Habit Formation and the Effect of Repeated Stress Exposures on Cognitive Flexibility Learning in Horses
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Management
2.2. Infrastructure/Stimuli
2.3. Data Collection
2.4. Physiological Samples
2.5. Experimental Sequence
2.5.1. Daily Preparation
- Day 1 Pre-test (PT)
- Days 2–3 Learning
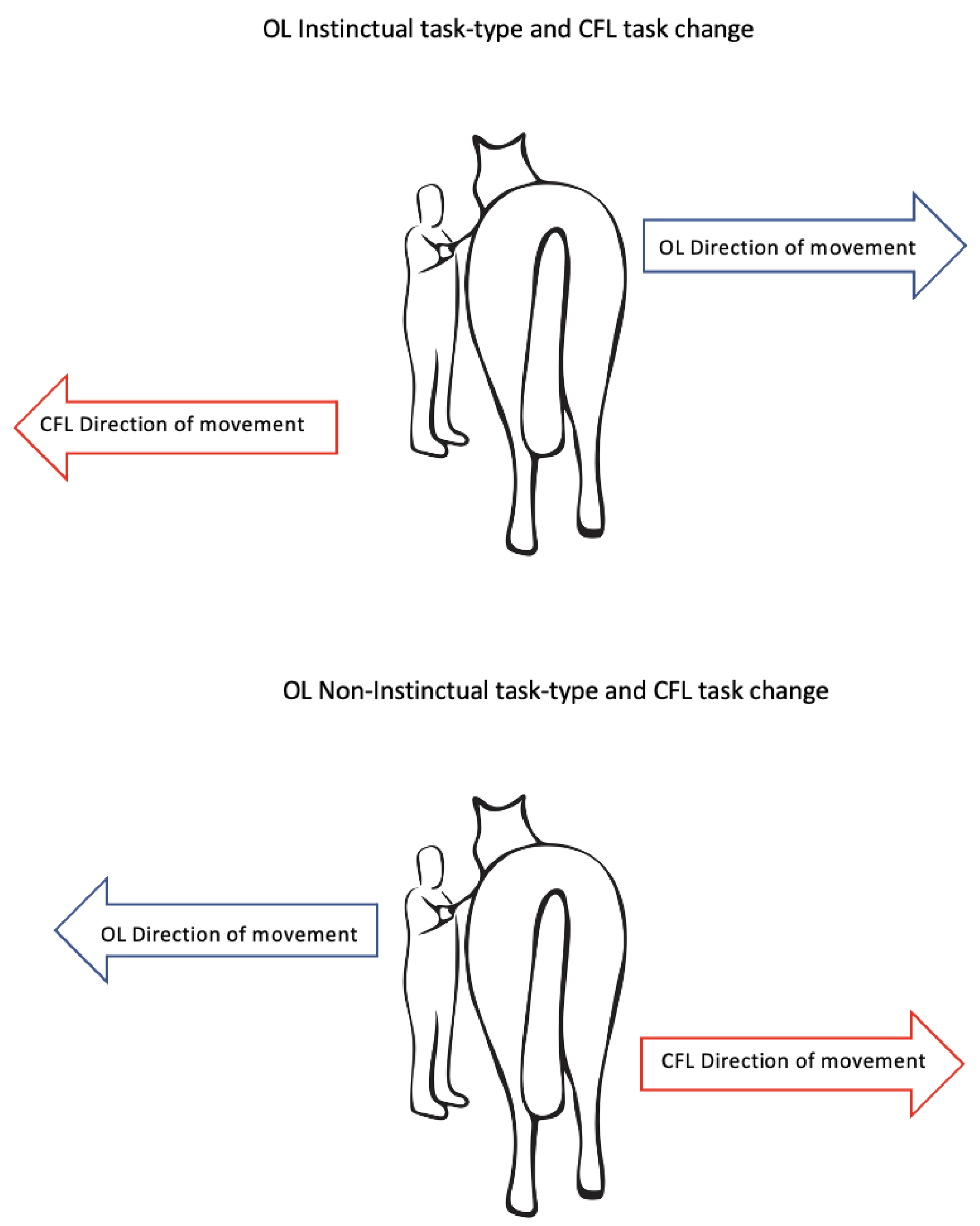
- Training procedure-original learning (OL)
- Days 3–8: Treatment
- Day 9: Cognitive Flexibility Learning (CFL)
2.5.2. Biochemical Analysis
- Cortisol
- Brain Derived Neurotrophic Factor
- HR data Analysis.
- Statistical analysis
3. Results
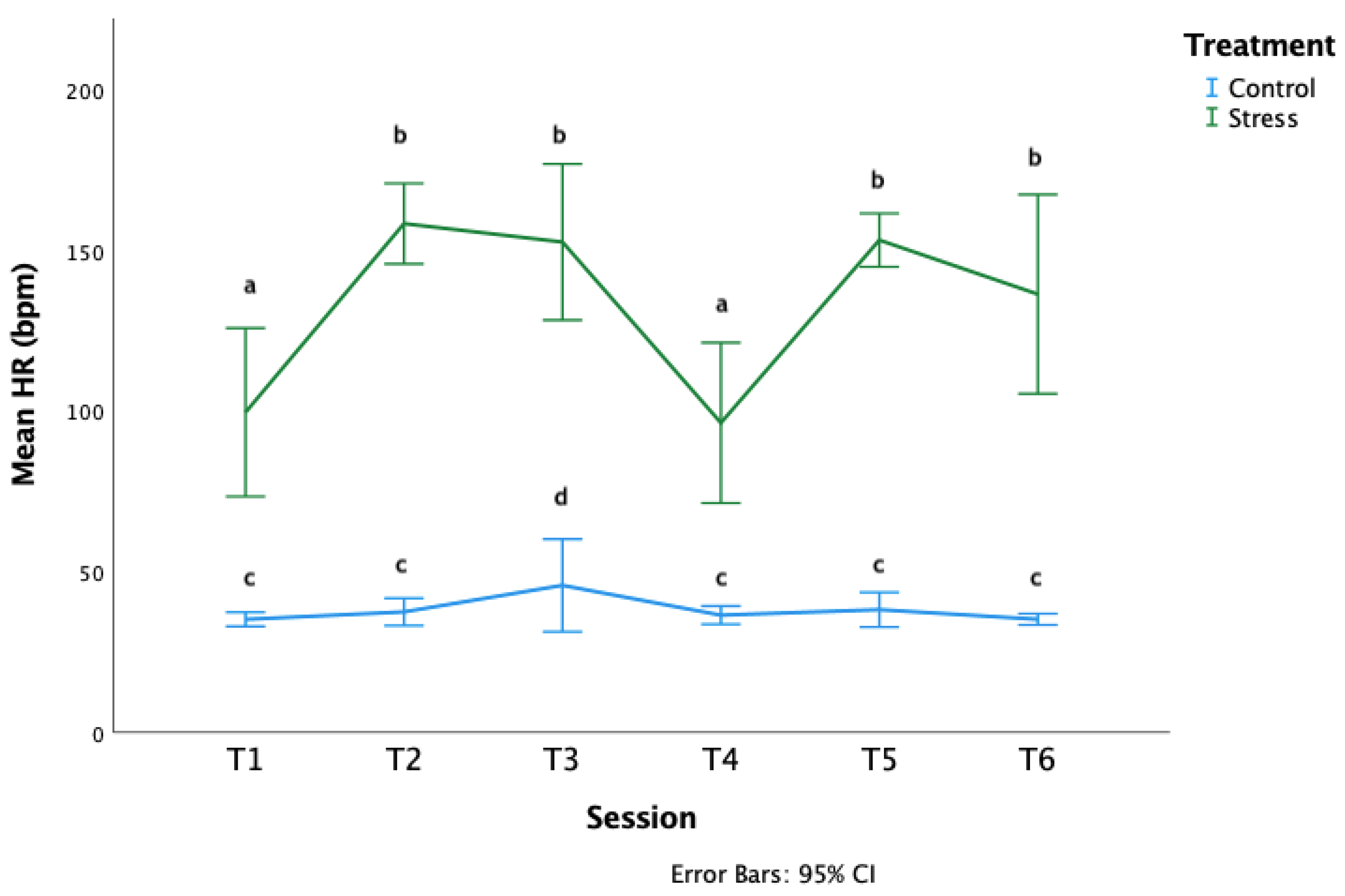
3.1. Pre-Test Physiology
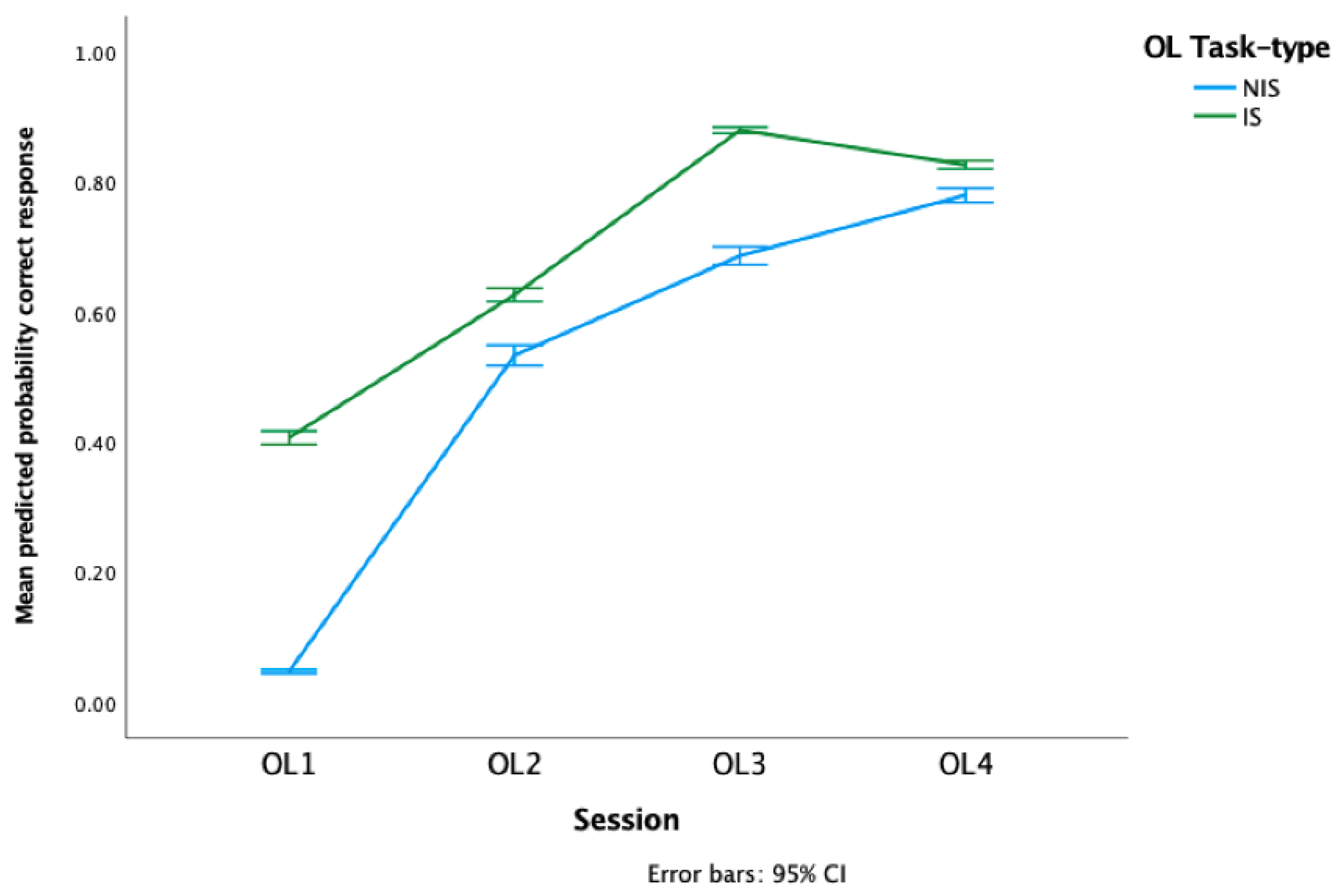
3.2. Original Learning
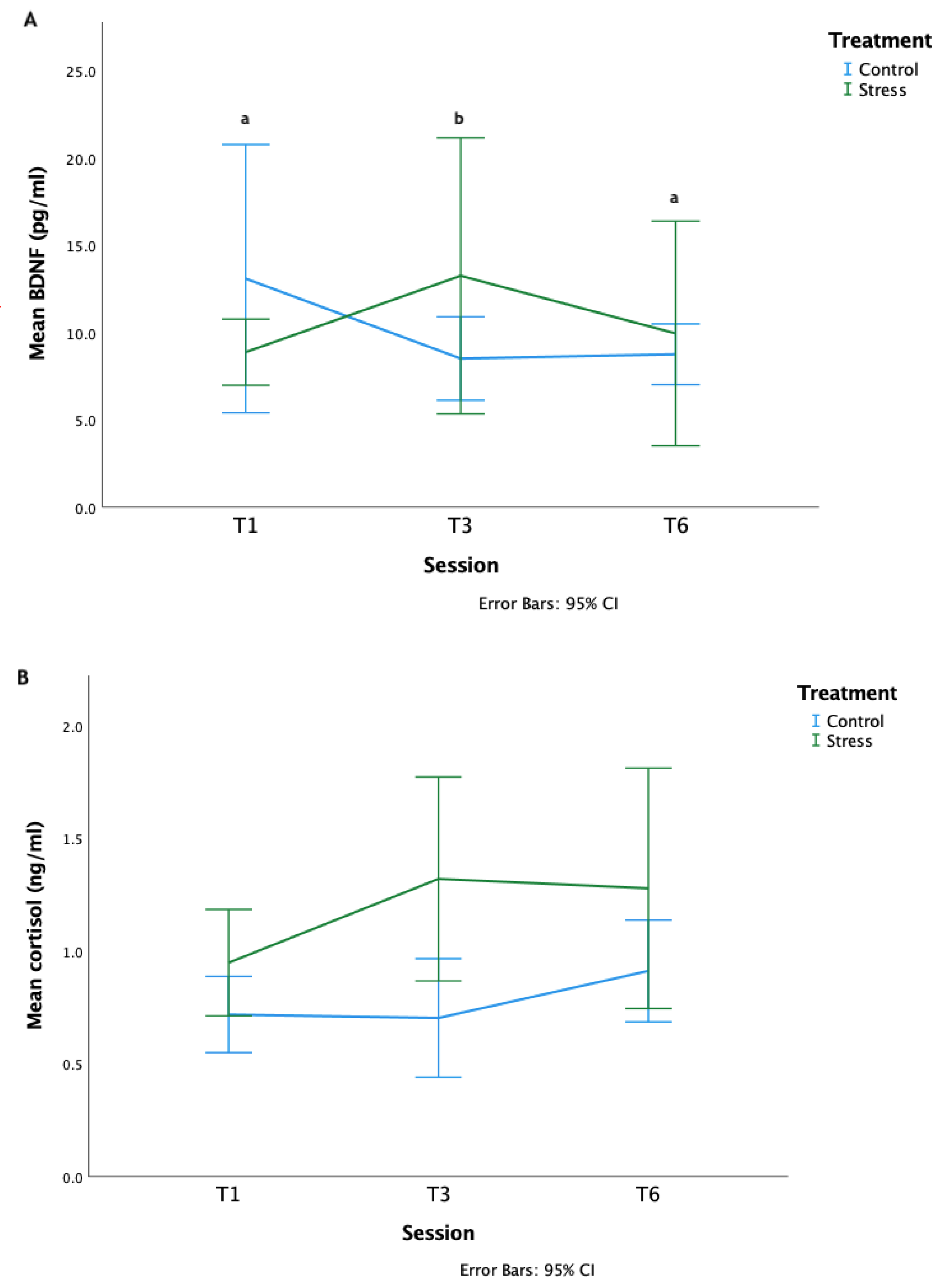
3.3. Treatment
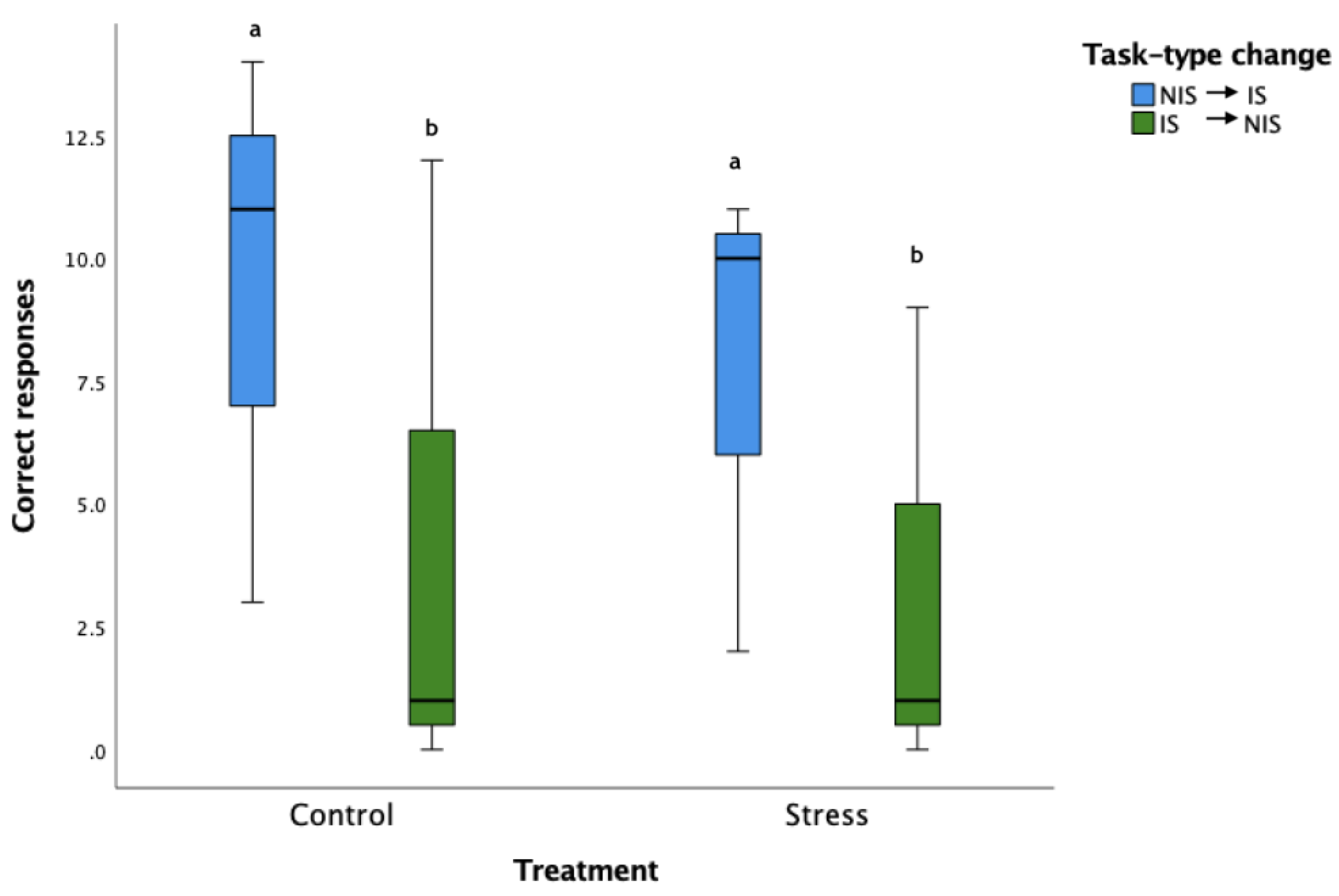
3.4. Cognitive Flexibility Learning Acquisition
3.5. Comparison of CFL to OL1
3.6. Relationships between Learning Outcomes and Physiological Indicators (Spearman’s Correlations)
4. Discussion
4.1. Differences in OL and IS-NIS Task-Type Acquisition
4.2. Lack of Stress Effect on Cognitive Flexibility Learning
4.3. Proxies for Neurotransmitter Release and Activity
4.4. Limitations
4.5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McGreevy, P.D.; McLean, A.N. Roles of learning theory and ethology in equitation. J. Vet. Behav. Clin. Appl. Res. 2007, 2, 108–118. [Google Scholar] [CrossRef]
- McLean, A.N.; McGreevy, P.D. Horse-training techniques that may defy the principles of learning theory and compromise welfare. J. Vet. Behav. Clin. Appl. Res. 2010, 5, 187–195. [Google Scholar] [CrossRef]
- Hurtubise, J.L.; Howland, J.G. Effects of stress on behavioral flexibility in rodents. Neuroscience 2017, 345, 176–192. [Google Scholar] [CrossRef] [PubMed]
- Waring, G.H. Horse Behavior, 2nd ed.; Noyes Publications: Norwich, NY, USA, 2003. [Google Scholar]
- McGreevy, P.; Hahn, C.N.; McLean, A.N. Equine behavior: A guide for veterinarians and equine scientists. In Equine Behavior: A Guide for Veterinarians and Equine Scientists; Saunders: London, UK, 2004; p. xiv + 369. [Google Scholar]
- Campese, V.D.; Sears, R.M.; Moscarello, J.M.; Diaz-Mataix, L.; Cain, C.K.; LeDoux, J.E. The Neural Foundations of Reaction and Action in Aversive Motivation. In Behavioral Neuroscience of Motivation; Eleanor, H., Simpson, P., Balsam, D., Eds.; Springer International Publishing: Cham, Swizterland, 2016; pp. 171–195. [Google Scholar]
- Bolles, R.C. Species-specific defense reactions and avoidance learning. Psychol. Rev. 1970, 77, 32–48. [Google Scholar] [CrossRef]
- Panksepp, J.; Fuchs, T.; Iacobucci, P. The basic neuroscience of emotional experiences in mammals: The case of subcortical FEAR circuitry and implications for clinical anxiety. Appl. Anim. Behav. Sci. 2011, 129, 1–17. [Google Scholar] [CrossRef]
- McLean, A.N.; Christensen, J.W. The application of learning theory in horse training. Appl. Anim. Behav. Sci. 2017, 190, 18–27. [Google Scholar] [CrossRef]
- Padalino, B. Effects of the different transport phases on equine health status, behavior, and welfare: A review. J. Vet. Behav. Clin. Appl. Res. 2015, 10, 272–282. [Google Scholar] [CrossRef]
- Munsters, C.C.B.M.; Visser, E.K.; Broek, J.V.D.; van Oldruitenborgh-Oosterbaan, M.M.S. Physiological and behavioral responses of horses during police training. Animal 2013, 7, 822–827. [Google Scholar] [CrossRef]
- Padalino, B.; Henshall, C.; Raidal, S.L.; Knight, P.; Celi, P.; Jeffcott, L.; Muscatello, G. Investigations Into Equine Transport-Related Problem Behaviors: Survey Results. J. Equine Vet. Sci. 2016, 48, 166–173.e162. [Google Scholar] [CrossRef]
