Dam (Canis familiaris) Welfare throughout the Peri-Parturient Period in Commercial Breeding Kennels
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Subjects and Facilities
2.3. Study Procedure
2.4. Behavioral and Physical Health Measures
2.5. Physiologic Measures
2.5.1. HCC
2.5.2. FGM, Fecal sIgA, and Presence of Parasites
2.5.3. FGM and Fecal sIgA Sample Processing
2.5.4. Cortisol and sIgA Immunoassays
Cortisol Enzyme Immunoassay
sIgA Enzyme-Linked Immunosorbant Assay
2.5.5. Presence of Intestinal Parasites
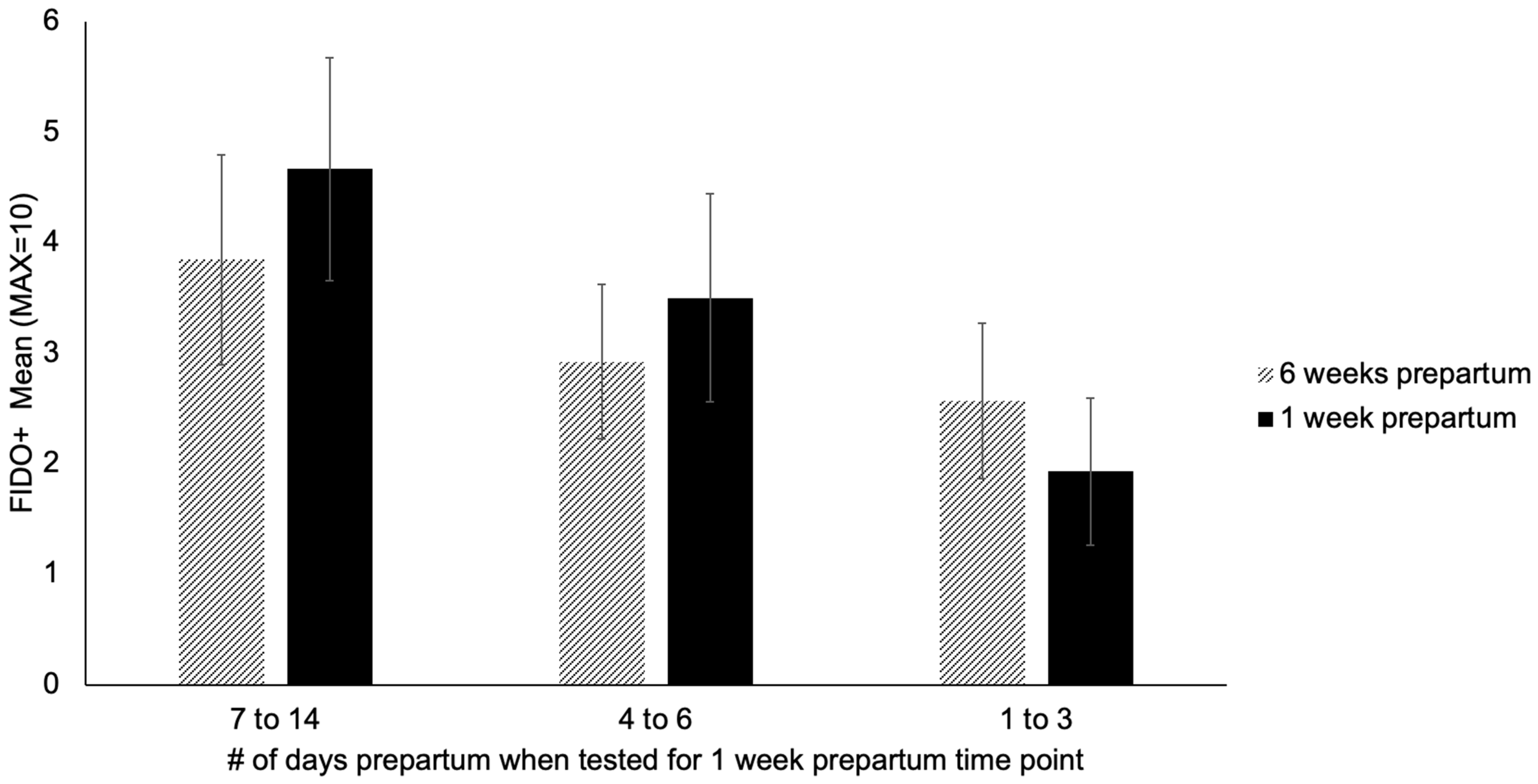
2.6. Deviation of Testing Date from Ideal Time Point and Data Point Exclusion
2.7. Statistical Analyses
2.7.1. Inter-Rater Reliability (IRR)
2.7.2. Changes in Behavior, HCC, FGM, and Fecal sIgA
2.7.3. Relationship between Physiologic Metrics
2.7.4. Parasites and Physical Health
3. Results
3.1. IRR
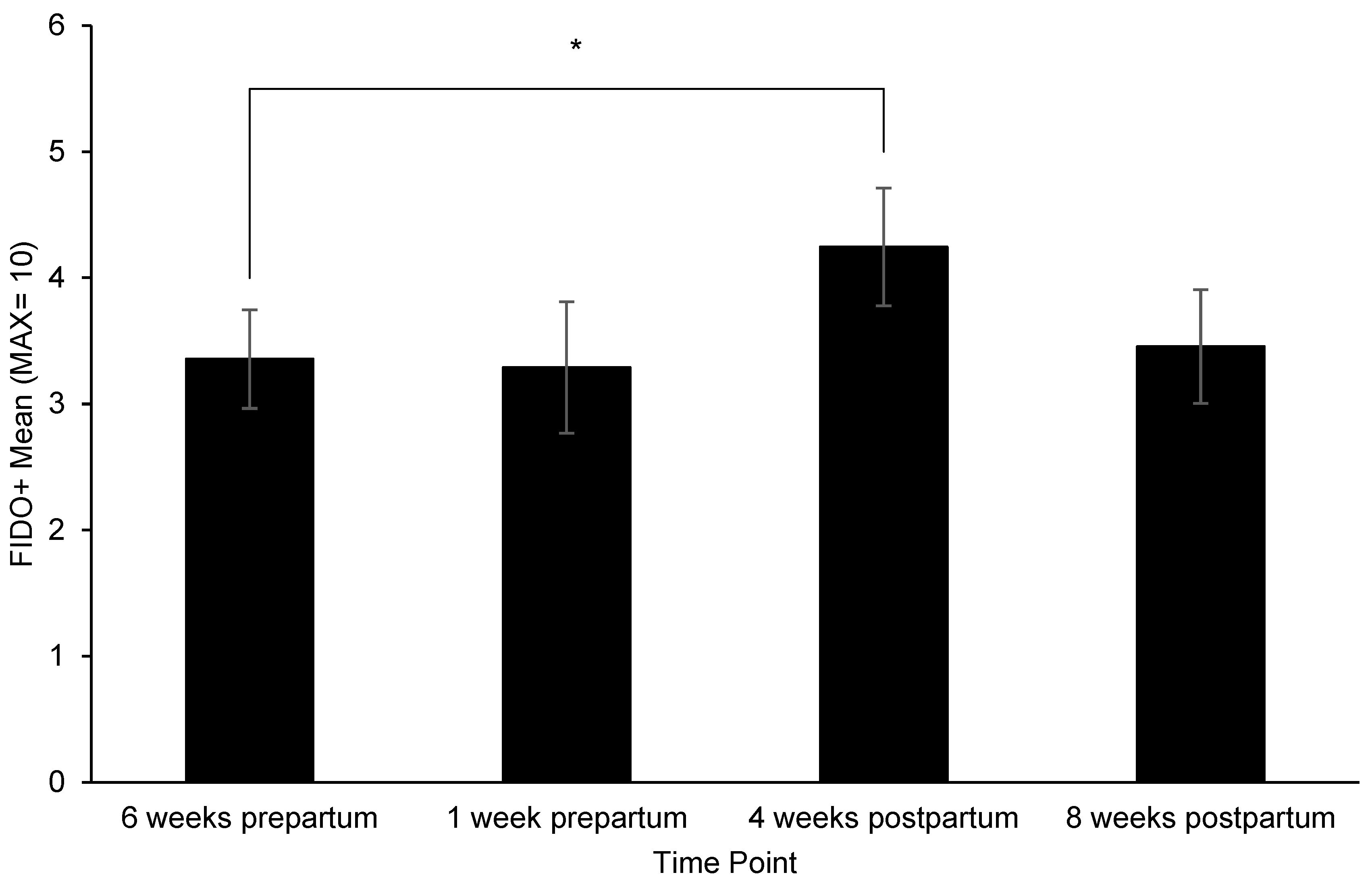
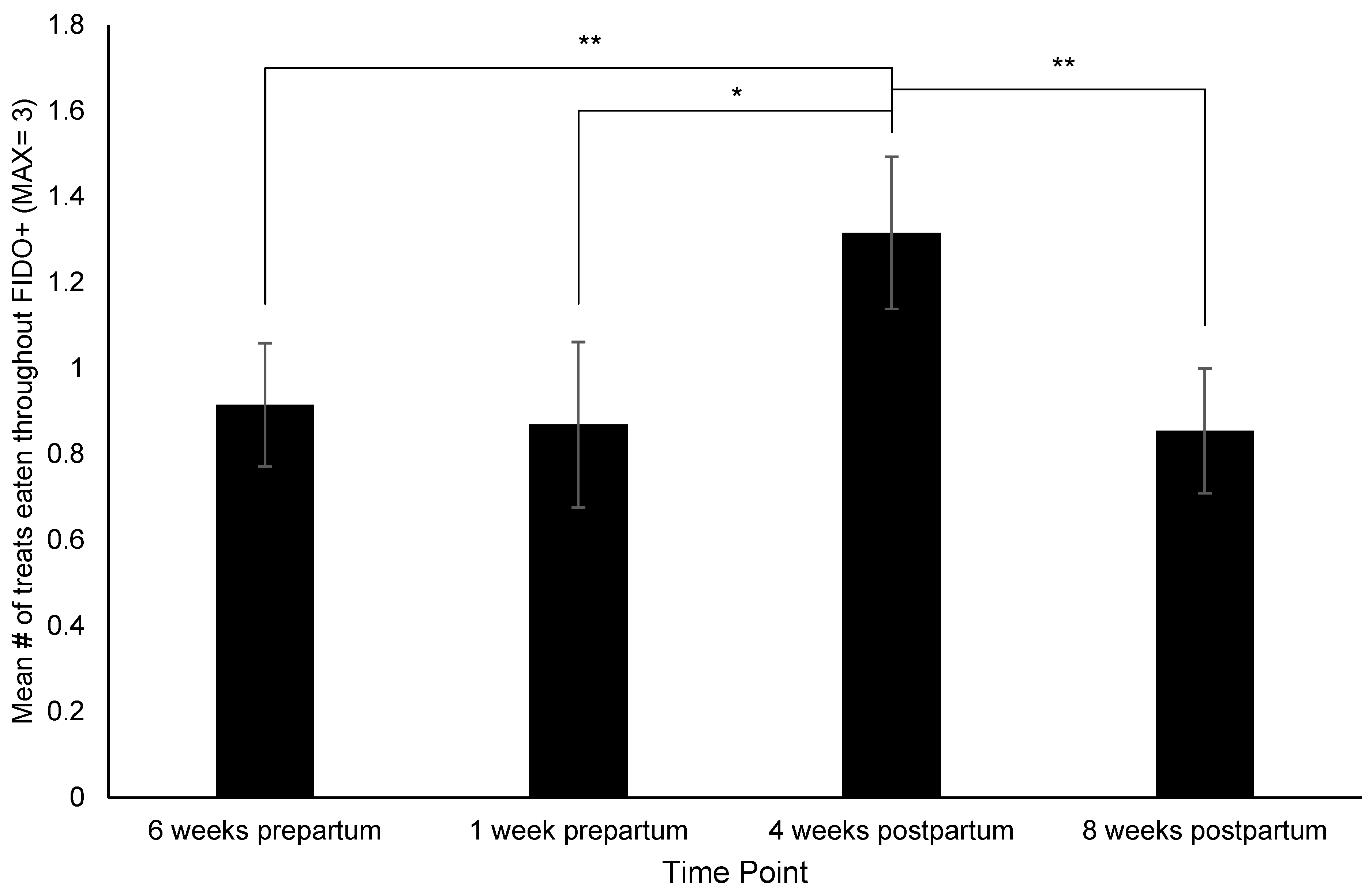
