC, USA; Arlington, VA, USA, 2005. [Google Scholar]
- Armstrong, T.A.; Cook, D.R.; Ward, M.M.; Williams, C.M.; Spears, J.W. Effect of dietary copper source (cupric citrate and cupric sulfate) and concentration on growth performance and fecal copper excretion in weanling pigs. J. Anim. Sci. 2004, 82, 1234–1240. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Giannouli, S.; Voulgarelis, M.; Ziakas, P.D.; Tzioufas, A.G. Anaemia in systemic lupus erythematosus: From pathophysiology to clinical assessment. Dis. Ann. Rheum. Dis. 2006, 65, 144–148. [Google Scholar] [CrossRef]
- Kals, J.; Blonk, R.J.; van der Mheen, H.W.; Schrama, J.W.; Verreth, J.A. Effect of different iron sources on the alleviation of nutritional anaemia in common sole (Solea solea). Aquaculture 2016, 451, 266–270. [Google Scholar] [CrossRef]
- Tsui, S.M.; Ahmed, R.; Amjad, N.; Ahmed, I.; Yang, J.; Manno, F.A.; Barman, I.; Shih, W.C.; Lau, C. Single red blood cell analysis reveals elevated hemoglobin in poikilocytes. J. Biomed. Opt. 2020, 25, 1–13. [Google Scholar] [CrossRef]
- Ettle, T.; Schlegel, P.; Roth, F.X. Investigations on iron bioavailability of different sources and supply levels in piglets. J. Anim. Physiol. An. N. 2008, 92, 35–43. [Google Scholar] [CrossRef]
- Peters, J.C.; Mahan, D.C. Effects of neonatal iron status, iron injections at birth, and weaning in young pigs from sows fed either organic or inorganic trace minerals. J. Anim. Sci. 2008, 86, 2261–2269. [Google Scholar] [CrossRef] [PubMed]
- Lechuga, G.C.; Borges, J.C.; Calvet, C.M.; de Araújo, H.P.; Zuma, A.A.; do Nascimento, S.B.; Bourguignon, S.C. Interactions between 4-aminoquinoline and heme: Promising mechanism against Trypanosoma cruzi. Int. J. Parasitol.-Drug 2016, 6, 154–164. [Google Scholar] [CrossRef][Green Version]
- Mateo, R.D.; Morrow, J.L.; Dailey, J.W.; Ji, F.; Kim, S.W. Use of δ-aminolevulinic acid in swine diet: Effect on growth performance, behavioral characteristics and hematological/immune status in nursery pigs. Asi. An. J. Anim. 2006, 19, 97–101. [Google Scholar] [CrossRef]
- Yan, L.; Kim, I.H. Evaluation of dietary supplementation of delta-aminolevulinic acid and chitooligosaccharide on growth performance, nutrient digestibility, blood characteristics, and fecal microbial shedding in weaned pigs. Anim. Feed. Sci.Tech. 2011, 169, 275–280. [Google Scholar] [CrossRef]
- Chen, Y.J.; Kim, I.H.; Cho, J.H.; Min, B.J.; Yoo, J.S.; Wang, Q. Effect of δ-aminolevulinic acid on growth performance, nutrient digestibility, blood parameters and the immune response of weanling pigs challenged with Escherichia coli lipopolysaccharide. Livest. Sci. 2008, 114, 108–116. [Google Scholar] [CrossRef]
- Nakamura, Y.; Haraguchi, A.; Shigeno, R.; Ito, A.; Horie, I.; Kawakami, A.; Abiru, N. A single-arm, open-label, intervention study to investigate the improvement of glucose tolerance after administration of the 5-aminolevulinic acid (5-ALA) in the patients with mitochondrial diabetes mellitus. Medicine 2021, 100, e25100. [Google Scholar] [CrossRef]
- Zhu, Y.; Hon, T.; Ye, W.; Zhang, L. Heme deficiency interferes with the Ras-mitogen-activated protein kinase signaling pathway and expression of a subset of neuronal genes. Cell Growth Differ. 2002, 13, 431–439. [Google Scholar]