- McLean, A. The Truth about Horses; Quarto Publishing: London, UK, 2003. [Google Scholar]
- LeDoux, J.; Daw, N.D. Surviving threats: Neural circuit and computational implications of a new taxonomy of defensive behaviour. Nat. Rev. Neurosci. 2018, 19, 269–282. [Google Scholar] [CrossRef]
- Maren, S. Seeking a Spotless Mind: Extinction, Deconsolidation, and Erasure of Fear Memory. Neuron 2011, 70, 830–845. [Google Scholar] [CrossRef] [PubMed]
- Ressler, R.L.; Maren, S. Synaptic encoding of fear memories in the amygdala. Curr. Opin. Neurobiol. 2019, 54, 54–59. [Google Scholar] [CrossRef] [PubMed]
- International Society for Equitation Science. Principles of Learning Theory in Equitation. 2018. Available online: https://equitationscience.com/learning-theory/ (accessed on 10 June 2021).
- McGreevy, P.; McLean, A. Domestic Horse: The Origins, Development and Management of Its Behaviour; Mills, D.S., McDonnell, S., Eds.; Cambridge University Press: Cambridge, UK, 2005; pp. 196–211. [Google Scholar]
- Audet, J.-N.; Lefebvre, L. What’s flexible in behavioral flexibility? Behav. Ecol. 2017, 28, 943–947. [Google Scholar] [CrossRef]
- McBride, S.D.; Parker, M.O.; Roberts, K.; Hemmings, A. Applied neurophysiology of the horse; implications for training, husbandry and welfare. Appl. Anim. Behav. Sci. 2017, 190, 90–101. [Google Scholar] [CrossRef]
- Yin, H.H.; Ostlund, S.B.; Knowlton, B.J.; Balleine, B.W. The role of the dorsomedial striatum in instrumental conditioning. Eur. J. Neurosci. 2005, 22, 513–523. [Google Scholar] [CrossRef]
- Balleine, B.W.; Liljeholm, M.; Ostlund, S.B. The integrative function of the basal ganglia in instrumental conditioning. Behav. Brain Res. 2009, 199, 43–52. [Google Scholar] [CrossRef]
- Corbit, L.H.; Leung, B.; Balleine, B. The Role of the Amygdala-Striatal Pathway in the Acquisition and Performance of Goal-Directed Instrumental Actions. J. Neurosci. 2013, 33, 17682–17690. [Google Scholar] [CrossRef]
- Izquierdo, A.; Brigman, J.; Radke, A.; Rudebeck, P.; Holmes, A. The neural basis of reversal learning: An updated perspective. Neuroscience 2017, 345, 12–26. [Google Scholar] [CrossRef]
- Yaple, Z.A.; Yu, R. Fractionating adaptive learning: A meta-analysis of the reversal learning paradigm. Neurosci. Biobehav. Rev. 2019, 102, 85–94. [Google Scholar] [CrossRef]
- Castro-Alamancos, M.A. Absence of Rapid Sensory Adaptation in Neocortex during Information Processing States. Neuron 2004, 41, 455–464. [Google Scholar] [CrossRef]
- Poremba, A.; Gabriel, M. Amygdala Neurons Mediate Acquisition But Not Maintenance of Instrumental Avoidance Behavior in Rabbits. J. Neurosci. 1999, 19, 9635–9641. [Google Scholar] [CrossRef] [PubMed]
- Killcross, S. Coordination of Actions and Habits in the Medial Prefrontal Cortex of Rats. Cereb. Cortex 2003, 13, 400–408. [Google Scholar] [CrossRef] [PubMed]
- Goldfarb, E.V.; Phelps, E.A. Stress and the trade-off between hippocampal and striatal memory. Curr. Opin. Behav. Sci. 2017, 14, 47–53. [Google Scholar] [CrossRef]
- Quinn, J.J.; Pittenger, C.; Lee, A.S.; Pierson, J.L.; Taylor, J.R. Striatum-dependent habits are insensitive to both increases and decreases in reinforcer value in mice. Eur. J. Neurosci. 2013, 37, 1012–1021. [Google Scholar] [CrossRef] [PubMed]
- Solomon, R.L.; Kamin, L.J.; Wynne, L.C. Traumatic avoidance learning: The outcomes of several extinction procedures with dogs. J. Abnorm. Soc. Psychol. 1953, 48, 291–302. [Google Scholar] [CrossRef]
