Effects of Dietary Supplementation with Aurantiochytrium sp. on Zebrafish Growth as Determined by Transcriptomics
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Source and Diet Preparation
2.2. Zebrafish Treatment and Sample Collection
2.3. Detection of Liver Biochemical Indicators
2.4. RNA-Seq and Bioinformatics Analysis
2.5. Real-Time Quantitative PCR (RT-qPCR) Validation
2.6. Calculation of Growth Performance and Statistical Analysis
3. Results
3.1. Effect of Dietary Aurantiochytrium sp. Crude Extract and Single Extract on Growth Performance in Zebrafish
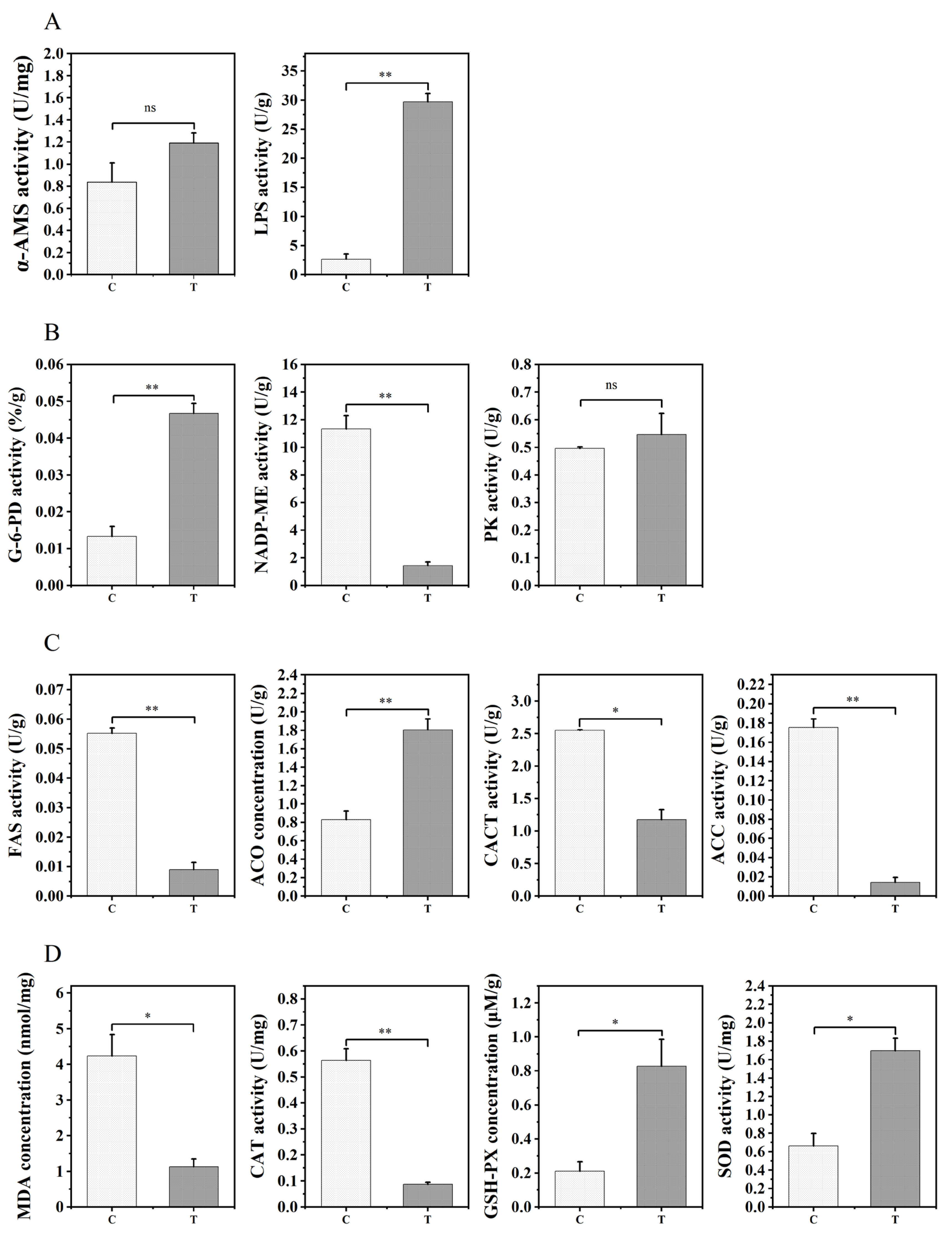
3.2. Effects of 1% Aurantiochytrium sp. Extract on Biochemical Indicators in the Zebrafish Liver
3.3. RNA-Seq of the Liver
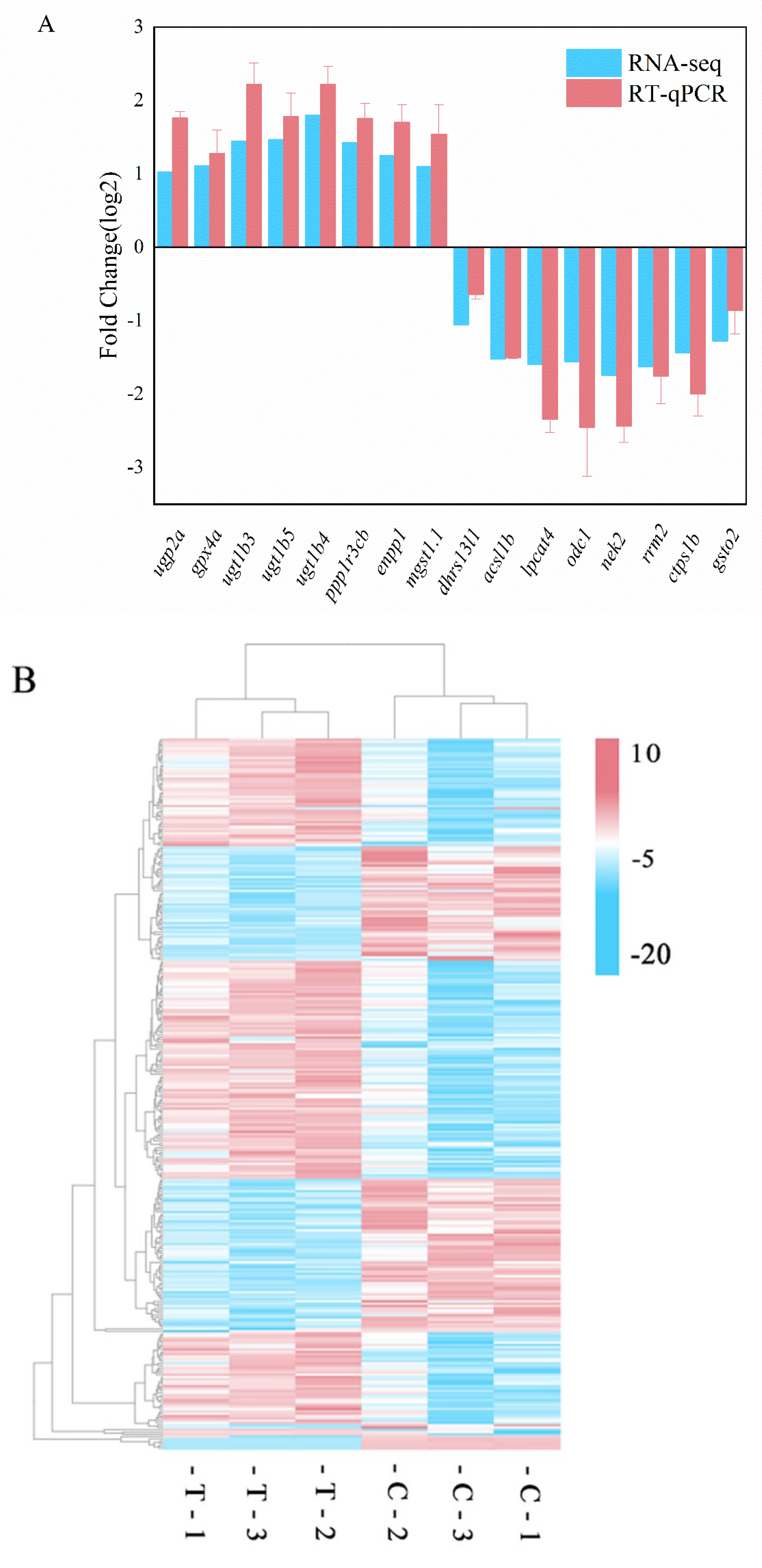
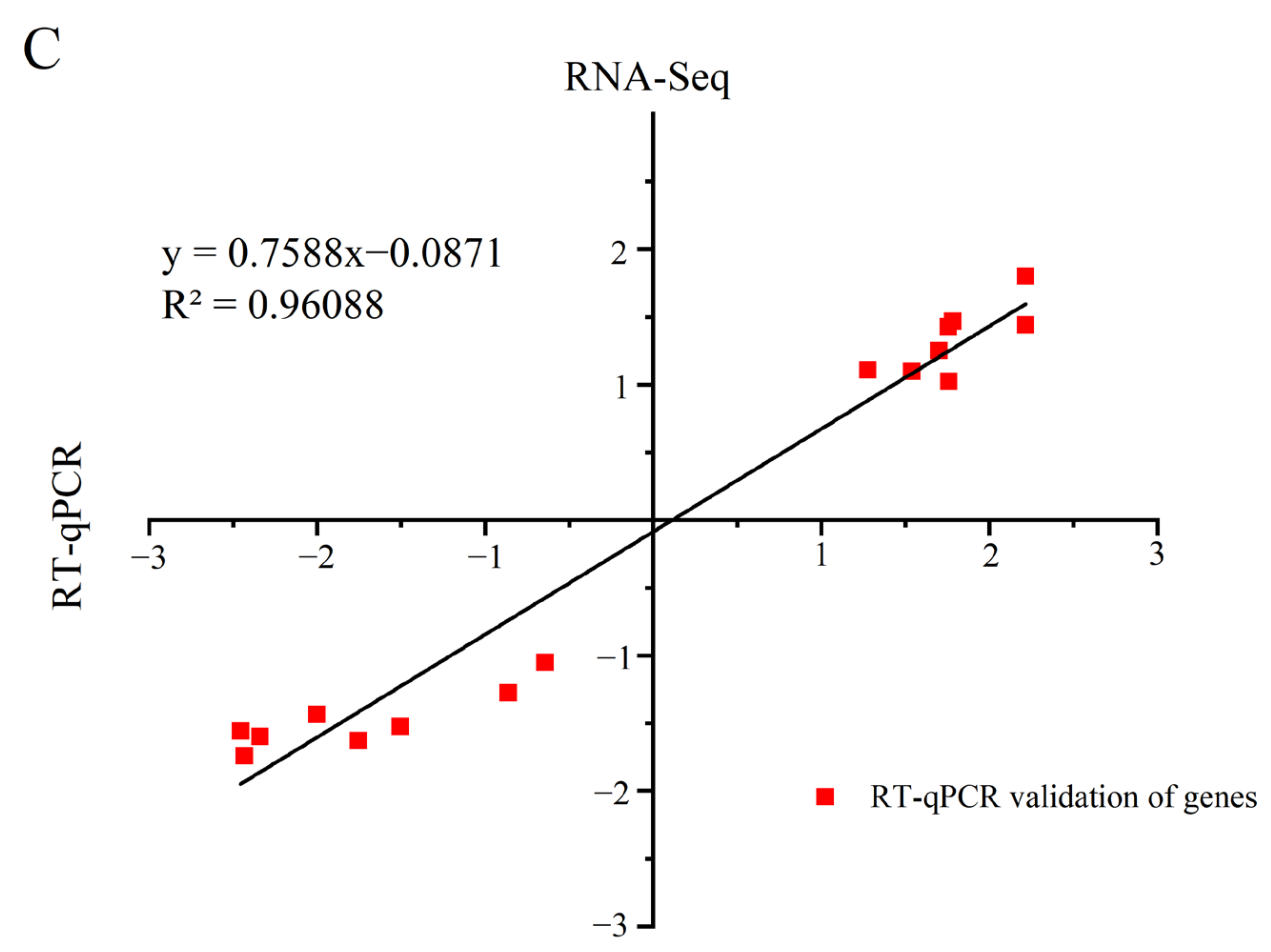
3.4. Differential Gene Expression Analysis
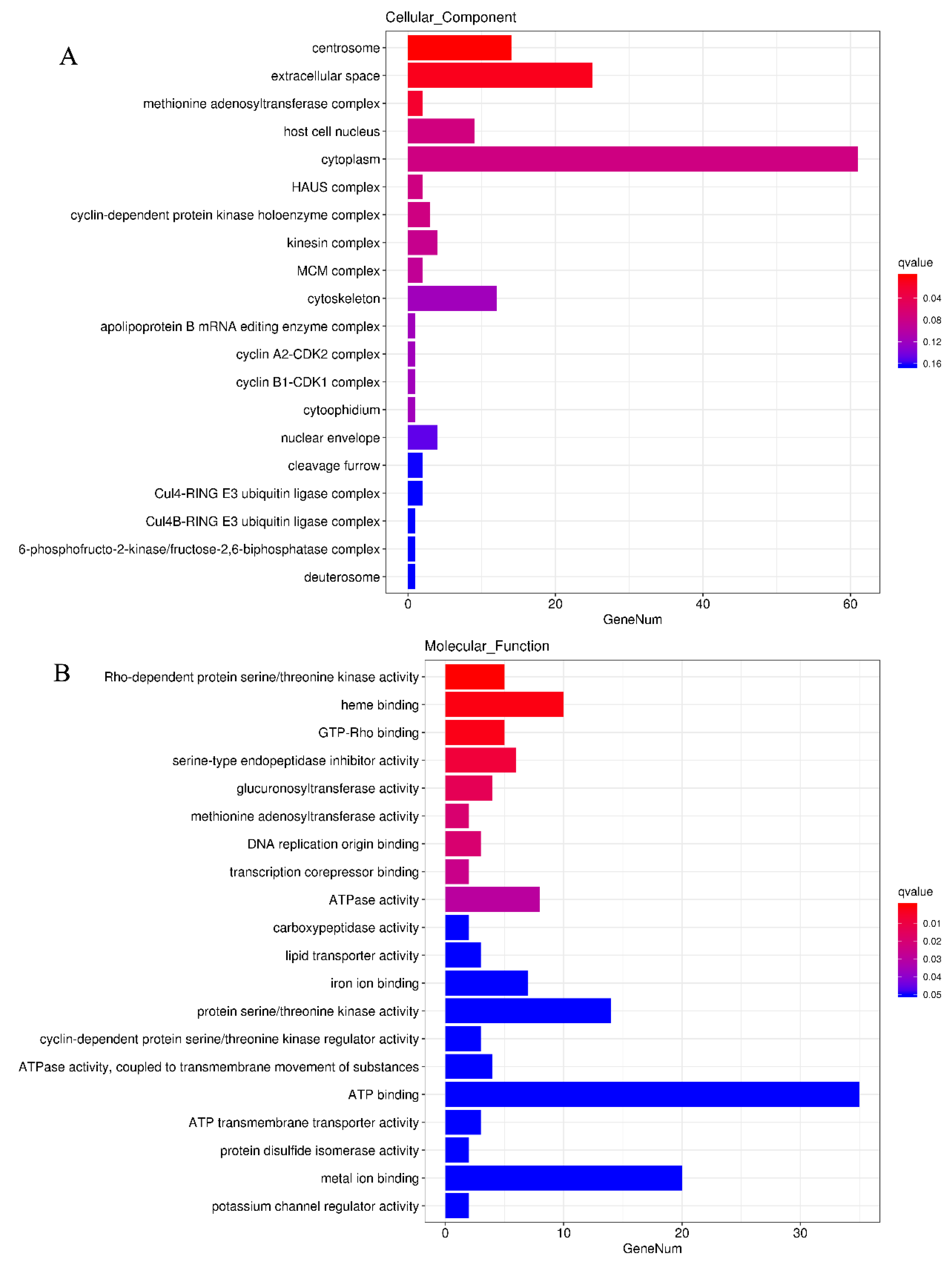
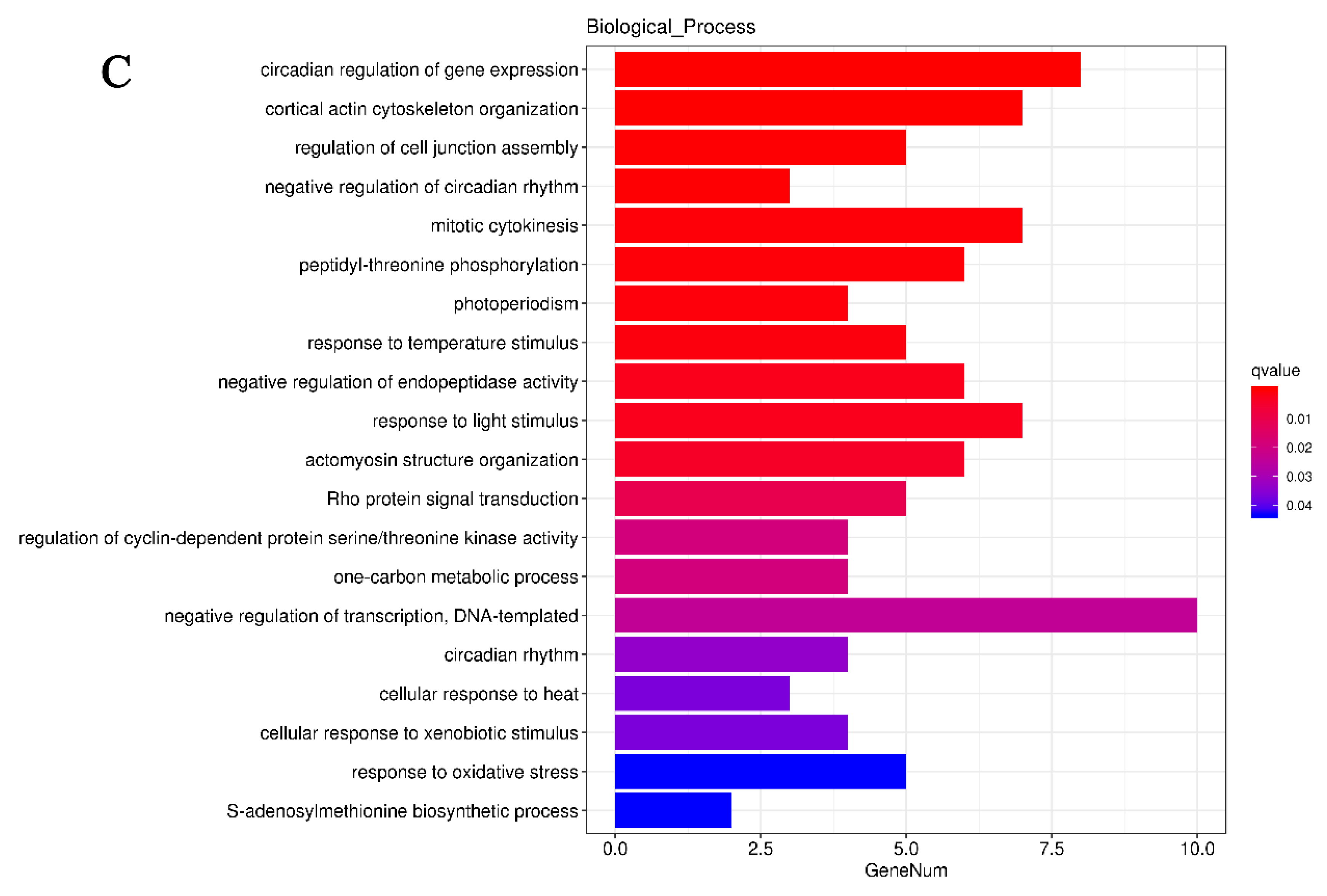
3.5. GO and KEGG Pathway Enrichment Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Naylor, R.L.; Hardy, R.W.; Buschmann, A.H.; Bush, S.R.; Cao, L.; Klinger, D.H.; Little, D.C.; Lubchenco, J.; Shumway, S.E.; Troell, M. A 20-year retrospective review of global aquaculture. Nature 2021, 591, 551–563. [Google Scholar] [CrossRef] [PubMed]
- Tocher, D.R. Omega-3 long-chain polyunsaturated fatty acids and aquaculture in perspective. Aquaculture 2015, 449, 94–107. [Google Scholar] [CrossRef]
- Steffens, W.; Wirth, M. Freshwater fish-an important source of n-3 polyunsaturated fatty acids: A review. Fish. Aquat. Life 2005, 13, 5–16. [Google Scholar]
- Wang, S.; Monroig, O.; Tang, G.; Zhang, L.; You, C.; Tocher, D.R.; Li, Y. Investigating long-chain polyunsaturated fatty acid biosynthesis in teleost fish: Functional characterization of fatty acyl desaturase (Fads2) and Elovl5 elongase in the catadromous species, Japanese eel Anguilla japonica. Aquaculture 2014, 434, 57–65. [Google Scholar] [CrossRef]
- Abedi, E.; Sahari, M.A. Long-chain polyunsaturated fatty acid sources and evaluation of their nutritional and functional properties. Food Sci. Nutr. 2014, 2, 443–463. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, K.; Arafiles, K.H.V.; Higashi, R.; Okamura, Y.; Tajima, T.; Matsumura, Y.; Nakashimada, Y.; Matsuyama, K.; Aki, T. Isolation of high carotenoid-producing Aurantiochytrium sp. mutants and improvement of astaxanthin productivity using metabolic information. J. Oleo Sci. 2018, 67, 571–578. [Google Scholar] [CrossRef] [PubMed]