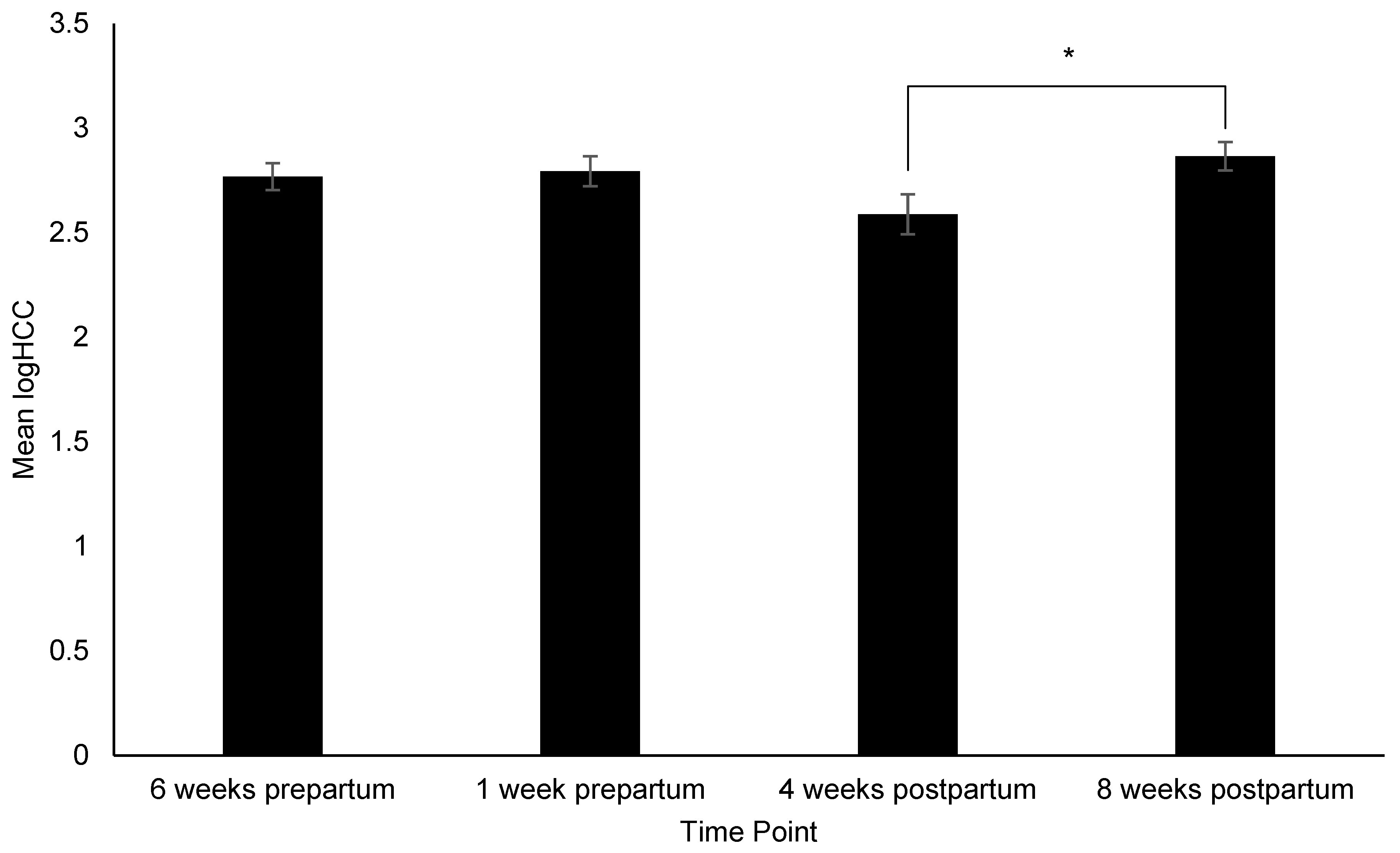
3.2. Changes in Behavior, Treat Consumption, HCC, FGM, and Fecal sIgA
3.3. Relationship between Physiologic Metrics
3.4. Parasites and Physical Health
4. Discussion
4.1. Behavior
4.2. HCC
4.3. FGM
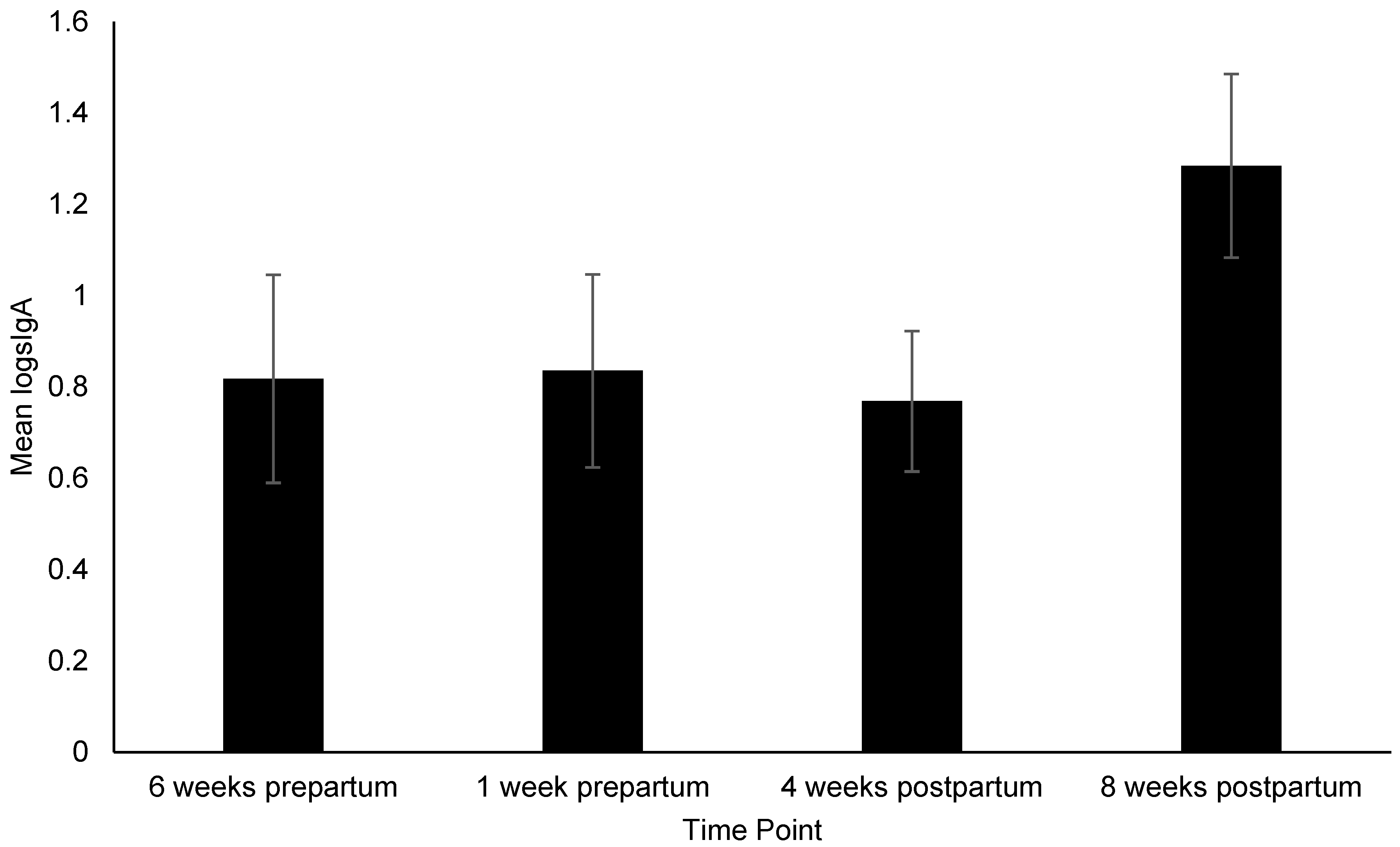
4.4. sIgA and Intestinal Parasites
4.5. Limitations
4.6. Implications and Future Directions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Francis, D.D.; Champagne, F.C.; Meaney, M.J. Variations in Maternal Behaviour Are Associated with Differences in Oxytocin Receptor Levels in the Rat. J. Neuroendocrinol. 2000, 12, 1145–1148. [Google Scholar] [CrossRef] [PubMed]
- Meaney, M.J. Maternal Care, Gene Expression, and the Transmission of Individual Differences in Stress Reactivity Across Generations. Annu. Rev. Neurosci. 2001, 24, 1161–1192. [Google Scholar] [CrossRef] [PubMed]