- Chen, J.J.; Zhang, S. Heme-regulated eIF2α kinase in erythropoiesis and hemoglobinopathies. Blood 2019, 134, 1697–1707. [Google Scholar] [CrossRef]
- Wang, J.P.; Kim, H.J.; Chen, Y.J.; Yoo, J.S.; Cho, J.H.; Kang, D.K.; Kim, I.H. Effects of delta-aminolevulinic acid and vitamin C supplementation on feed intake, backfat, and iron status in sows. J. Anim. Sci. 2009, 87, 3589–3595. [Google Scholar] [CrossRef]
- Papanikolaou, G.; Pantopoulos, K. Iron metabolism and toxicity. Toxicol. Appl. Pharm. 2005, 202, 199–211. [Google Scholar] [CrossRef]
- Osei-Owusu, J.; Yang, J.; Leung, K.H.; Ruan, Z.; Lü, W.; Krishnan, Y.; Qiu, Z. Proton-activated chloride channel PAC regulates endosomal acidification and transferrin receptor-mediated endocytosis. Cell Rep. 2021, 34, 108683. [Google Scholar] [CrossRef]
- Goswami, G.; Panda, D.; Samanta, R.; Boro, R.C.; Modi, M.K.; Bujarbaruah, K.M.; Barooah, M. Bacillus megaterium adapts to acid stress condition through a network of genes: Insight from a genome-wide transcriptome analysis. Sci. Rep. 2018, 8, 16105. [Google Scholar] [CrossRef] [PubMed]
- Ong, S.T.; Ho, J.Z.S.; Ho, B.; Ding, J.L. Iron-withholding strategy in innate immunity. Immunobiology 2006, 211, 295–314. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Lv, Q.; Gao, J.; Long, L.; Duan, Z.; Liang, H.; Lu, F. Coinfection with HIV-1 alleviates iron accumulation in patients with chronic hepatitis C virus infection. PLoS ONE 2014, 9, e98039. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Andersson, O.; Hellström-Westas, L.; Andersson, D.; Domellöf, M. Effect of delayed versus early umbilical cord clamping on neonatal outcomes and iron status at 4 months: A randomised controlled trial. BMJ 2011, 343, d7157. [Google Scholar] [CrossRef]
- Sylvestre, T.F.; Cavalcante, R.D.S.; da Silva, J.D.F.; Paniago, A.M.M.; Weber, S.S.; Pauletti, B.A.; Mendes, R.P. Ceruloplasmin, transferrin and apolipoprotein A-II play important role in treatment’s follow-up of paracoccidioidomycosis patients. PLoS ONE 2018, 13, e0206051. [Google Scholar] [CrossRef]
- Wang, J.P.; Yan, L.; Lee, J.H.; Zhou, T.X.; Kim, I.H. Effects of dietary delta-aminolevulinic acid and vitamin C on growth performance, immune organ weight and ferrum status in broiler chicks. Livest. Sci. 2011, 135, 148–152. [Google Scholar] [CrossRef]
- Skikne, B.S.; Punnonen, K.; Caldron, P.H.; Bennett, M.T.; Rehu, M.; Gasior, G.H.; Southwick, P.C. Improved differential diagnosis of anemia of chronic disease and iron deficiency anemia: A prospective multicenter evaluation of soluble transferrin receptor and the sTfR/log ferritin index. Am. J. Hematol. 2011, 86, 923–927. [Google Scholar] [CrossRef]
- Bhattacharya, P.T.; Misra, S.R.; Hussain, M. Nutritional aspects of essential trace elements in oral health and disease: An extensive review. Scientifica 2016, 2016, 5464373. [Google Scholar] [CrossRef]
- Williams, H.E.; DeRouchey, J.M.; Woodworth, J.C.; Dritz, S.S.; Tokach, M.D.; Goodband, R.D.; Gebhardt, J.T. Effects of increasing Fe dosage in newborn pigs on suckling and subsequent nursery performance and hematological and immunological criteria. J. Anim. Sci. 2020, 98, skaa221. [Google Scholar] [CrossRef]