- Iwasaka, H.; Koyanagi, R.; Satoh, R.; Nagano, A.; Watanabe, K.; Hisata, K.; Satoh, N.; Aki, T. A possible trifunctional β-carotene synthase gene identified in the draft genome of Aurantiochytrium sp. strain KH105. Genes 2018, 9, 200. [Google Scholar] [CrossRef] [PubMed]
- Kaya, K.; Nakazawa, A.; Matsuura, H.; Honda, D.; Inouye, I.; Watanabe, M.M. Thraustochytrid Aurantiochytrium sp. 18W-13a accummulates high amounts of squalene. Biosci. Biotechnol. Biochem. 2011, 75, 2246–2248. [Google Scholar] [CrossRef]
- Chen, G.; Fan, K.-W.; Lu, F.-P.; Li, Q.; Aki, T.; Chen, F.; Jiang, Y. Optimization of nitrogen source for enhanced production of squalene from thraustochytrid Aurantiochytrium sp. New Biotechnol. 2010, 27, 382–389. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, M.; Ioki, M.; Matsuura, H.; Hashimoto, A.; Kaya, K.; Nakajima, N.; Watanabe, M.M. Diverse steroidogenic pathways in the marine alga Aurantiochytrium. J. Appl. Phycol. 2020, 32, 1631–1642. [Google Scholar] [CrossRef]
- Bagul, V.P.; Annapure, U.S. Isolation and characterization of docosahexaenoic acid-producing novel strain Aurantiochytrium sp. ICTFD5: A sterol with vitamin d-cholecalciferol, and cellulase and lipase producing thraustochytrid. Bioresour. Technol. Rep. 2021, 14, 100688. [Google Scholar] [CrossRef]
- Du, F.; Wang, Y.-Z.; Xu, Y.-S.; Shi, T.-Q.; Liu, W.-Z.; Sun, X.-M.; Huang, H. Biotechnological production of lipid and terpenoid from thraustochytrids. Biotechnol. Adv. 2021, 48, 107725. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Sen, B.; Wang, G. Mining terpenoids production and biosynthetic pathway in thraustochytrids. Bioresour. Technol. 2017, 244, 1269–1280. [Google Scholar] [CrossRef] [PubMed]
- Aasen, I.M.; Ertesvåg, H.; Heggeset, T.M.B.; Liu, B.; Brautaset, T.; Vadstein, O.; Ellingsen, T.E. Thraustochytrids as production organisms for docosahexaenoic acid (DHA), squalene, and carotenoids. Appl. Microbiol. Biotechnol. 2016, 100, 4309–4321. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.-J.; Mo, K.-Q.; Ren, L.-J.; Li, G.-L.; Huang, J.