- Patin, V.; Lordi, B.; Vincent, A.; Thoumas, J.L.; Vaudry, H.; Caston, J. Effects of Prenatal Stress on Maternal Behavior in the Rat. Dev. Brain Res. 2002, 139, 1–8. [Google Scholar] [CrossRef]
- Barbazanges, A.; Piazza, V.; le Moal, M.; Maccari, S. Maternal Glucocorticoid Secretion Mediates Long-Term Effects of Prenatal Stress. J. Neurosci. 1996, 16, 3943–3949. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caldji, C.; Tannenbaum, B.; Sharma, S.; Francis, D.; Plotsky, P.M.; Meaney, M.J. Maternal Care during Infancy Regulates the Development of Neural Systems Mediating the Expression of Fearfulness in the Rat. Proc. Natl. Acad. Sci. USA 1998, 95, 5335–5340. [Google Scholar] [CrossRef] [Green Version]
- Liu, D.; Diorio, J.; Tannenbaum, B.; Caldji, C.; Francis, D.; Freedman, A.; Sharma, S.; Pearson, D.; Plotsky, P.M.; Meaney, M.J. Maternal Care, Hippocampal Glucocorticoid Receptors, and Hypothalamic- Pituitary-Adrenal Responses to Stress. Science 1997, 277, 1659–1662. [Google Scholar] [CrossRef] [Green Version]
- Liu, D.; Diorio, J.; Day, J.C.; Francis, D.D.; Meaney, M.J. Maternal Care, Hippocampal Synaptogenesis and Cognitive Development in Rats. Nat. Neurosci. 2000, 3, 799–806. [Google Scholar] [CrossRef]
- Braastad, B.O.; Osadchuk, L.V.; Lund, G.; Bakken, M. Effects of Prenatal Handling Stress on Adrenal Weight and Function and Behaviour in Novel Situations in Blue Fox Cubs (Alopex lagopus). Appl. Anim. Behav. Sci. 1998, 57, 157–169. [Google Scholar] [CrossRef]
- Schneider, M.L. Prenatal Stress Exposure Alters Postnatal Behavioral Expression under Conditions of Novelty Challenge in Rhesus Monkey Infants. Dev. Psychobiol. 1992, 25, 529–540. [Google Scholar] [CrossRef]
- United States Department of Agriculture Animal Welfare Act and Animal Welfare Regulations. Available online: https://www.aphis.usda.gov/animal_welfare/downloads/bluebook-ac-awa.pdf (accessed on 26 September 2022).