- Hendawy, A.O.; Shirai, M.; Takeya, H.; Sugimura, S.; Miyanari, S.; Taniguchi, S.; Sato, K. Effects of 5-aminolevulinic acid supplementation on milk production, iron status, and immune response of dairy cows. J. Dairy Sci. 2019, 102, 11009–11015. [Google Scholar] [CrossRef] [PubMed]
- Park, C.H.; Valore, E.V.; Waring, A.J.; Ganz, T. Hepcidin, a urinary antimicrobial peptide synthesized in the liver. J. Biolo. Chem. 2001, 276, 7806–7810. [Google Scholar] [CrossRef] [PubMed]
- Loréal, O.; Haziza-Pigeon, C.; Troadec, M.B.; Detivaud, L.; Turlin, B.; Courselaud, B.; Brissot, P. Hepcidin in iron metabolism. Curr. Protein Pept. Sc. 2005, 6, 279–291. [Google Scholar] [CrossRef]
- Nemeth, E.; Ganz, T. The role of hepcidin in iron metabolism. Acta Haematol. 2009, 122, 78–86. [Google Scholar] [CrossRef]
- Nemeth, E.; Tuttle, M.S.; Powelson, J.; Vaughn, M.B.; Donovan, A.; Ward, D.M.; Kaplan, J. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science 2004, 306, 2090–2093. [Google Scholar] [CrossRef]
- Hentze, M.W.; Muckenthaler, M.U.; Galy, B.; Camaschella, C. Two to tango: Regulation of Mammalian iron metabolism. Cell 2010, 142, 24–38. [Google Scholar] [CrossRef]
- Nguyen, N.B.; Callaghan, K.D.; Ghio, A.J.; Haile, D.J.; Yang, F. Hepcidin expression and iron transport in alveolar macrophages. Am. J. Physiolo-Lung C. 2006, 291, L417–L425. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.S.; Song, S.H.; Lee, J.H.; Kim, H.J.; Yang, H.R. Serum hepcidin levels and iron parameters in children with iron deficiency. Korean J. Hematol. 2012, 47, 286–292. [Google Scholar] [CrossRef]
- Dong, Z.; Wan, D.; Li, G.; Zhang, Y.; Yang, H.; Wu, X.; Yin, Y. Comparison of oral and parenteral iron administration on iron homeostasis, oxidative and immune status in anemic neonatal pigs. Biolo. Trace Eleme Res. 2020, 195, 117–124. [Google Scholar] [CrossRef]
- Mazgaj, R.; Szudzik, M.; Lipiński, P.; Jończy, A.; Smuda, E.; Kamyczek, M.; Starzyński, R.R. Effect of oral supplementation of healthy pregnant sows with sucrosomial ferric pyrophosphate on maternal iron status and hepatic iron stores in newborn piglets. Animals 2020, 10, 1113. [Google Scholar] [CrossRef]
- Ganz, T.; Nemeth, E. Hepcidin and disorders of iron metabolism. Annu. Rev. Med. 2011, 62, 347–360. [Google Scholar] [CrossRef] [PubMed]
- Nicolas, G.; Chauvet, C.; Viatte, L.; Danan, J.L.; Bigard, X.; Devaux, I.; Vaulont, S. The gene encoding the iron regulatory peptide hepcidin is regulated by anemia, hypoxia, and inflammation. J. Clin. Investig. 2002, 110, 1037–1044. [Google Scholar] [CrossRef] [PubMed]
- Vokurka, M.; Krijt, J.; Sulc, K.; Necas, E. Hepcidin mRNA levels in mouse liver respond to inhibition of erythropoiesis. Physiol. Res. 2006, 55, 667–674. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhang, X.; Zhao, J.; Tang, X.; Wang, F.; Du, H. Split iron supplementation is beneficial for newborn piglets. Biomed. Pharmacother. 2019, 120, 109479. [Google Scholar] [CrossRef]
- Bergamaschi, G.; Villani, L. Serum hepcidin: A novel diagnostic tool in disorders of iron metabolism. Haematologica 2009, 94, 1631. [Google Scholar] [CrossRef]