-Z.; Huang, H. Genome sequence of Schizochytrium sp. CCTCC M209059, an effective producer of docosahexaenoic acid-rich lipids. Genome Announc. 2015, 3, e00819-15. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Barrow, C.J.; Puri, M. Omega-3 biotechnology: Thraustochytrids as a novel source of omega-3 oils. Biotechnol. Adv. 2012, 30, 1733–1745. [Google Scholar] [CrossRef]
- Nobrega, R.O.; Batista, R.O.; Corrêa, C.F.; Mattioni, B.; Filer, K.; Pettigrew, J.E.; Fracalossi, D.M. Dietary supplementation of Aurantiochytrium sp. meal, a docosahexaenoic-acid source, promotes growth of Nile tilapia at a suboptimal low temperature. Aquaculture 2019, 507, 500–509. [Google Scholar] [CrossRef]
- Kousoulaki, K.; Berge, G.M.; Turid, M.; Aleksei, K.; Grete, B.; Trine, Y.; Mats, C.; John, S.; Bente, R. Microalgal Schizochytrium limacinum Biomass Improves Growth and Filet Quality When Used Long-Term as a Replacement for Fish Oil, in Modern Salmon Diets. Front. Mar. Sci. 2020, 7, 57. [Google Scholar] [CrossRef]
- Sprague, M.; Walton, J.; Campbell, P.J.; Strachan, F.; Dick, J.R.; Bell, J.G. Replacement of fish oil with a DHA-rich algal meal derived from Schizochytrium sp. on the fatty acid and persistent organic pollutant levels in diets and flesh of Atlantic salmon (Salmo salar, L.) post-smolts. Food Chem. 2015, 185, 413–421. [Google Scholar] [CrossRef]
- Shi, Y.; Cao, X.; Ye, Z.; Xu, Y.; Wang, Y.; Li, Z.; Hang, W.; He, N. Role of dietary Schizochytrium sp. in improving disease resistance of zebrafish through metabolic and microbial analysis. Aquaculture 2021, 539, 736631. [Google Scholar] [CrossRef]
- Fukada, H.; Kitagima, R.; Shinagawa, J.; Morino, H.; Masumoto, T. Effects of complete replacement of fish oil with plant oil mixtures and algal meal on growth performance and fatty acid composition in juvenile yellowtail Seriola quinqueradiata. Fisheries Science 2020, 86, 107–118. [Google Scholar] [CrossRef]
- Kumar, V.; Habte-Tsion, H.M.; Allen, K.M.; Bowman, B.A.; Thompson, K.R.; El-Haroun, E.; Filer, K.; Tidwell, J.H. Replacement of fish oil with Schizochytrium meal and its impacts on the growth and lipid metabolism of Pacific white shrimp (Litopenaeus vannamei). Aquac. Nutr. 2018, 24, 1769–1781. [Google Scholar] [CrossRef]