- Indiana State Veterinarian for the Indiana State Board of Animal Health Indiana State Board of Animal Health Indiana Commercial Dog Breeder and Broker Program Guidance Document. Available online: https://www.in.gov/boah/files/Dog-Breeder-Guidance-_12-2020_Ver1_02.pdf (accessed on 23 August 2022).
- Stella, J.L.; Bauer, A.E.; Croney, C.C. A Cross-Sectional Study to Estimate Prevalence of Periodontal Disease in a Population of Dogs (Canis familiaris) in Commercial Breeding Facilities in Indiana and Illinois. PLoS ONE 2018, 13, e0191395. [Google Scholar] [CrossRef]
- Stella, J.; Hurt, M.; Bauer, A.; Gomes, P.; Ruple, A.; Beck, A.; Croney, C. Does Flooring Substrate Impact Kennel and Dog Cleanliness in Commercial Breeding Facilities? Animals 2018, 8, 59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stella, J.; Shreyer, T.; Ha, J.; Croney, C. Improving Canine Welfare in Commercial Breeding (CB) Operations: Evaluating Rehoming Candidates. Appl. Anim. Behav. Sci. 2019, 220, 104861. [Google Scholar] [CrossRef]
- Mugenda, L.; Shreyer, T.; Croney, C. Refining Canine Welfare Assessment in Kennels: Evaluating the Reliability of Field Instantaneous Dog Observation (FIDO) Scoring. Appl. Anim. Behav. Sci. 2019, 221, 104874. [Google Scholar] [CrossRef]
- Bauer, A.E.; Jordan, M.; Colon, M.; Shreyer, T.; Croney, C.C. Evaluating FIDO: Developing and Pilot Testing the Field Instantaneous Dog Observation Tool. Pet. Behaviour. Sci. 2017, 4, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Broom, D.M. Animal Welfare: An Aspect of Care, Sustainability, and Food Quality Required by the Public. J. Vet. Med. Educ. 2010, 37, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Croney, C.C. Turning up the Volume on Man’s Best Friend: Ethical Issues Associated with Commercial Dog Breeding. J. Appl. Anim. Ethics Res. 2019, 1, 230–252. [Google Scholar] [CrossRef] [Green Version]
- Sandøe, P.; Corr, S.; Palmer, C. Companion Animal Ethics; John Wiley & Sons: Hoboken, NJ, USA, 2015; ISBN 1118376714. [Google Scholar]
- Fraser, D.; Weary, D.M.; Pajor, E.A.; Milligan, B.N. A Scientific Conception of Animal Welfare That Reflects Ethical Concerns. Anim. Welf. 1997, 6, 187–205. [Google Scholar]
- Mason, G.; Mendl, M. Why Is There No Simple Way of Measuring Animal Welfare? Anim. Welf. 1993, 2, 301–319. [Google Scholar]
- Barnard, S.; Flint, H.; Shreyer, T.; Croney, C. Evaluation of an Easy-to-Use Protocol for Assessing Behaviors of Dogs Retiring from Commercial Breeding Kennels. PLoS ONE 2021, 16, e0255883. [Google Scholar] [CrossRef]
- Sheriff, M.J.; Dantzer, B.; Delehanty, B.; Palme, R.; Boonstra, R. Measuring Stress in Wildlife: Techniques for Quantifying Glucocorticoids. Oecologia 2011, 166, 869–887. [Google Scholar] [CrossRef]
- Broom, D.M. The Scientific Assessment of Animal Welfare. Appl. Anim. Behav. Sci. 1988, 20, 5–19. [Google Scholar] [CrossRef]
- Mormède, P.; Andanson, S.; Aupérin, B.; Beerda, B.; Guémené, D.; Malmkvist, J.; Manteca, X.; Manteuffel, G.; Prunet, P.; van Reenen, C.G.; et al. Exploration of the Hypothalamic–Pituitary–Adrenal Function as a Tool to Evaluate Animal Welfare. Physiol. Behav. 2007, 92, 317–339. [Google Scholar] [CrossRef] [PubMed]
- Concannon, P.W.; Butler, W.R.; Hansel, W.; Knight, P.J.; Hamilton, J.M. Parturition and Lactation in the Bitch: Serum Progesterone, Cortisol and Prolactin. Biol. Reprod. 1978, 19, 1113–1118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cardinali, L.; Troisi, A.; Verstegen, J.P.; Menchetti, L.; Elad Ngonput, A.; Boiti, C.; Canello, S.; Zelli, R.; Polisca, A. Serum Concentration Dynamic of Energy Homeostasis Hormones, Leptin, Insulin, Thyroid Hormones, and Cortisol throughout Canine Pregnancy and Lactation. Theriogenology 2017, 97, 154–158. [Google Scholar] [CrossRef] [PubMed]
- Groppetti, D.; Meazzi, S.; Filipe, J.F.S.; Colombani, C.; Panseri, S.; Zanzani, S.A.; Palestrini, C.; Cannas, S.; Giordano, A.; Pecile, A. Maternal and Neonatal Canine Cortisol Measurement in Multiple Matrices during the Perinatal Period: A Pilot Study. PLoS ONE 2021, 16, e0254842. [Google Scholar] [CrossRef] [PubMed]
- Fusi, J.; Peric, T.; Probo, M.; Cotticelli, A.; Faustini, M.; Veronesi, M.C. How Stressful Is Maternity? Study about Cortisol and Dehydroepiandrosterone-Sulfate Coat and Claws Concentrations in Female Dogs from Mating to 60 Days Post-Partum. Animals 2021, 11, 1632. [Google Scholar] [CrossRef]
- Mack, Z.; Fokidis, H.B. A Novel Method for Assessing Chronic Cortisol Concentrations in Dogs Using the Nail as a Source. Domest. Anim. Endocrinol. 2017, 59, 53–57. [Google Scholar] [CrossRef]