- Manning, E.E.; Bradfield, L.A.; Iordanova, M.D. Adaptive behaviour under conflict: Deconstructing extinction, reversal, and active avoidance learning. Neurosci. Biobehav. Rev. 2020, 120, 526–536. [Google Scholar] [CrossRef]
- Cain, C.K. Avoidance problems reconsidered. Curr. Opin. Behav. Sci. 2019, 26, 9–17. [Google Scholar] [CrossRef]
- LeDoux, J.E.; Moscarello, J.; Sears, R.; Campese, V. The birth, death and resurrection of avoidance: A reconceptualization of a troubled paradigm. Mol. Psychiatry 2016, 22, 24–36. [Google Scholar] [CrossRef]
- O’Malley, J.J.; Bruning, J.L. Aversive stimulation and reversal learning. Psychon. Sci. 1969, 15, 40. [Google Scholar] [CrossRef]
- Quirk, G.J.; Mueller, D. Neural Mechanisms of Extinction Learning and Retrieval. Neuropsychopharmacology 2008, 33, 56–72. [Google Scholar] [CrossRef]
- Trapp, S.; O’Doherty, J.P.; Schwabe, L. Stressful Events as Teaching Signals for the Brain. Trends Cogn. Sci. 2018, 22, 475–478. [Google Scholar] [CrossRef] [PubMed]
- Iordanova, M.D.; Yau, J.O.-Y.; McDannald, M.A.; Corbit, L.H. Neural substrates of appetitive and aversive prediction error. Neurosci. Biobehav. Rev. 2021, 123, 337–351. [Google Scholar] [CrossRef] [PubMed]
- Lammel, S.; Lim, B.K.; Malenka, R.C. Reward and aversion in a heterogeneous midbrain dopamine system. Neuropharmacology 2014, 76, 351–359. [Google Scholar] [CrossRef]
- Schultz, W. Dopamine reward prediction error coding. Dialog-Clin. Neurosci. 2016, 18, 23–32. [Google Scholar] [CrossRef]
- Peters, A.; McEwen, B.S.; Friston, K. Uncertainty and stress: Why it causes diseases and how it is mastered by the brain. Prog. Neurobiol. 2017, 156, 164–188. [Google Scholar] [CrossRef] [PubMed]
- Wenzel, J.M.; Rauscher, N.A.; Cheer, J.F.; Oleson, E.B. A Role for Phasic Dopamine Release within the Nucleus Accumbens in Encoding Aversion: A Review of the Neurochemical Literature. ACS Chem. Neurosci. 2015, 6, 16–26. [Google Scholar] [CrossRef]
- Hemmings, A.; McBride, S.; Hale, C. Perseverative responding and the aetiology of equine oral stereotypy. Appl. Anim. Behav. Sci. 2007, 104, 143–150. [Google Scholar] [CrossRef]
- Kirsty, R.; Andrew, H.; Meriel, M.-C.; Catherine, H. Cognitive differences in horses performing locomotor versus oral stereotypic behaviour. Appl. Anim. Behav. Sci. 2015, 168, 37–44. [Google Scholar] [CrossRef]
- Freymond, S.B.; Ruet, A.; Grivaz, M.; Fuentes, C.; Zuberbühler, K.; Bachmann, I.; Briefer, E.F. Stereotypic horses (Equus caballus) are not cognitively impaired. Anim. Cogn. 2019, 22, 17–33. [Google Scholar] [CrossRef]
- Fortin, M.; Valenchon, M.; Lévy, F.; Calandreau, L.; Arnould, C.; Lansade, L. Emotional state and personality influence cognitive flexibility in horses (Equus caballus). J. Comp. Psychol. 2018, 132, 130–140. [Google Scholar] [CrossRef]
- Lansade, L.; Marchand, A.R.; Coutureau, E.; Ballé, C.; Polli, F.; Calandreau, L. Personality and predisposition to form habit behaviours during instrumental conditioning in horses (Equus caballus). PLoS ONE 2017, 12, e0171010. [Google Scholar] [CrossRef] [PubMed]
- McGreevy, P.; McLean, A. Equitation Science; Wiley Blackwell: London, UK, 2010. [Google Scholar]
- Zhang, Y.; Shao, F.; Wang, Q.; Xie, X.; Wang, W. Neuroplastic Correlates in the mPFC Underlying the Impairment of Stress-Coping Ability and Cognitive Flexibility in Adult Rats Exposed to Chronic Mild Stress during Adolescence. Neural Plast. 2017, 2017, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Jett, J.D.; Bulin, S.E.; Hatherall, L.C.; McCartney, C.M.; Morilak, D.A. Deficits in cognitive flexibility induced by chronic unpredictable stress are associated with impaired glutamate neurotransmission in the rat medial prefrontal cortex. Neuroscience 2017, 346, 284–297. [Google Scholar] [CrossRef] [PubMed]