- Osmond, A.T.; Arts, M.T.; Hall, J.R.; Rise, M.L.; Bazinet, R.P.; Armenta, R.E.; Colombo, S.M. Schizochytrium sp. (T18) Oil as a Fish Oil Replacement in Diets for Juvenile Rainbow Trout (Oncorhynchus mykiss): Effects on Growth Performance, Tissue Fatty Acid Content, and Lipid-Related Transcript Expression. Animals 2021, 11, 1185. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wang, B.; Liu, L.; Song, Y.; Lv, C.; Zhu, X.; Luo, Y.; Cheng, C.H.; Chen, H.; Yang, X. Enhanced Growth Performance Physiological and Biochemical Indexes of Trachinotus ovatus Fed With Marine Microalgae Aurantiochytrium sp. Rich in n-3 Polyunsaturated Fatty Acids. Front. Mar. Sci. 2021, 7, 609837. [Google Scholar] [CrossRef]
- Nguyen, P.; Leray, V.; Diez, M.; Serisier, S.; Bloc’h, J.L.; Siliart, B.; Dumon, H. Liver lipid metabolism. J. Anim. Physiol. Anim. Nutr. 2008, 92, 272–283. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Chen, H.-M.; Qian, X.-Q.; Gui, J.-F. Transcriptome analysis of grass carp (Ctenopharyngodon idella) between fast-and slow-growing fish. Comp. Biochem. Physiol. Part D Genom. Proteom. 2020, 35, 100688. [Google Scholar] [CrossRef]
- Caballero-Solares, A.; Xue, X.; Parrish, C.C.; Foroutani, M.B.; Taylor, R.G.; Rise, M.L. Changes in the liver transcriptome of farmed Atlantic salmon (Salmo salar) fed experimental diets based on terrestrial alternatives to fish meal and fish oil. BMC Genom. 2018, 19, 796. [Google Scholar] [CrossRef]
- Liu, L.; Hu, Z.; Li, S.; Yang, H.; Li, S.; Lv, C.; Zaynab, M.; Cheng, C.H.K.; Chen, H.; Yang, X. Comparative Transcriptomic Analysis Uncovers Genes Responsible for the DHA Enhancement in the Mutant Aurantiochytrium sp. Microorganisms 2020, 8, 529. [Google Scholar] [CrossRef]
- Li, B.; Dewey, C.N. RSEM: Accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinform. 2011, 12, 323. [Google Scholar] [CrossRef]
- Young, M.D.; Wakefield, M.J.; Smyth, G.K.; Oshlack, A. Gene ontology analysis for RNA-seq: Accounting for selection bias. Genome Biol. 2010, 11, R14. [Google Scholar] [CrossRef]
- Mao, X.; Cai, T.; Olyarchuk, J.G.; Wei, L. Automated genome annotation and pathway identification using the KEGG Orthology (KO) as a controlled vocabulary. Bioinformatics 2005, 21, 3787–3793. [Google Scholar] [CrossRef] [PubMed]