- Corthésy, B. Multi-Faceted Functions of Secretory IgA at Mucosal Surfaces. Front. Immunol. 2013, 4, 185. [Google Scholar] [CrossRef] [Green Version]
- Mantis, N.J.; Rol, N.; Corthésy, B. Secretory IgA’s Complex Roles in Immunity and Mucosal Homeostasis in the Gut. Mucosal Immunol. 2011, 4, 603–611. [Google Scholar] [CrossRef] [Green Version]
- Campos-Rodríguez, R.; Godínez-Victoria, M.; Abarca-Rojano, E.; Pacheco-Yépez, J.; Reyna-Garfias, H.; Barbosa-Cabrera, R.E.; Drago-Serrano, M.E. Stress Modulates Intestinal Secretory Immunoglobulin A. Front. Integr. Neurosci. 2013, 7, 86. [Google Scholar] [CrossRef] [Green Version]
- Staley, M.; Conners, M.G.; Hall, K.; Miller, L.J. Linking Stress and Immunity: Immunoglobulin A as a Non-Invasive Physiological Biomarker in Animal Welfare Studies. Horm. Behav. 2018, 102, 55–68. [Google Scholar] [CrossRef] [PubMed]
- Grellet, A.; Mila, H.; Heilmann, R.M.; Feugier, A.; Gruetzner, N.; Suchodolski, J.S.; Steiner, J.M.; Chastant-Maillard, S. Effect of Age, Gestation and Lactation on Faecal IgA and Calprotectin Concentrations in Dogs. J. Nutr. Sci. 2014, 3, e41. [Google Scholar] [CrossRef] [PubMed]
- Alekseeva, G.S.; Loshchagina, J.A.; Erofeeva, M.N.; Naidenko, S.V. Stressed by Maternity: Changes of Cortisol Level in Lactating Domestic Cats. Animals 2020, 10, 903. [Google Scholar] [CrossRef]
- United States Department of Agriculture [USDA] Regulatory Compliance for Commercial Dog Breeders: Introductory Course. Available online: https://www.aphis.usda.gov/aphis/ourfocus/animalwelfare/caw/education+and+training/ct_commercial_dog_breeders (accessed on 21 July 2019).
- Mugenda, L.W. Refining and Validating an On-Site Canine Welfare Assessment Tool Developed for Use in Commercial Breeding Kennels. Master’s Thesis, Purdue University, West Lafayette, IN, USA, 2019. [Google Scholar]
- Davenport, M.D.; Tiefenbacher, S.; Lutz, C.K.; Novak, M.A.; Meyer, J.S. Analysis of Endogenous Cortisol Concentrations in the Hair of Rhesus Macaques. Gen. Comp. Endocrinol. 2006, 147, 255–261. [Google Scholar] [CrossRef]
- Palme, R.; Rettenbacher, S.; Touma, C.; El-Bahr, S.M.; Möstl, E. Stress Hormones in Mammals and Birds: Comparative Aspects Regarding Metabolism, Excretion, and Noninvasive Measurement in Fecal Samples. Ann. N. Y. Acad. Sci. 2005, 1040, 162–171. [Google Scholar] [CrossRef] [PubMed]
- Munro, C.J.; Lasley, B.L. Non-Radiometric Methods for Immunoassay of Steroid Hormones. Prog. Clin. Biol. Res. 1988, 285, 289–329. [Google Scholar]
- Zajac, A.; Conboy, G.; Little, S.; Reichard, M. Veterinary Clinical Parasitology, 9th ed.; John Wiley and Sons Inc.: Hoboken, NJ, USA, 2021. [Google Scholar]
- Concannon, P.W. Canine Pregnancy and Parturition. Vet. Clin. N. Am. Small Anim. Pract. 1986, 16, 453–475. [Google Scholar] [CrossRef]
- Lloyd, S. Effect of Pregnancy and Lactation upon Infection. Vet. Immunol. Immunopathol. 1983, 4, 153–176. [Google Scholar] [CrossRef]
- Lloyd, S.; Amerasinghe, P.H.; Soulsby, E.J.L. Periparturient Immunosuppression in the Bitch and Its Influence on Infection with Toxocara Canis. J. Small Anim. Pract. 1983, 24, 237–247. [Google Scholar] [CrossRef]
- Veronesi, M.C.; Battocchio, M.; Marinelli, L.; Faustini, M.; Kindahl, H.; Cairoli, F. Correlations Among Body Temperature, Plasma Progesterone, Cortisol and Prostaglandin F2α of the Periparturient Bitch. J. Vet. Med. Ser. A 2002, 49, 264–268. [Google Scholar] [CrossRef]
- Wells, D. Behaviour of Dogs. In The Ethology of Domestic Animals: An Introductory Text; Jensen, P., Ed.; CABI: Wallingford, UK, 2009; pp. 228–240. [Google Scholar]
- von Heimendahl, A.; Cariou, M. Normal Parturition and Management of Dystocia in Dogs and Cats. Pract. 2009, 31, 254–261. [Google Scholar] [CrossRef]
- Signorell, A. DescTools: Tools for Descriptive Statistics, R Package Version 0.99.42; R Foundation for Statistical Computing: Vienna, Austria, 2015. [Google Scholar]
- Koo, T.K.; Li, M.Y. A Guideline of Selecting and Reporting Intraclass Correlation Coefficients for Reliability Research. J. Chiropr. Med. 2016, 15, 155–163. [Google Scholar] [CrossRef] [Green Version]
- Gamer, M.; Lemon, J.; Singh, I. Irr: Various Coefficients of Interrater Reliability and Agreement, Version 0.84.1; R Foundation for Statistical Computing: Vienna, Austria, 2019. [Google Scholar]
- McHugh, M.L. Interrater Reliability: The Kappa Statistic. Biochem. Med. 2012, 22, 276–282. [Google Scholar] [CrossRef]
- Hornung, R.W.; Reed, L.D. Estimation of Average Concentration in the Presence of Nondetectable Values. Appl. Occup. Environ. Hyg. 1990, 5, 46–51. [Google Scholar] [CrossRef]