- Hermans, E.J.; Henckens, M.J.; Joels, M.; Fernández, G. Dynamic adaptation of large-scale brain networks in response to acute stressors. Trends Neurosci. 2014, 37, 304–314. [Google Scholar] [CrossRef] [PubMed]
- Shields, G.S.; Bonner, J.C.; Moons, W.G. Does cortisol influence core executive functions? A meta-analysis of acute cortisol administration effects on working memory, inhibition, and set-shifting. Psychoneuroendocrinology 2015, 58, 91–103. [Google Scholar] [CrossRef]
- Bondi, C.; Rodriguez, G.; Gould, G.; Frazer, A.; Morilak, D.A. Chronic Unpredictable Stress Induces a Cognitive Deficit and Anxiety-Like Behavior in Rats that is Prevented by Chronic Antidepressant Drug Treatment. Neuropsychopharmacology 2008, 33, 320–331. [Google Scholar] [CrossRef]
- Douma, E.H.; de Kloet, E.R. Stress-induced plasticity and functioning of ventral tegmental dopamine neurons. Neurosci. Biobehav. Rev. 2020, 108, 48–77. [Google Scholar] [CrossRef]
- Padalino, B.; Raidal, S.L.; Carter, N.; Celi, P.; Muscatello, G.; Jeffcott, L.; de Silva, K. Immunological, clinical, haematological and oxidative responses to long distance transportation in horses. Res. Vet. Sci. 2017, 115, 78–87. [Google Scholar] [CrossRef]
- Lesimple, C. Indicators of Horse Welfare: State-of-the-Art. Animals 2020, 10, 294. [Google Scholar] [CrossRef]
- Nogueira, G.P.; Barnabe, R.C. Is the Thoroughbred race-horse under chronic stress? Braz. J. Med. Biol. Res. 1997, 30, 1237–1239. [Google Scholar] [CrossRef]
- Alexander, S.L.; Irvine, C.H.G. The effect of social stress on adrenal axis activity in horses: The importance of monitoring corticosteroid-binding globulin capacity. J. Endocrinol. 1998, 157, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Cousillas, H.; Oger, M.; Rochais, C.; Pettoello, C.; Ménoret, M.; Henry, S.; Hausberger, M. An Ambulatory Electroencephalography System for Freely Moving Horses: An Innovating Approach. Front. Veter-Sci. 2017, 4, 57. [Google Scholar] [CrossRef] [PubMed]
- Mott, R.O.; Hawthorne, S.J.; McBride, S.D. Blink rate as a measure of stress and attention in the domestic horse (Equus caballus). Sci. Rep. 2020, 10, 21409. [Google Scholar] [CrossRef] [PubMed]
- Schwabe, L.; Wolf, O.T. Learning under stress impairs memory formation. Neurobiol. Learn. Mem. 2010, 93, 183–188. [Google Scholar] [CrossRef]
- Balleine, B.W.; O’Doherty, J.P. Human and Rodent Homologies in Action Control: Corticostriatal Determinants of Goal-Directed and Habitual Action. Neuropsychopharmacology 2010, 35, 48–69. [Google Scholar] [CrossRef]
- Peeters, M.; Sulon, J.; Beckers, J.-F.; LeDoux, D.; Vandenheede, M. Comparison between blood serum and salivary cortisol concentrations in horses using an adrenocorticotropic hormone challenge. Equine Vet. J. 2011, 43, 487–493. [Google Scholar] [CrossRef]
- Barman, S.M.; Yates, B. Deciphering the Neural Control of Sympathetic Nerve Activity: Status Report and Directions for Future Research. Front. Neurosci. 2017, 11, 730. [Google Scholar] [CrossRef]
- Kurosawa, M.; Nagata, S.-I.; Takeda, F.; Mima, K.; Hiraga, A.; Kai, M.; Taya, K. Plasma Catecholamine, Adrenocorticotropin and Cortisol Responses to Exhaustive Incremental Treadmill Exercise of the Thoroughbred Horse. J. Equine Sci. 1998, 9, 9–18. [Google Scholar] [CrossRef]
- Baragli, P.; Pacchini, S.; Gatta, D.; Ducci, M.; Sighieri, C. Brief note about plasma catecholamines kinetics and submaximal exercise in untrained standardbreds. Ann. Dell’istituto Super. Di Sanità 2010, 46, 96–100. [Google Scholar] [CrossRef]