- Tang, R.; Dodd, A.; Lai, D.; McNabb, W.C.; Love, D.R. Validation of zebrafish (Danio rerio) reference genes for quantitative real-time RT-PCR normalization. Acta Biochim. Biophys. Sin. 2007, 39, 384–390. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2 −ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Li, M.H.; Robinson, E.H.; Tucker, C.S.; Manning, B.B.; Khoo, L. Effects of dried algae Schizochytrium sp., a rich source of docosahexaenoic acid, on growth, fatty acid composition, and sensory quality of channel catfish Ictalurus punctatus. Aquaculture 2009, 292, 232–236. [Google Scholar] [CrossRef]
- Xie, J.; Fang, H.; Liao, S.; Guo, T.; Yin, P.; Liu, Y.; Tian, L.; Niu, J. Study on Schizochytrium sp. improving the growth performance and non-specific immunity of golden pompano (Trachinotus ovatus) while not affecting the antioxidant capacity. Fish Shellfish Immunol. 2019, 95, 617–623. [Google Scholar] [CrossRef]
- Thongprajukaew, K.; Kovitvadhi, U.; Kovitvadhi, S.; Engkagul, A.; Rungruangsak-Torrissen, K. Evaluation of growth performance and nutritional quality of diets using digestive enzyme markers and in vitro digestibility in Siamese fighting fish (Betta splendens Regan, 1910). Afr. J. Biotechnol. 2013, 12, 6400–6410. [Google Scholar] [CrossRef][Green Version]
- Ciftci, M.; Turkoglu, V.; Coban, T.A. Effects of some drugs on hepatic glucose 6-phosphate dehydrogenase activity in Lake Van Fish (Chalcalburnus Tarischii Pallas, 1811). J. Hazard. Mater. 2007, 143, 415–418. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, Z.; Hoshino, A.; Zheng, H.D.; Morley, M.; Arany, Z.; Rabinowitz, J.D. NADPH production by the oxidative pentose-phosphate pathway supports folate metabolism. Nat. Metab. 2019, 1, 404–415. [Google Scholar] [CrossRef]
- Xiong, Q.; Feng, D.; Wang, Z.; Ying, Y.; Xu, C.; Wei, Q.; Zeng, S.; Yang, L. Fatty Acid Synthase Is the Key Regulator of Fatty Acid Metabolism and Is Related to Immunotherapy in Bladder Cancer. Front. Immunol. 2022, 13, 836939. [Google Scholar] [CrossRef]
- Lu, K.-L.; Xu, W.-N.; Wang, L.-N.; Zhang, D.-D.; Zhang, C.-N.; Liu, W.-B. Hepatic β-oxidation and regulation of carnitine palmitoyltransferase (CPT) I in blunt snout bream Megalobrama amblycephala fed a high fat diet. PLoS ONE 2014, 9, e93135. [Google Scholar] [CrossRef]