- Pleil, J.D. Imputing Defensible Values for Left-Censored ‘below Level of Quantitation’ (LoQ) Biomarker Measurements. J. Breath Res. 2016, 10, 045001. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, J.; Bates, D.; DebRoy, S.; Sarkar, D.; R Core Team. Nlme: Linear and Nonlinear Mixed Effects Models, R Package Version 3.1-152; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- Fox, J.; Weisburg, S. An R Companion to Applied Regression, 3rd ed.; Sage Publications: Thousand Oaks, CA, USA, 2019. [Google Scholar]
- Lenth, R.V. Least-Squares Means: The R Package Lsmeans. J. Stat. Softw. 2016, 69, 1–33. [Google Scholar] [CrossRef] [Green Version]
- Bakdash, J.; Marusich, L. Rmcorr: Repeated Measures Correlation; Version 0.4.4; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- Santos, N.R.; Beck, A.; Fontbonne, A. A Review of Maternal Behaviour in Dogs and Potential Areas for Further Research. J. Small Anim. Pract. 2020, 61, 85–92. [Google Scholar] [CrossRef] [Green Version]
- Scott, J.P.; Fuller, J.L. Genetics and the Social Behavior of the Dog; University of Chicago Press: Chicago, IL, USA, 1965. [Google Scholar] [CrossRef]
- Shrout, P.E.; Fleiss, J.L. Intraclass Correlations: Uses in Assessing Rater Reliability. Psychol. Bull. 1979, 86, 420–428. [Google Scholar] [CrossRef]
- Fontaine, E. Food Intake and Nutrition During Pregnancy, Lactation and Weaning in the Dam and Offspring. Reprod. Domest. Anim. 2012, 47, 326–330. [Google Scholar] [CrossRef]
- Slattery, D.A.; Neumann, I.D. No Stress Please! Mechanisms of Stress Hyporesponsiveness of the Maternal Brain. J. Physiol. 2008, 586, 377–385. [Google Scholar] [CrossRef]
- Garvey, M.; Stella, J.; Croney, C. Auditory Stress: Implications for Kenneled Dog Welfare; Center of animal welfare science, Department of Comparative Pathobiology, College of Veterinary Medicine, Purdue University: West Lafayette, IN, USA, 2016. [Google Scholar]
- Coppola, C.L.; Enns, R.M.; Grandin, T. Noise in the Animal Shelter Environment: Building Design and the Effects of Daily Noise Exposure. J. Appl. Anim. Welf. Sci. 2006, 9, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Conley, M.J.; Fisher, A.D.; Hemsworth, P.H. Effects of Human Contact and Toys on the Fear Responses to Humans of Shelter-Housed Dogs. Appl. Anim. Behav. Sci. 2014, 156, 62–69. [Google Scholar] [CrossRef]
- Bergamasco, L.; Osella, M.C.; Savarino, P.; Larosa, G.; Ozella, L.; Manassero, M.; Badino, P.; Odore, R.; Barbero, R.; Re, G. Heart Rate Variability and Saliva Cortisol Assessment in Shelter Dog: Human-Animal Interaction Effects. Appl. Anim. Behav. Sci. 2010, 125, 56–68. [Google Scholar] [CrossRef]
- Hubrecht, R.C. A Comparison of Social and Environmental Enrichment Methods for Laboratory Housed Dogs. Appl. Anim. Behav. Sci. 1993, 37, 345–361. [Google Scholar] [CrossRef]
- Brunton, P.J.; Russell, J.A.; Douglas, A.J. Adaptive Responses of the Maternal Hypothalamic-Pituitary-Adrenal Axis during Pregnancy and Lactation. J. Neuroendocrinol. 2008, 20, 764–776. [Google Scholar] [CrossRef]
- Sapolsky, R. Why Zebras Don’t Get Ulcers: The Acclaimed Guide to Stress, Stress-Related Diseases, and Coping, 3rd ed.; Henry Holt and Company: New York, NY, USA, 2004. [Google Scholar]
- Durocher, L.L.; Hinchcliff, K.W.; Williamson, K.K.; McKenzie, E.C.; Holbrook, T.C.; Willard, M.; Royer, C.M.; Davis, M.S. Effect of Strenuous Exercise on Urine Concentrations of Homovanillic Acid, Cortisol, and Vanillylmandelic Acid in Sled Dogs. Am. J. Vet. Res. 2007, 68, 107–111. [Google Scholar] [CrossRef]
- Angle, C.T.; Wakshlag, J.J.; Gillette, R.L.; Stokol, T.; Geske, S.; Adkins, T.O.; Gregor, C. Hematologic, Serum Biochemical, and Cortisol Changes Associated with Anticipation of Exercise and Short Duration High-Intensity Exercise in Sled Dogs. Vet. Clin. Pathol. 2009, 38, 370–374. [Google Scholar] [CrossRef]
- Siiteri, P.K.; Stites, D.P. Immunologic and Endocrine Interrelationships in Pregnancy. Biol. Reprod. 1982, 26, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Walker, J.K.; Waran, N.K.; Phillips, C.J.C. The Effect of Conspecific Removal on the Behaviour and Physiology of Pair-Housed Shelter Dogs. Appl. Anim. Behav. Sci. 2014, 158, 46–56. [Google Scholar] [CrossRef] [Green Version]
- Carlsson, H.E.; Lyberg, K.; Royo, F.; Hau, J. Quantification of Stress Sensitive Markers in Single Fecal Samples Do Not Accurately Predict Excretion of These in the Pig. Res. Vet. Sci. 2007, 82, 423–428. [Google Scholar] [CrossRef]