- Hada, T.; Onaka, T.; Kusunose, R.; Yagi, K. Effects of Novel Environmental Stimuli on Neuroendocrine Activity in Thoroughbred Horses. J. Equine Sci. 2001, 12, 33–38. [Google Scholar] [CrossRef]
- Wascher, C.A.F. Heart rate as a measure of emotional arousal in evolutionary biology. Philos. Trans. R. Soc. B Biol. Sci. 2021, 376, 20200479. [Google Scholar] [CrossRef]
- Kongoun, S.; Chanda, M.; Piyachaturawat, P.; Saengsawang, W. Exercise increases brain-derived neurotrophic factor level in serum of horses. Livest. Sci. 2015, 180, 253–256. [Google Scholar] [CrossRef]
- Und, T.B.; Lessmann, V. BDNF: A regulator of learning and memory with clinical relevance. Neuroforum 2014, 20, 166–177. [Google Scholar]
- Halbach, O.V.B.U.; Halbach, V.V.B.U. BDNF effects on dendritic spine morphology and hippocampal function. Cell Tissue Res. 2018, 373, 729–741. [Google Scholar] [CrossRef] [PubMed]
- Zagrebelsky, M.; Tacke, C.; Korte, M. BDNF signaling during the lifetime of dendritic spines. Cell Tissue Res. 2020, 382, 185–199. [Google Scholar] [CrossRef] [PubMed]
- Bathina, S.; Das, U.N. Brain-derived neurotrophic factor and its clinical implications. Arch. Med. Sci. 2015, 11, 1164–1178. [Google Scholar] [CrossRef] [PubMed]
- Pluchino, N.; Russo, M.; Santoro, A.; Litta, P.; Cela, V.; Genazzani, A. Steroid hormones and BDNF. Neuroscience 2013, 239, 271–279. [Google Scholar] [CrossRef]
- Jeanneteau, F.; Borie, A.; Chao, M.V.; Garabedian, M.J. Bridging the Gap between Brain-Derived Neurotrophic Factor and Glucocorticoid Effects on Brain Networks. Neuroendocrinology 2019, 109, 277–284. [Google Scholar] [CrossRef]
- Linz, R.; Puhlmann, L.M.C.; Apostolakou, F.; Mantzou, E.; Papassotiriou, I.; Chrousos, G.P.; Engert, V.; Singer, T. Acute psychosocial stress increases serum BDNF levels: An antagonistic relation to cortisol but no group differences after mental training. Neuropsychopharmacology 2019, 44, 1797–1804. [Google Scholar] [CrossRef]
- Barfield, E.T.; Gerber, K.J.; Zimmermann, K.S.; Ressler, K.J.; Parsons, R.G.; Gourley, S.L. Regulation of actions and habits by ventral hippocampal trkB and adolescent corticosteroid exposure. PLoS Biol. 2017, 15, e2003000. [Google Scholar] [CrossRef]
- Klein, A.B.; Williamson, R.; Santini, M.A.; Clemmensen, C.; Ettrup, A.; Rios, M.; Knudsen, G.M.; Aznar, S. Blood BDNF concentrations reflect brain-tissue BDNF levels across species. Int. J. Neuropsychopharmacol. 2011, 14, 347–353. [Google Scholar] [CrossRef]
- Elfving, B.; Plougmann, P.H.; Wegener, G. Detection of brain-derived neurotrophic factor (BDNF) in rat blood and brain preparations using ELISA: Pitfalls and solutions. J. Neurosci. Methods 2010, 187, 73–77. [Google Scholar] [CrossRef] [PubMed]
- Piepmeier, A.T.; Etnier, J.L. Brain-derived neurotrophic factor (BDNF) as a potential mechanism of the effects of acute exercise on cognitive performance. J. Sport Health Sci. 2015, 4, 14–23. [Google Scholar] [CrossRef]
- McGreevy, P.D.; McLean, A.N. Punishment in horse-training and the concept of ethical equitation. J. Vet. Behav. Clin. Appl. Res. 2009, 4, 193–197. [Google Scholar] [CrossRef]
- Schmidt, A.; Möstl, E.; Wehnert, C.; Aurich, J.; Müller, J.; Aurich, C. Cortisol release and heart rate variability in horses during road transport. Horm. Behav. 2010, 57, 209–215. [Google Scholar] [CrossRef]
- Charmandari, E.; Tsigos, C.; Chrousos, G. Endocrinology of The Stress Response. Annu. Rev. Physiol. 2005, 67, 259–284. [Google Scholar] [CrossRef]
- Henshall, C.; Randle, H.; Francis, N.; Freire, R. The effect of stress and exercise on the learning performance of horses. Sci. Rep. 2022, 12, 1918. [Google Scholar] [CrossRef]