- Pang, Y.; Xu, X.; Xiang, X.; Li, Y.; Zhao, Z.; Li, J.; Gao, S.; Liu, Q.; Mai, K.; Ai, Q. High Fat Activates O-GlcNAcylation and Affects AMPK/ACC Pathway to Regulate Lipid Metabolism. Nutrients 2021, 13, 1740. [Google Scholar] [CrossRef] [PubMed]
- Morais, S.; Knoll-Gellida, A.; André, M.; Barthe, C.; Babin, P.J. Conserved expression of alternative splicing variants of peroxisomal acyl-CoA oxidase 1 in vertebrates and developmental and nutritional regulation in fish. Physiol. Genom. 2007, 28, 239–252. [Google Scholar] [CrossRef] [PubMed]
- Moore, K.; Roberts, L.J. Measurement of lipid peroxidation. Free Radic. Res. 1998, 28, 659–671. [Google Scholar] [CrossRef] [PubMed]
- Xiao, F.; Xing, J.; Li, H.; Xu, X.; Hu, Z.; Ji, H. Effects of the defatted Schizochytrium sp. on growth performance, fatty acid composition, histomorphology and antioxidant status of juvenile mirror carp (Cyprinus carpio var. specularis). Aquac. Res. 2021, 52, 3062–3076. [Google Scholar] [CrossRef]
- Tsuduki, T.; Honma, T.; Nakagawa, K.; Ikeda, I.; Miyazawa, T. Long-term intake of fish oil increases oxidative stress and decreases lifespan in senescence-accelerated mice. Nutrition 2011, 27, 334–337. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Lu, T.; Yan, J.; Li, J.; Zhou, H.; Pan, X.; Lu, Y.; He, N.; Ling, X. Regulation of polyunsaturated fatty acids synthesis by enhancing carotenoid-mediated endogenous antioxidant capacity in Schizochytrium sp. Algal Res. 2021, 55, 102238. [Google Scholar] [CrossRef]
- Qian, X.; Ba, Y.; Zhuang, Q.; Zhong, G. RNA-Seq technology and its application in fish transcriptomics. Omics J. Integr. Biol. 2014, 18, 98–110. [Google Scholar] [CrossRef]
- Calviello, G.; Serini, S.; Palozza, P. n-3 polyunsaturated fatty acids as signal transduction modulators and therapeutical agents in cancer. Curr. Signal Transduct. Ther. 2006, 1, 255–271. [Google Scholar] [CrossRef]
- Glauser, D.A.; Schlegel, W. The emerging role of FOXO transcription factors in pancreatic β cells. J Endocrinol. 2007, 193, 195–207. [Google Scholar] [CrossRef]
- Chen, Y.-J.; Chen, C.-C.; Li, T.-K.; Wang, P.-H.; Liu, L.-R.; Chang, F.-Y.; Wang, Y.-C.; Yu, Y.-H.; Lin, S.-P.; Mersmann, H.J.; et al. Docosahexaenoic acid suppresses the expression of FoxO and its target genes. J Nutr Biochem. 2012, 23, 1609–1616. [Google Scholar] [CrossRef]