- Millspaugh, J.J.; Washburn, B.E. Within-Sample Variation of Fecal Glucocorticoid Measurements. Gen. Comp. Endocrinol. 2003, 132, 21–26. [Google Scholar] [CrossRef]
- Palme, R.; Fischer, P.; Schildorfer, H.; Ismail, M.N. Excretion of Infused 14C-Steroid Hormones via Faeces and Urine in Domestic Livestock. Anim. Reprod. Sci. 1996, 43, 43–63. [Google Scholar] [CrossRef]
- Skandakumar, S.; Stodulski, G.; Hau, J. Salivary IgA: A Possible Stress Marker in Dogs. Anim. Welf. 1995, 4, 339–350. [Google Scholar]
- Royo, F.; Björk, N.; Carlsson, H.E.; Mayo, S.; Hau, J. Impact of Chronic Catheterization and Automated Blood Sampling (Accusampler) on Serum Corticosterone and Fecal Immunoreactive Corticosterone Metabolites and Immunoglobulin A in Male Rats. J. Endocrinol. 2004, 180, 145–153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jarillo-Luna, R.A.; Rivera-Aguilar, V.; Pacheco-Yépez, J.; Godínez-Victoria, M.; Oros-Pantoja, R.; Miliar-García, A.; Campos-Rodríguez, R. Nasal IgA Secretion in a Murine Model of Acute Stress. The Possible Role of Catecholamines. J. Neuroimmunol. 2015, 278, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Svobodová, I.; Chaloupková, H.; Končel, R.; Bartoš, L.; Hradecká, L.; Jebavý, L. Cortisol and Secretory Immunoglobulin A Response to Stress in German Shepherd Dogs. PLoS ONE 2014, 9, e90820. [Google Scholar] [CrossRef]
- Gourkow, N.; LaVoy, A.; Dean, G.A.; Phillips, C.J.C. Associations of Behaviour with Secretory Immunoglobulin A and Cortisol in Domestic Cats during Their First Week in an Animal Shelter. Appl. Anim. Behav. Sci. 2014, 150, 55–64. [Google Scholar] [CrossRef]
- Paramastri, Y.; Royo, F.; Eberova, J.; Carlsson, H.E.; Sajuthi, D.; Fernstrom, A.L.; Pamungkas, J.; Hau, J. Urinary and Fecal Immunoglobulin A, Cortisol and 11-17 Dioxoandrostanes, and Serum Cortisol in Metabolic Cage Housed Female Cynomolgus Monkeys (Macaca fascicularis). J. Med. Primatol. 2007, 36, 355–364. [Google Scholar] [CrossRef]

| Time Point | Metrics Collected |
|---|---|
| 6 weeks prepartum | FIDO+ |
| HCC | |
| FGM | |
| Fecal sIgA | |
| Presence of intestinal parasites | |
| Physical health assessment | |
| 1 week prepartum | FIDO+ |
| HCC | |
| FGM | |
| Fecal sIgA | |
| Presence of intestinal parasites | |
| Physical health assessment | |
| 4 weeks postpartum | FIDO+ (with litter present) |
| HCC | |
| FGM | |
| Fecal sIgA | |
| Presence of intestinal parasites | |
| Physical health assessment | |
| 8 weeks postpartum | FIDO+ |
| HCC | |
| FGM | |
| Fecal sIgA | |
| Presence of intestinal parasites | |
| Physical health assessment |
| Metric | 6 Weeks Prepartum | 1 Week Prepartum | 4 Weeks Postpartum | 8 Weeks Postpartum |
|---|---|---|---|---|
| FIDO+ | n = 59 | n = 38 | n = 57 | n = 55 |
| HCC | n = 59 | n = 43 | n = 41 | n = 53 |
| FGM | n = 34 | n = 37 | n = 53 | n = 52 |
| Fecal sIgA | n = 34 | n = 37 | n = 53 | n = 51 |
| Parasites | n = 18 | n = 27 | n = 48 | n = 46 |
| Parasite | 6 Weeks Prepartum | 1 Week Prepartum | 4 Weeks Postpartum | 8 Weeks Postpartum |
|---|---|---|---|---|
| Cryptosporidium | 0/18 (0%) | 0/27 (0%) | 1/48 (2%) | 0/46 (0%) |
| Cystoisospora a | 0/18 (0%) | 0/27 (0%) | 5/48 (10%) | 4/46 (9%) |
| Giardia | 1/18 (6%) | 1/27 (4%) | 7/48 (15%) | 3/46 (7%) |
| Trichuris (whipworm) | 1/18 (6%) | 0/27 (0%) | 0/48 (0%) | 0/46 (0%) |
| Total | 2/18 (11%) | 1/27 (4%) | 13/48 (27%) b | 7/46 (15%) |
| Metric | 6 Weeks Prepartum | 1 Week Prepartum | 4 Weeks Postpartum | 8 Weeks Postpartum |
|---|---|---|---|---|
| BCS a | 3.21 | N/A | 3.07 | 3.07 |
| Cleanliness–0% b | 86% | 85% | 77% | 67% |
| Ocular discharge–‘present’ b | 7% | 5% | 11% | 9% |
| Tear staining–‘moderate’ or ‘severe’ b | 29% | 21% | 26% | 35% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Romaniuk, A.C.; Barnard, S.; Weller, J.E.; Weng, H.-Y.; Dangoudoubiyam, S.; Croney, C. Dam (Canis familiaris) Welfare throughout the Peri-Parturient Period in Commercial Breeding Kennels. Animals 2022, 12, 2820. https://doi.org/10.3390/ani12202820
Romaniuk AC, Barnard S, Weller JE, Weng H-Y, Dangoudoubiyam S, Croney C. Dam (Canis familiaris) Welfare throughout the Peri-Parturient Period in Commercial Breeding Kennels. Animals. 2022; 12(20):2820. https://doi.org/10.3390/ani12202820
Chicago/Turabian StyleRomaniuk, Aynsley C., Shanis Barnard, Jennifer E. Weller, Hsin-Yi Weng, Sriveny Dangoudoubiyam, and Candace Croney. 2022. "Dam (Canis familiaris) Welfare throughout the Peri-Parturient Period in Commercial Breeding Kennels" Animals 12, no. 20: 2820. https://doi.org/10.3390/ani12202820