- Tovote, P.; Fadok, J.P.; Lüthi, A. Neuronal circuits for fear and anxiety. Nat. Rev. Neurosci. 2015, 16, 317–331. [Google Scholar] [CrossRef]
- Deng, H.; Xiao, X.; Wang, Z. Periaqueductal Gray Neuronal Activities Underlie Different Aspects of Defensive Behaviors. J. Neurosci. 2016, 36, 7580–7588. [Google Scholar] [CrossRef]
- Arnsten, A.F.T. Stress weakens prefrontal networks: Molecular insults to higher cognition. Nat. Neurosci. 2015, 18, 1376–1385. [Google Scholar] [CrossRef]
- Maier, S.F.; Seligman, M.E.P. Learned helplessness at fifty: Insights from neuroscience. Psychol. Rev. 2016, 123, 349–367. [Google Scholar] [CrossRef]
- Fernández-Teruel, A.; Tobeña, A. Revisiting the role of anxiety in the initial acquisition of two-way active avoidance: Pharmacological, behavioural and neuroanatomical convergence. Neurosci. Biobehav. Rev. 2020, 118, 739–758. [Google Scholar] [CrossRef] [PubMed]
- Ahrendt, L.P.; Labouriau, R.; Malmkvist, J.; Nicol, C.; Christensen, J.W. Development of a standard test to assess negative reinforcement learning in horses. Appl. Anim. Behav. Sci. 2015, 169, 38–42. [Google Scholar] [CrossRef]
- Fenner, K.; Webb, H.; Starling, M.J.; Freire, R.; Buckley, P.; McGreevy, P. Effects of pre-conditioning on behavior and physiology of horses during a standardised learning task. PLoS ONE 2017, 12, e0174313. [Google Scholar] [CrossRef]
- Byström, A.; Clayton, H.; Hernlund, E.; Rhodin, M.; Egenvall, A. Equestrian and biomechanical perspectives on laterality in the horse. Comp. Exerc. Physiol. 2020, 16, 35–45. [Google Scholar] [CrossRef]
- Roozendaal, B.; Okuda, S.; Van der Zee, E.A.; McGaugh, J.L. Glucocorticoid enhancement of memory requires arousal-induced noradrenergic activation in the basolateral amygdala. Proc. Natl. Acad. Sci. USA 2006, 103, 6741–6746. [Google Scholar] [CrossRef] [PubMed]
- Snow, D.H.; Harris, R.C.; Macdonald, I.A.; Forster, C.D.; Marlin, D.J. Effects of high-intensity exercise on plasma catecholamines in the Thoroughbred horse. Equine Vet. J. 1992, 24, 462–467. [Google Scholar] [CrossRef] [PubMed]
- Gruber, A.J.; McDonald, R.J. Context, emotion, and the strategic pursuit of goals: Interactions among multiple brain systems controlling motivated behavior. Front. Behav. Neurosci. 2012, 6, 50. [Google Scholar] [CrossRef]
- Schultz, W. Updating dopamine reward signals. Curr. Opin. Neurobiol. 2013, 23, 229–238. [Google Scholar] [CrossRef]
- Evans, D.A.; Stempel, A.V.; Vale, R.; Ruehle, S.; Lefler, Y.; Branco, T. A synaptic threshold mechanism for computing escape decisions. Nature 2018, 558, 590–594. [Google Scholar] [CrossRef]
- de Jong, J.W.; Afjei, S.A.; Dorocic, I.P.; Peck, J.R.; Liu, C.; Kim, C.K.; Tian, L.; Deisseroth, K.; Lammel, S. A Neural Circuit Mechanism for Encoding Aversive Stimuli in the Mesolimbic Dopamine System. Neuron 2019, 101, 133–151.e7. [Google Scholar] [CrossRef]
- Oleson, E.B.; Gentry, R.N.; Chioma, V.C.; Cheer, J. Subsecond Dopamine Release in the Nucleus Accumbens Predicts Conditioned Punishment and Its Successful Avoidance. J. Neurosci. 2012, 32, 14804–14808. [Google Scholar] [CrossRef] [PubMed]
- Gentry, R.N.; Schuweiler, D.R.; Roesch, M.R. Dopamine signals related to appetitive and aversive events in paradigms that manipulate reward and avoidability. Brain Res. 2019, 1713, 80–90. [Google Scholar] [CrossRef] [PubMed]
- Stelly, C.E.; Haug, G.C.; Fonzi, K.M.; Garcia, M.A.; Tritley, S.C.; Magnon, A.P.; Ramos, M.A.P.; Wanat, M.J. Pattern of dopamine signaling during aversive events predicts active avoidance learning. Proc. Natl. Acad. Sci. USA 2019, 116, 13641–13650. [Google Scholar] [CrossRef] [PubMed]