- Maiorino, M.; Thomas, J.P.; Girotti, A.W.; Ursini, F. Reactivity of phospholipid hydroperoxide glutathione peroxidase with membrane and lipoprotein lipid hydroperoxides. Free Radic Res Commun. 1991, 12, 131–135. [Google Scholar] [CrossRef] [PubMed]
- Pignatti, C.; Tantini, B.; Stefanelli, C.; Flamigni, F. Signal transduction pathways linking polyamines to apoptosis. Amino Acids 2004, 27, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Webster, D.M.; Teo, C.F.; Sun, Y.; Wloga, D.; Gay, S.; Klonowski, K.D.; Wells, L.; Dougan, S.T. O-GlcNAc modifications regulate cell survival and epiboly during zebrafish development. BMC Dev Biol. 2009, 9, 28. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.-L.; Chen, S.; Xu, J.-H.; Yi, L.-F.; Peng, Y.-X.; Pan, Q.; Shen, X.; Dong, Z.-G.; Zhang, X.-Q.; Wang, W.-X. Molecular cloning and nutrient regulation analysis of long chain acyl-CoA synthetase 1 gene in grass carp, Ctenopharyngodon idella L. Comp Biochem Physiol B Biochem Mol Biol. 2017, 204, 61–68. [Google Scholar] [CrossRef] [PubMed]
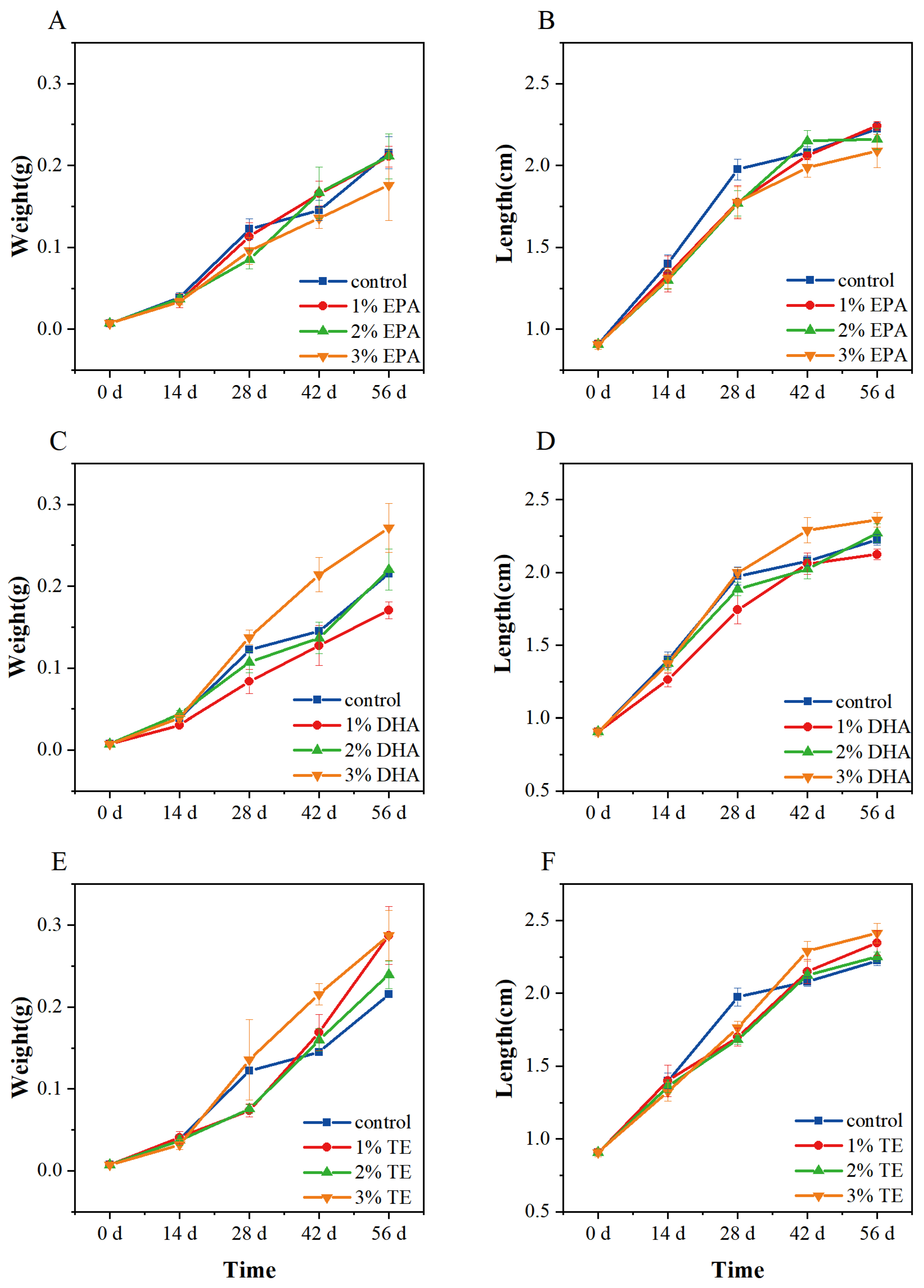


| Growth Parameters | Control | 1% EPA | 2% EPA | 3% EPA |
| RWG (%/day) | 60.52 ± 4.63 | 59.02 ± 3.28 | 59.10 ± 3.35 | 37.81 ± 2.32 ** |
| SGR (%/day) | 6.33 ± 0.14 | 6.29 ± 0.10 | 6.30 ± 0.10 | 5.53 ± 0.11 ** |
| CF (g/cm3) | 2.07 ± 0.10 | 2.03 ± 0.14 | 2.14 ± 0.08 | 2.11 ± 0.13 |
| SR (%) | 93.33 ± 0.27 | 90.00 ± 0.47 | 90.00 ± 0.82 | 91.67 ± 0.27 |
| Growth Parameters | Control | 1% DHA | 2% DHA | 3% DHA |
| RWG(%/day) | 60.52 ± 4.63 | 47.44 ± 2.99 * | 61.52 ± 2.11 | 76.26 ± 3.12 * |
| SGR (%/day) | 6.33 ± 0.14 | 5.92 ± 0.11 * | 6.37 ± 0.06 | 6.75 ± 0.07 * |
| CF (g/cm3) | 2.07 ± 0.10 | 2.02 ± 0.05 | 1.90 ± 0.14 | 2.19 ± 0.23 |
| SR (%) | 93.33 ± 0.27 | 88.33 ± 0.27 | 90.00 ± 0.47 | 93.33 ± 0.54 |
| Growth Parameters | Control | 1% TE | 2% TE | 3% TE |
| RWG (%/day) | 60.52 ± 4.63 | 80.73 ± 2.33 ** | 80.80 ± 3.74 ** | 67.35 ± 4.80 |
| SGR (%/day) | 6.33 ± 0.14 | 6.84 ± 0.05 * | 6.84 ± 0.08 * | 6.52 ± 0.12 |
| CF (g/cm3) | 2.07 ± 0.10 | 2.23 ± 0.03 | 2.19 ± 0.09 | 2.00 ± 0.04 |
| SR (%) | 93.33 ± 0.27 | 91.67 ± 0.72 | 95.00 ± 0.47 | 93.33 ± 0.27 |
| Samples | Clean Reads | Clean Bases | GC Content | ≥Q30% |
|---|---|---|---|---|
| C-1 | 20,237,339 | 6,043,571,206 | 48.47% | 93.81% |
| C-2 | 22,833,729 | 6,803,261,102 | 48.57% | 94.47% |