- Recalcati, S.; Correnti, M.; Gammella, E.; Raggi, C.; Invernizzi, P.; Cairo, G. Iron metabolism in liver cancer stem cells. Front. Oncol. 2019, 9, 149. [Google Scholar] [CrossRef] [PubMed]
- Graham, R.M.; Chua, A.C.; Herbison, C.E.; Olynyk, J.K.; Trinder, D. Liver iron transport. WJG 2007, 13, 4725. [Google Scholar] [CrossRef]


| Item | Content |
|---|---|
| Ingredients, % | |
| Corn starch | 53.83 |
| Casein | 20.00 |
| Sucrose | 9.00 |
| Soybean oil | 7.00 |
| Fiber | 5.00 |
| Mineral mixture 1 | 3.50 |
| Vitamin mixture 2 | 1.00 |
| L-Tryptophan | 0.02 |
| DL-Methionine | 0.40 |
| Choline bitartrate | 0.25 |
| Total | 100.00 |
| Nutrient levels | |
| DE/(MJ/kg) | 16.46 |
| Crude protein, % | 17.96 |
| Tryptophan, % | 0.29 |
| Methionine, % | 0.90 |
| Iron, mg/kg 3 | 277.00 |
| Genes | Primer Sequence (5′ to 3′) | Length/bp | GeneBank No. |
|---|---|---|---|
| β-actin | F: TCTACAATGAGCTGCGTGTG | 100 | NM_031144.3 |
| R: ACATGGCTGGGGTGTTGAA | |||
| Fpn1 | F: TCATTGGCTGTGGTTTCATT | 228 | AF394785 |
| R: ATTCAAGTTCACGGATGTTAGAG | |||
| TfR1 | F: CGAAGTCCAGTGTGGGAACA | 140 | NM_022712.1 |
| R: GGCACCAACAGCTCCATAGT | |||
| Hepcidin | F: TGATGCTGAAGCGAAGGAAG | 116 | NM_053469.1 |
| R: AAGGCTCTTGGCTCTCTATGTTAT |
| Items 1 | CON | 5-ALA50 | 5-ALA100 2 | SEM 3 | p-Value |
|---|---|---|---|---|---|
| RBC, 1012/L | 5.40 b | 5.58 b | 5.82 a | 0.035 | 0.003 |
| HGB, g/L | 111.75 c | 116.75 b | 122.00 a | 0.486 | <0.001 |
| HCT, % | 39.23 b | 41.95 a | 43.35 a | 0.445 | 0.013 |
| Items 1 | CON | 5-ALA50 | 5-ALA100 2 | SEM 3 | p-Value |
|---|---|---|---|---|---|
| Liver, μg/g | 540.55 | 669.66 | 657.61 | 21.031 | 0.085 |
| SI, μmol/L | 28.73 b | 34.81 a | 35.81 a | 0.547 | 0.001 |
| TIBC, μmol/L | 76.33 a | 65.88 b | 75.32 a | 0.915 | 0.002 |
| TSAT, % | 37.66 c | 52.89 a | 47.72 a | 1.050 | 0.001 |
| Items | CON | 5-ALA50 | 5-ALA100 1 | SEM 2 | p-Value |
|---|---|---|---|---|---|
| Serum, μg/L | 105.11 c | 149.88 a | 140.78 b | 0.662 | <0.001 |
| Liver, μg/L | 56.46 b | 73.68 a | 72.18 a | 0.512 | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Xie, Y.; Li, M.; Zhang, S.; Cheng, Q.; Yang, W. Effects of Feeding 5-Aminolevulinic Acid on Iron Status in Weaned Rats from the Female Rats during Gestation and Lactation. Animals 2022, 12, 2869. https://doi.org/10.3390/ani12202869
Li J, Xie Y, Li M, Zhang S, Cheng Q, Yang W. Effects of Feeding 5-Aminolevulinic Acid on Iron Status in Weaned Rats from the Female Rats during Gestation and Lactation. Animals. 2022; 12(20):2869. https://doi.org/10.3390/ani12202869
Chicago/Turabian StyleLi, Junhui, Yuhuai Xie, Min Li, Shaotao Zhang, Qun Cheng, and Weiren Yang. 2022. "Effects of Feeding 5-Aminolevulinic Acid on Iron Status in Weaned Rats from the Female Rats during Gestation and Lactation" Animals 12, no. 20: 2869. https://doi.org/10.3390/ani12202869
APA StyleLi, J., Xie, Y., Li, M., Zhang, S., Cheng, Q., & Yang, W. (2022). Effects of Feeding 5-Aminolevulinic Acid on Iron Status in Weaned Rats from the Female Rats during Gestation and Lactation. Animals, 12(20), 2869. https://doi.org/10.3390/ani12202869