- Pultorak, K.J.; Schelp, S.; Isaacs, D.P.; Krzystyniak, G.; Oleson, E.B. A Transient Dopamine Signal Represents Avoidance Value and Causally Influences the Demand to Avoid. Eneuro 2018, 1–18. [Google Scholar] [CrossRef]
- Wendler, E.; Gaspar, J.C.; Ferreira, T.L.; Barbiero, J.K.; Andreatini, R.; Vital, M.A.; Blaha, C.D.; Winn, P.; Da Cunha, C. The roles of the nucleus accumbens core, dorsomedial striatum, and dorsolateral striatum in learning: Performance and extinction of Pavlovian fear-conditioned responses and instrumental avoidance responses. Neurobiol. Learn. Mem. 2014, 109, 27–36. [Google Scholar] [CrossRef]
- Hulme, S.R.; Jones, O.D.; Abraham, W.C. Emerging roles of metaplasticity in behaviour and disease. Trends Neurosci. 2013, 36, 353–362. [Google Scholar] [CrossRef]
- Parsons, R.G. Behavioral and neural mechanisms by which prior experience impacts subsequent learning. Neurobiol. Learn. Mem. 2018, 154, 22–29. [Google Scholar] [CrossRef]
- Bailey, C.H.; Kandel, E.R.; Harris, K. Structural Components of Synaptic Plasticity and Memory Consolidation. Cold Spring Harb. Perspect. Biol. 2015, 7, a021758. [Google Scholar] [CrossRef]
- Joëls, M.; Pasricha, N.; Karst, H. The interplay between rapid and slow corticosteroid actions in brain. Eur. J. Pharmacol. 2013, 719, 44–52. [Google Scholar] [CrossRef]
- Quaedflieg, C.W.E.M.; Schwabe, L. Memory dynamics under stress. Memory 2018, 26, 364–376. [Google Scholar] [CrossRef]
- Conrad, C.D. A critical review of chronic stress effects on spatial learning and memory. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2010, 34, 742–755. [Google Scholar] [CrossRef] [PubMed]
- Dias-Ferreira, E.; Sousa, J.C.; Melo, I.; Morgado, P.; Mesquita, A.R.; Cerqueira, J.J.; Costa, R.M.; Sousa, N. Chronic Stress Causes Frontostriatal Reorganization and Affects Decision-Making. Science 2009, 325, 621–625. [Google Scholar] [CrossRef] [PubMed]
- Taylor, S.; Anglin, J.; Paode, P.; Riggert, A.; Olive, F.; Conrad, C. Chronic stress may facilitate the recruitment of habit- and addiction-related neurocircuitries through neuronal restructuring of the striatum. Neuroscience 2014, 280, 231–242. [Google Scholar] [CrossRef] [PubMed]
- Cockrem, J.F. Individual variation in glucocorticoid stress responses in animals. Gen. Comp. Endocrinol. 2013, 181, 45–58. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Morán, S.; Palència, M.; Mont-Cardona, C.; Cañete, T.; Blázquez, G.; Martínez-Membrives, E.; López-Aumatell, R.; Tobeña, A.; Fernández-Teruel, A. Coping style and stress hormone responses in genetically heterogeneous rats: Comparison with the Roman rat strains. Behav. Brain Res. 2012, 228, 203–210. [Google Scholar] [CrossRef]
- Smeets, T.; van Ruitenbeek, P.; Hartogsveld, B.; Quaedflieg, C.W. Stress-induced reliance on habitual behavior is moderated by cortisol reactivity. Brain Cogn. 2019, 133, 60–71. [Google Scholar] [CrossRef]
- Sauer, F.J.; Hermann, M.; Ramseyer, A.; Burger, D.; Riemer, S.; Gerber, V. Effects of breed, management and personality on cortisol reactivity in sport horses. PLoS ONE 2019, 14, e0221794. [Google Scholar] [CrossRef]
- Valenchon, M.; Lévy, F.; Moussu, C.; Lansade, L. Stress affects instrumental learning based on positive or negative reinforcement in interaction with personality in domestic horses. PLoS ONE 2017, 12, e0170783. [Google Scholar] [CrossRef]
- Valenchon, M.; Lévy, F.; Prunier, A.; Moussu, C.; Calandreau, L.; Lansade, L. Stress Modulates Instrumental Learning Performances in Horses (Equus caballus) in Interaction with Temperament. PLoS ONE 2013, 8, e62324. [Google Scholar] [CrossRef]