| C-3 | 29,402,492 | 8,774,618,350 | 48.43% | 94.45% |
| T-1 | 22,681,184 | 6,757,938,492 | 48.19% | 94.47% |
| T-2 | 22,571,833 | 6,730,129,406 | 48.49% | 94.35% |
| T-3 | 21,741,708 | 6,489,332,016 | 48.26% | 94.21% |
| Sample | Total Reads | Mapped Reads | Uniq Mapped Reads | Multiple Map Reads | Reads Map to ‘+’ | Reads Map to ‘−’ |
|---|---|---|---|---|---|---|
| C-1 | 40,474,678 | 36,863,629 (91.08%) | 34,099,049 (84.25%) | 2,764,580 (6.83%) | 18,396,027 (45.45%) | 18,428,782 (45.53%) |
| C-2 | 45,667,458 | 42,081,877 (92.15%) | 39,375,737 (86.22%) | 2,706,140 (5.93%) | 20,994,119 (45.97%) | 21,028,413 (46.05%) |
| C-3 | 58,804,984 | 53,960,399 (91.76%) | 50,048,173 (85.11%) | 3,912,226 (6.65%) | 26,914,470 (45.77%) | 26,978,224 (45.88%) |
| T-1 | 45,362,368 | 41,432,683 (91.34%) | 37,606,267 (82.90%) | 3,826,416 (8.44%) | 20,648,798 (45.52%) | 20,715,844 (45.67%) |
| T-2 | 45,143,666 | 40,850,423 (90.49%) | 37,875,132 (83.90%) | 2,975,291 (6.59%) | 20,380,508 (45.15%) | 20,420,021 (45.23%) |
| T-3 | 43,483,416 | 39,832,673 (91.60%) | 36,965,709 (85.01%) | 2,866,964 (6.59%) | 19,868,815 (45.69%) | 19,905,921 (45.78%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, H.; Huang, Y.; Li, Z.; Guo, Y.; Li, S.; Huang, H.; Yang, X.; Li, G.; Chen, H. Effects of Dietary Supplementation with Aurantiochytrium sp. on Zebrafish Growth as Determined by Transcriptomics. Animals 2022, 12, 2794. https://doi.org/10.3390/ani12202794
Yang H, Huang Y, Li Z, Guo Y, Li S, Huang H, Yang X, Li G, Chen H. Effects of Dietary Supplementation with Aurantiochytrium sp. on Zebrafish Growth as Determined by Transcriptomics. Animals. 2022; 12(20):2794. https://doi.org/10.3390/ani12202794
Chicago/Turabian StyleYang, Hao, Yanlin Huang, Zhiyuan Li, Yuwen Guo, Shuangfei Li, Hai Huang, Xuewei Yang, Guangli Li, and Huapu Chen. 2022. "Effects of Dietary Supplementation with Aurantiochytrium sp. on Zebrafish Growth as Determined by Transcriptomics" Animals 12, no. 20: 2794. https://doi.org/10.3390/ani12202794
APA StyleYang, H., Huang, Y., Li, Z., Guo, Y., Li, S., Huang, H., Yang, X., Li, G., & Chen, H. (2022). Effects of Dietary Supplementation with Aurantiochytrium sp. on Zebrafish Growth as Determined by Transcriptomics. Animals, 12(20), 2794. https://doi.org/10.3390/ani12202794