- Lakshminarasimhan, H.; Chattarji, S. Stress Leads to Contrasting Effects on the Levels of Brain Derived Neurotrophic Factor in the Hippocampus and Amygdala. PLoS ONE 2012, 7, e30481. [Google Scholar] [CrossRef]
- Bennett, M.; Lagopoulos, J. Stress and trauma: BDNF control of dendritic-spine formation and regression. Prog. Neurobiol. 2014, 112, 80–99. [Google Scholar] [CrossRef] [PubMed]
- Jeanneteau, F.; Chao, M. Are BDNF and glucocorticoid activities calibrated? Neuroscience 2013, 239, 173–195. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, P.; Brassard, P.; Adser, H.; Pedersen, M.V.; Leick, L.; Hart, E.; Secher, N.H.; Pedersen, B.K.; Pilegaard, H. Evidence for a release of brain-derived neurotrophic factor from the brain during exercise. Exp. Physiol. 2009, 94, 1062–1069. [Google Scholar] [CrossRef] [PubMed]
- Walsh, J.J.; Tschakovsky, M.E. Exercise and circulating BDNF: Mechanisms of release and implications for the design of exercise interventions. Appl. Physiol. Nutr. Metab. 2018, 43, 1095–1104. [Google Scholar] [CrossRef] [PubMed]
- Conrad, C.D.; Ortiz, J.B.; Judd, J.M. Chronic stress and hippocampal dendritic complexity: Methodological and functional considerations. Physiol. Behav. 2017, 178, 66–81. [Google Scholar] [CrossRef]
- Vyas, A.; Mitra, R.; Rao, B.S.S.; Chattarji, S. Chronic Stress Induces Contrasting Patterns of Dendritic Remodeling in Hippocampal and Amygdaloid Neurons. J. Neurosci. 2002, 22, 6810–6818. [Google Scholar] [CrossRef]
- Maroun, M.; Ioannides, P.J.; Bergman, K.L.; Kavushansky, A.; Holmes, A.; Wellman, C.L. Fear extinction deficits following acute stress associate with increased spine density and dendritic retraction in basolateral amygdala neurons. Eur. J. Neurosci. 2013, 38, 2611–2620. [Google Scholar] [CrossRef]


| Horse | Sex | Age | Breed | Original Task-Type Group | Treatment Group |
|---|---|---|---|---|---|
| 1 | Mare | 7 | Thoroughbred | Instinctual | Stress |
| 2 | Mare | 7 | Thoroughbred | Non-Instinctual | Control |
| 3 | Mare | 14 | Thoroughbred x Riding pony | Instinctual | Control |
| 4 | Mare | 17 | Quarter Horse | Non-Instinctual | Stress |
| 5 | Gelding | 13 | Welsh Pony | Instinctual | Stress |
| 6 | Gelding | 16 | Australian Stockhorse | Non-Instinctual | Control |
| 7 | Mare | 9 | Welsh pony | Instinctual | Control |
| 8 | Mare | 13 | Welsh Pony | Non-Instinctual | Stress |
| 9 | Mare | 7 | Thoroughbred | Instinctual | Control |
| 10 | Gelding | 11 | Pony | Non-Instinctual | Stress |
| 11 | Mare | 14 | Australian Stock Horse | Instinctual | Stress |
| 12 | Gelding | 17 | Paint/Quarter Horse | Non-Instinctual | Control |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Henshall, C.; Randle, H.; Francis, N.; Freire, R. Habit Formation and the Effect of Repeated Stress Exposures on Cognitive Flexibility Learning in Horses. Animals 2022, 12, 2818. https://doi.org/10.3390/ani12202818
Henshall C, Randle H, Francis N, Freire R. Habit Formation and the Effect of Repeated Stress Exposures on Cognitive Flexibility Learning in Horses. Animals. 2022; 12(20):2818. https://doi.org/10.3390/ani12202818
Chicago/Turabian StyleHenshall, Cathrynne, Hayley Randle, Nidhish Francis, and Rafael Freire. 2022. "Habit Formation and the Effect of Repeated Stress Exposures on Cognitive Flexibility Learning in Horses" Animals 12, no. 20: 2818. https://doi.org/10.3390/ani12202818
APA StyleHenshall, C., Randle, H., Francis, N., & Freire, R. (2022). Habit Formation and the Effect of Repeated Stress Exposures on Cognitive Flexibility Learning in Horses. Animals, 12(20), 2818. https://doi.org/10.3390/ani12202818






